A self-immolative dendritic glucuronide prodrug of doxorubicin†
Marion
Grinda
a,
Jonathan
Clarhaut
bc,
Brigitte
Renoux
a,
Isabelle
Tranoy-Opalinski
a and
Sébastien
Papot
*a
aUniversité de Poitiers, UMR-CNRS 6514, 4 rue Michel Brunet, BP 633, 86022, Poitiers, France. E-mail: sebastien.papot@univ-poitiers.fr; Fax: +33 549 453 501; Tel: +33 549 453 862
bUniversité de Poitiers, UMR-CNRS 6187, 1 rue Georges Bonnet, BP 633, 86022, Poitiers, France
cINSERM CIC 0802, 2 rue de la Milétrie, CHU de Poitiers, 86021, Poitiers, France
First published on 19th October 2011
Abstract
The first self-immolative dendritic glucuronide prodrug of doxorubicin was studied with the aim to target β-glucuronidase overexpressed in the microenvironment of numerous tumors. This compound includes a chemical amplifier programmed to release two molecules of doxorubicin after a single enzymatic activation step. Upon β-glucuronidase activation, the dendritic prodrug was twice more toxic than its monomeric counterpart against H661 lung cancer cells.
Introduction
Nowadays, cancer chemotherapy is not entirely effective against many common solid tumor types. Most anticancer drugs lack any intrinsic antitumor selectivity causing severe side effects due to massive destruction of normal tissues. To circumvent this drawback, many drug carriers designed to deliver potent cytostatic agents exclusively at the tumor site have been investigated.1 In this context, the use of non-toxic prodrugs that can be activated by an enzyme naturally overexpressed in the tumor microenvironment has emerged as a promising strategy to enhance the selectivity of chemotherapy.2 In this approach, enzymatic prodrug activation is followed by the release of the parent drug thereby restoring its antitumor activity selectively in malignant tissues.β-Glucuronidase has been detected at high concentration in a wide range of malignancies including breast, lung, colon and ovarian carcinomas.3 In 1988, Tietze4 was the first who proposed to target this tumor specificity by means of enzyme-responsive prodrugs in the course of a prodrug monotherapy (PMT3b). Ever since then, the validity of this targeting strategy was demonstrated with several glucuronide prodrugs.5 Within this framework, one of us has been involved in the development of a glucuronide prodrug of doxorubicin, HMR 1826,6 which led to superior therapeutic efficacy compared to standard treatment in various xenograft models.7 However, the efficiency of HMR 1826 was limited by the poor turnover of β-glucuronidase in the tumor microenvironment. Indeed, the optimal pH for activity of β-glucuronidase is approximately 4 whereas that of the tumor extracellular medium is 6–7. Thus, above a certain threshold dose of prodrug the enzyme is saturated and consequently the amount of drug liberated in targeted tissues is not sufficient to induce total and lasting remission of the tumor.
As proposed earlier by Shabat et al.,8 de Groot et al.9 and McGrath et al.,10 this problem could be overcome by the use of self-immolative dendrimers allowing the release of several drug units after a single enzymatic activation step. Thus, we decided to study the dendritic glucuronide prodrug 1 designed for the selective targeting of two doxorubicins (Fig. 1). This targeting system is composed of five units comprising an enzymatic trigger, a self-immolative linker11 and two drug molecules articulated around a chemical amplifier. With this design, enzymatic hydrolysis of the glycosidic bond should result in the formation of the phenol intermediate 2 which will induce the release of the aniline 3via an 1,6 elimination. The amplifier unit will then conduct to the expulsion of two doxorubicins through successive 1,4 and 1,6 elimination processes as depicted in Fig. 1.12 Therefore, in the presence of the same quantity of β-glucuronidase, the dendritic glucuronide prodrug 1 should be twice more toxic than its monomeric counterpart HMR 1826.
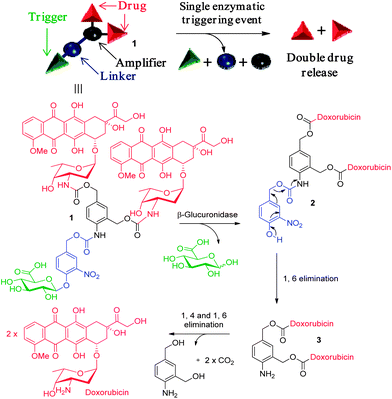 | ||
| Fig. 1 β-Glucuronidase-catalysed drug release mechanism. | ||
Results and discussion
Chemistry
In order to access the dendritic glucuronide prodrug 1, our initial efforts focused upon the synthetic route illustrated in Scheme 1. Thus, the β-O-glucuronyl carbamate 6 was synthesized by coupling the readily accessible carbonate 4 (ref. 13) with the aniline 5 (ref. 14) (70%). The two primary alcohols were then activated in the presence of 4-nitrophenyl chloroformate to produce the bis-carbonate 7 in an excellent 98% yield after purification by flash column chromatography. The synthesis was pursued by the introduction of one molecule of doxorubicin on each carbonate to form the protected dendritic prodrug 8 (57%). The deprotection of the glucuronide moiety was envisioned under classical conditions in the course of a two step strategy. The O-acetyl groups of compound 8 were first removed using a catalytic amount of MeONa to afford the hydroxyl free derivative 9 (81%).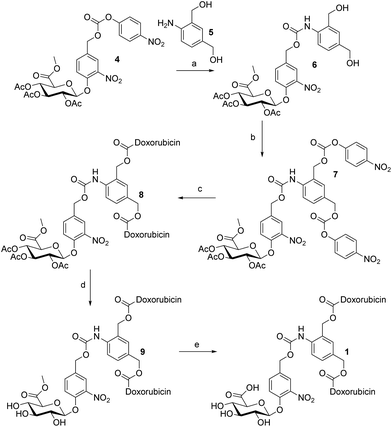 | ||
| Scheme 1 Reagents and conditions: (a) 5, HOBt, DMF, 50 °C, 3 h, 70%; (b) p-nitrophenyl chloroformate, pyridine, CH2Cl2, 0 °C to RT, 1 h, 98%; (c) doxorubicin (HCl), Et3N, HOBt, DMF, RT, 3 h, 57%; (d) MeONa/MeOH, −10 °C, 3 h, 81%; and (e) NaOH, H2O/THF, −5 °C or −15 °C, 15 min or 2 h. | ||
The cleavage of the methyl ester was undertaken with NaOH in H2O/THF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) at −5 °C for 2 hours. In this case, the synthesis of prodrug 1 was confirmed by HPLC/MS analysis of the crude mixture. However, this was accompanied by the formation of several side products making impossible the purification of compound 1 (see the ESI†). Other attempts at a lower temperature (−15 °C) or with a shorter reaction time (15 min) gave similar results. At this stage, although the reasons of this failure remain unclear, we considered it preferable to turn our attention to other protecting groups for the carbohydrate moiety. Thus, we decided to start again the synthesis of prodrug 1 from the fully allyl protected bis-carbonate 10 recently prepared in our laboratory (Scheme 2).15 Indeed, both allyl ester and carbonates are compatible with the presence of either alkali- or acid-sensitive anticancer drugs.16 Furthermore, the entire deprotection of the glucuronide can be achieved in a one step procedure under mild conditions in the presence of Pd(0) as a catalyst.
1) at −5 °C for 2 hours. In this case, the synthesis of prodrug 1 was confirmed by HPLC/MS analysis of the crude mixture. However, this was accompanied by the formation of several side products making impossible the purification of compound 1 (see the ESI†). Other attempts at a lower temperature (−15 °C) or with a shorter reaction time (15 min) gave similar results. At this stage, although the reasons of this failure remain unclear, we considered it preferable to turn our attention to other protecting groups for the carbohydrate moiety. Thus, we decided to start again the synthesis of prodrug 1 from the fully allyl protected bis-carbonate 10 recently prepared in our laboratory (Scheme 2).15 Indeed, both allyl ester and carbonates are compatible with the presence of either alkali- or acid-sensitive anticancer drugs.16 Furthermore, the entire deprotection of the glucuronide can be achieved in a one step procedure under mild conditions in the presence of Pd(0) as a catalyst.
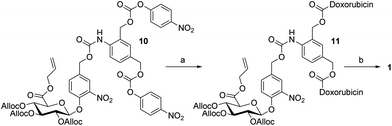 | ||
| Scheme 2 Reagents and conditions: (a) doxorubicin (HCl), Et3N, HOBt, DMF, RT, 3 h, 58%; and (b) Pd(PPh3)4, aniline, MeOH/CH2Cl2, RT, 24 h, 80%. | ||
Compound 10 reacted with doxorubicin to yield the bis-carbamate 11 (58%). No difference of reactivity was recorded between the bis-carbonates 7 and 10 for this coupling reaction. Finally, the dendritic prodrug 1 was obtained by the cleavage of protecting groups performed in the presence of a catalytic amount of Pd(PPh3)4 and two equivalents of aniline (80%). As expected, under such conditions the full deprotection of the glucuronide moiety proceeded cleanly avoiding the formation of the side products observed previously.
Enzymatic assays
The stability of prodrug 1 was examined in a 0.02 M phosphate buffer (pH 7) at 37 °C. The evolution of the mixture was followed by HPLC over a period of 10 days. No detectable decomposition of 1 has been observed in the course of this experiment. The stability of prodrug 1 was further investigated at 37 °C in bovine serum for 24 h. Again, the prodrug appeared stable with no detectable degradation. Enzymatic hydrolysis of glucuronide 1 was then conducted with β-glucuronidase under physiological conditions (phosphate buffer, pH 7, 37 °C) and the kinetics of drug release was monitored by HPLC (Fig. 2).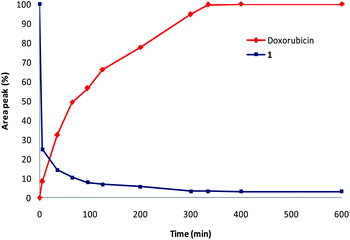 | ||
| Fig. 2 E. coli β-glucuronidase-catalysed release of doxorubicin from prodrug 1 (phosphate buffer, pH 7, 37 °C). | ||
As soon as incubated with the enzyme, prodrug 1 rapidly disappeared concomitantly with the appearance of doxorubicin. Thanks to the presence of the nitrobenzylphenoxy carbamate linker, the glucuronide trigger is substantially far away from the two bulky drug units to allow an easy recognition of 1 by β-glucuronidase. Only six hours after the addition of the enzyme, the liberation of doxorubicin was completed. Thus, in spite of the complexity of the double drug release mechanism from prodrug 1, it appeared that the whole process occurred relatively rapidly.
Biological evaluations
Prodrug 1 was then tested for its anti-proliferative activity against H661 lung cancer cell line and compared to that of HMR 1826 and doxorubicin (Fig. 3).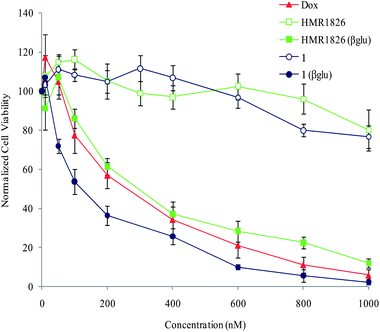 | ||
| Fig. 3 Cell viability of H661 cells treated 3 days with indicated compounds from 10 to 1000 nM. Graphs represent mean +/− SEM of four independent experiments performed in triplicate. | ||
When incubated alone compound 1 did not exhibit significant toxicity until the highest tested dose of 1000 nM. This result indicated that the derivatisation of doxorubicin in the form of the prodrug 1 markedly reduced its anti-proliferative activity. The reduced toxicity could be attributed to the hydrophilicity imparted by the glucuronide trigger which probably prevented passive cellular uptake and further intracellular activation of prodrug 1 by lysosomal β-glucuronidase. On the other hand, addition of the enzyme in the culture medium produced a dramatic anti-proliferative effect with an IC50 value of 110 nM. In contrast, the recorded cytotoxicity for HMR 1826 in the presence of the same quantity of β-glucuronidase was approximately 2-fold lower and similar to that obtained for doxorubicin (IC50 = 280 nM and 250 nM respectively). All together, these results confirm the β-glucuronidase-catalysed double drug release from prodrug 1 in the cell culture medium.
Conclusions
In conclusion, we developed the first self-immolative dendritic glucuronide prodrug programmed to release two molecules of the potent doxorubicin after a single enzymatic activation step. Upon β-glucuronidase activation, the dendritic prodrug is twice more toxic than its monomeric counterpart as well as doxorubicin at the same concentration. This targeting system could represent an important step toward the quest for the magic bullet in cancer chemotherapy. Further developments in this direction are underway in our laboratory.Acknowledgements
The authors thank CNRS, La Ligue Nationale contre le Cancer (comité charente-maritime) for financial support of this study and Prof. J.-P. Gesson for many fruitful discussions.Notes and references
- F. Kratz, I. A. Müller, C. Ryppa and A. Warnecke, ChemMedChem, 2008, 3, 20–53 CrossRef CAS.
- M. Rooseboom, J. N. M. Commandeur and N. P. E. Vermeulen, Pharm. Rev., 2004, 56, 53–102 CrossRef CAS.
- (a) T. A. Connors and M. E. Whisson, Nature, 1966, 210, 866–867 CrossRef CAS; (b) K. Bosslet, J. Czecha and D. Hoffmann, Tumor Targeting, 1995, 1, 45–50 CAS.
- L. F. Tietze, R. Seele, B. Leiting and T. Krach, Carbohydr. Res., 1988, 180, 253–262 CrossRef CAS.
- (a) For reviews see: M. de Graaf, E. Boven, H. W. Scheeren, H. J. Haisma and H. M. Pinedo, Curr. Pharm. Des., 2002, 8, 1391–1403 CrossRef CAS; (b) X. Chen, B. Wu and P. G. Wang, Curr. Med. Chem.: Anti-Cancer Agents, 2003, 3, 139–150 CrossRef CAS.
- J.-C. Florent, X. Dong, G. Gaudel, S. Mitaku, C. Monneret, J.-P. Gesson, J.-C. Jacquesy, M. Mondon, B. Renoux, S. Andrianomenjanahary, S. Michel, M. Koch, F. Tillequin, M. Gerken, J. Czech, R. Straub and K. Bosslet, J. Med. Chem., 1998, 41, 3572–3581 CrossRef CAS.
- K. Bosslet, R. Straub, M. Blumrich, J. Czech, M. Gerken, B. Sperker, H. K. Kroemer, J.-P. Gesson, M. Koch and C. Monneret, Cancer Res., 1998, 58, 1195–1201 CAS.
- (a) R. J. Amir, N. Pessah, M. Shamis and D. Shabat, Angew. Chem., Int. Ed., 2003, 42, 4494–4499 CrossRef CAS; (b) A. Gopin, S. Ebner, B. Attali and D. Shabat, Bioconjugate Chem., 2006, 17, 1432–1440 CrossRef CAS; (c) A. Sagi, E. Segal, R. Satchi-Fainaro and D. Shabat, Bioorg. Med. Chem., 2007, 15, 3720–3727 CrossRef CAS; (d) M. Shamis and D. Shabat, Chem.–Eur. J., 2007, 13, 4523–4528 CrossRef CAS; (e) R. Erez, E. Segal, K. Miller, R. Satchi-Fainaro and D. Shabat, Bioorg. Med. Chem., 2009, 17, 4327–4335 CrossRef CAS.
- F. M. H. De Groot, C. Albrecht, R. Koekkoek, P. H. Beusker and H. W. Scheeren, Angew. Chem., Int. Ed., 2003, 42, 4490–4494 CrossRef CAS.
- S. Li, M. L. Szalai, R. M. Kevwitch and D. V. McGrath, J. Am. Chem. Soc., 2003, 125, 10516–10517 CrossRef CAS.
- (a) For reviews see: S. Papot, I. Tranoy, F. Tillequin, J.-C. Florent and J.-P. Gesson, Curr. Med. Chem.: Anti-Cancer Agents, 2002, 2, 155–185 CrossRef CAS; (b) I. Tranoy-Opalinski, A. Fernandes, M. Thomas, J.-P. Gesson and S. Papot, Anti-Cancer Agents Med. Chem., 2008, 8, 618–637 CAS.
- A. Warnecke and F. Kratz, J. Org. Chem., 2008, 73, 1546–1552 CrossRef CAS.
- S. Papot, D. Combaud, K. Bosslet, M. Gerken, J. Czech and J.-P. Gesson, Bioorg. Med. Chem. Lett., 2000, 10, 1835–1837 CrossRef CAS.
- R. Erez and D. Shabat, Org. Biomol. Chem., 2008, 6, 2669–2672 CAS.
- M. Grinda, J. Clarhaut, I. Tranoy-Opalinski, B. Renoux, A. Monvoisin, L. Cronier and S. Papot, ChemMedChem, DOI:10.1002/cmdc.201100355, in press.
- A. El Alaoui, F. Schmidt, C. Monneret and J.-C. Florent, J. Org. Chem., 2006, 71, 9628–9636 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: General experimental procedures, chemical structure of HMR 1826, synthesis and NMR spectra of the compounds, cell culture and cell proliferation assays. See DOI: 10.1039/c1md00193k |
| This journal is © The Royal Society of Chemistry 2012 |
