Research highlights
Šeila
Selimović
ab,
Mehmet R.
Dokmeci
ab and
Ali
Khademhosseini
*abcd
aCenter for Biomedical Engineering, Department of Medicine, Brigham and Women's Hospital, Harvard Medical School, Cambridge, Massachusetts 02139, USA. E-mail: alik@rics.bwh.harvard.edu
bHarvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139, USA
cWyss Institute for Biologically Inspired Engineering, Harvard University, Boston, Massachusetts 02115, USA
dWorld Premier International – Advanced Institute for Materials Research (WPI-AIMR), Tohoku University, Sendai 980-8577, Japan
First published on 14th March 2012
Network logic on a chip
A network can be represented as a set of nodes connected by paths (e.g. channels, wires, roads, and biological signaling pathways). One of the main functions of a network is the transportation of components – such as droplets, energy, vehicles, molecules etc.1–5 An abstract network consisting of lines, nodes and externally driven (passive) discrete components can be modeled mathematically, if it is sufficiently simple. However, most real networks are highly complex and irregular. General solutions, such as finding the shortest path, do not always apply and the local environment may be more important than the global. With this in mind, Whitesides and colleagues created a fluidic model of a branched network to study the transport and response of discrete components to local and global system characteristics.The model developed by Choi et al.6 was based on a series of interconnected poly(dimethylsiloxane) (PDMS)-based microfluidic channels filled with surfactant-containing water and nitrogen gas bubbles. In most experiments, the distance between two gas bubbles was large enough so that one bubble had completely passed through the network before the next one entered it. The gas bubbles were larger than the channel diameter, thereby increasing the resistance along the path, but due to the low capillary number, the bubbles did not break at channel junctions. The flow was pressure-driven and laminar and a no-slip condition was maintained.
The authors first approached the problem of path selection in a branched network by single phase fluid (water). The flow in such networks is analogous to electrical circuits, such that the drop in pressure is an analog to a drop in voltage and the fluidic resistance is similar to electrical resistance (i.e. Ohm's Law). Calculations of the flow distributions confirmed that the flow depended on the global structure of the network.
Surprising observations were made when the authors added gas bubbles to the water. At a junction between a low-resistance and high-resistance path, (e.g. the two paths varied in length), a bubble would choose the shorter (low-resistance) path, as expected. However, when faced with two possible paths whose total lengths and therefore resistances were equal, but which had local variations in width, a bubble would repeatedly enter the channel in which it initially had a faster velocity. Thus, in a globally symmetric structure, the flow path of bubbles was determined by local variations.
The effect of local flow rate differences was also the deciding factor in asymmetrical networks. In Fig. 1, path A is the shortest path of all possible choices, and is much shorter than path B. Thus, path A had a higher flow rate than path B. Yet, bubbles consistently preferred to travel along the longer path. It was concluded that here, too, the flow rate in the channels branching from each junction, not along the entire path, was the determining factor in the choice of path, and that bubbles at a T-junction chose to enter the channel which had a higher flow rate.
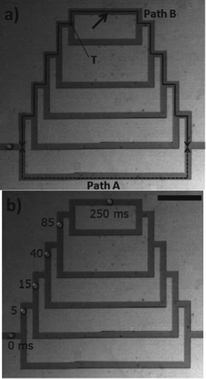 | ||
| Fig. 1 A bubble in a branched microfluidic network chooses longer path B over path A. Scale bar: 1 mm. Figure adapted and reprinted with permission from the Royal Society of Chemistry from Choi et al.6 | ||
In an even more complex network, which transported two or more bubbles at the same time, the path choice was additionally influenced by the presence of other bubbles downstream. Each bubble entering a channel increased the fluidic resistance of that channel, which in turn influenced the flow path of other bubbles.
As one of the first fundamental studies of component transport in moderately complex networks, this research revealed a stark contrast between local and global structures. Single phase flows are determined by the global architecture, while discrete components such as bubbles are subject to various local variations. As such, this study could lead to more advanced results, including a potential analytical description of the system, which might lead to predictive results for more complex networks.
Bubble-free microfluidics
The ability to control flow inside microfluidic devices is crucial for conducting experiments reliably.7 For example, in droplet-forming devices the flow rates of all fluids have to be tightly regulated to produce droplets of a desired size with a necessary spacing. Microscale geometries that rely on diffusion-driven processes, such as most concentration gradient generators, also require precisely controlled flows. In addition, cell culture-on-a-chip relies on controlled fluid flows that do not cause a loss of cellular viability.One of the most challenging problems in maintaining cells inside a microfluidic chip is the presence of gas bubbles inside microfluidic structures. In their latest design, Günther and co-workers developed a simple, yet powerful method for the removal of gas bubbles. The bubble trap designed by Lochovsky et al.8 consisted of a circular chamber, cylindrical trapping pillars and a separate vacuum channel (Fig. 2), all of which were fabricated using a single layer PDMS chip. The bubble trap was attached to the input fluidic channel, such that the liquid carrying a gas bubble would enter the trapping chamber, deposit the bubble behind the pillars and continue to flow along the fluidic path towards other microscale structures. At moderate pressures, bubbles 0.07 nl and larger could not be sufficiently deformed to pass between the pillars and remained stuck in the trapping chamber. At the same time, the pillars were designed to maintain a low fluidic resistance in the device to prevent high shear stresses and device failure.
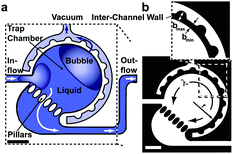 | ||
| Fig. 2 Illustration (a) and design of the bubble trap (b). In (b), black indicates the solid device material and white indicates fluidic structures. Scale bars: 500 μm. Figure adapted and reprinted with permission from the Royal Society of Chemistry from Lochovsky et al.8 | ||
Aside from the pillars, which were designed to remove the bubbles from the fluidic path, the trap featured a vacuum channel for complete extraction of the gas. This channel was placed adjacent to and surrounding the trapping chamber. When a vacuum line was connected to this channel, the trapped bubble diffused through the gas-permeable PDMS wall from the trapping chamber into the vacuum channel.
As expected, small gas bubbles were removed faster than large ones, however all bubbles on the order of μl or less were extracted within a few minutes. An increase in the pressure difference between the trapping chamber and the vacuum channel (75–95 kPa) also sped up the degassing process. Furthermore, the trapping feature proved reliable, as over the course of a 24 h study over 4000 gas bubbles (∼0.02 μl) were removed in regular intervals during continuous flow without bubbles being detected downstream. In addition, the authors showed that adding several traps in parallel increased the operating efficiency of the device. An array of 8 traps could remove bubbles more than three times faster than a single trap.
With an area of less than 10 mm2, the gas bubble trap can be easily included at the inlet of any PDMS device. Also, the bubble traps appear robust and capable of removing bubbles from a range of aqueous and surfactant solutions with different interfacial properties. Thus, this structure seems suitable for a variety of microfluidic applications. Despite numerous merits, future technological advances could further enhance this approach. For example, an ideal bubble removal device would also be applicable at larger flow rates, and it may distinguishing between small particles (e.g. colloids or cells) and bubbles of the same size, and should be compatible with gas-impermeable materials such as polyester and glass.
Squishy bubbles-on-chips
The term rheology refers to the study of flow of matter, including liquids, suspensions, and viscoelastic materials, such as gels. Examples of such ‘soft’ materials include bodily fluids, foods such as honey or peanut butter, slush and various polymers. Microrheology, then, is the probing of these systems at the microscale by tracking the Brownian motion of suspended tracer particles.9,10 Microfluidic devices are versatile experimental platforms for microrheological studies as they offer excellent control over flow, require small amounts of reagents, and are usually compatible with video microscopy setups.In this context, Schultz and Furst11 have recently illustrated the applicability of a simple microfluidic device for high-throughput microrheological investigations. They fabricated a PDMS chip on a glass substrate for encapsulation of aqueous rheological samples in droplets surrounded by oil. The flow channels were relatively large (1800 μm wide and 700 μm tall) and could accommodate droplets that were large enough to avoid hydrodynamic interactions between the tracer particles and the inner drop surface. The channels were rendered fully hydrophobic to allow for a stable water-in-oil emulsion.
The chip contained two T-junctions for mixing a semi-dilute aqueous particle solution with glycerin in varying ratios and encapsulating it in droplets sheared off by silicone oil. The droplets, each of which had a slightly different composition of the two solutions to form a large-scale concentration gradient, were continuously being generated until they filled a serpentine storage channel. The shape of this channel aided the mixing of the two solutions, as the droplets experienced chaotic advection when turning corners. Upon loading, the device ports were sealed with UV-sensitive resin and the drops remained stationary inside the storage channel.
Each droplet was recorded at a high magnification (63×) using a high-speed video camera that was focused on the center of the drop to avoid inclusion of edge effects. At least 100 tracer particles were recorded in each movie to allow for ensemble-averaging of their trajectories and mean-square displacement. By applying the generalized Stokes–Einstein equation, Schultz and Furst related this mean-square displacement to the materials' creep compliance and obtained the viscosity of the sample.
Measurements of glycerine viscosities at different concentrations using the described technique corresponded well with published results. As expected, the sample viscosity increased with incremental glycerin concentrations. Furthermore, the measurement error increased as the limit of the measurement method due to high sample viscosity (∼75% wt glycerin) was approached.
The same experimental and data analysis approach was taken to determine the viscosity of different high molecular weight heparin solutions. The measured viscosity values were comparable to those obtained from discrete microrheology and rolling-ball viscometry experiments. Further, log–log plots of the specific viscosity of the polymer as a function of its concentration revealed the presence of distinct dilute and semi-dilute physical regimes. Specific viscosity describes the contribution of the solute (heparin) to the overall sample viscosity, hence the measurement of this parameter offers additional information about the mutual interactions of the polymer molecules. Here, the overlap concentration between the two regimes, ∼6% wt of heparin, was in agreement with the calculated value.
The operating limit of the presented technique is defined by two factors: the ability to trace freely diffusing particles inside the droplets without the use of an external force and to form droplets in the required size range. The first constraint limits the technique to samples of 0.15 Pa s viscosity or 5 Pa equilibrium modulus, when 1 μm beads are used; alternatively, smaller tracer particles can be chosen. The second constraint can be addressed by adjusting the device geometry. Despite these limitations, the presented method is still noteworthy, as it combines the principles of microrheology with smart microfluidics engineering to arrive at a high-throughput measurement technique for the study of soft materials.
References
- K. Börner, S. Sanyal and A. Vespignani, Network science, Annual Review of Information Science and Technology, 2007, 41(1), 537–607 CrossRef.
- A.-L. Barabasi and Z. N. Oltvai, Network biology: understanding the cell's functional organization, Nat. Rev. Genet., 2004, 5(2), 101–113 CrossRef CAS.
- H. Yang and M. G. H. Bell, Models and algorithms for road network design: a review and some new developments, Transp. Rev., 1998, 18(3), 257–278 CrossRef.
- T. G. Lewis, Frontmatter, in Network Science. 2008, John Wiley & Sons, Inc. p. i-xi Search PubMed.
- C. H. Hauser, D. E. Bakken and A. Bose, A failure to communicate: next generation communication requirements, technologies, and architecture for the electric power grid, Power and Energy Magazine, IEEE, 2005, 3(2), 47–55 CrossRef.
- W. Choi, et al., Bubbles navigating through networks of microchannels, Lab Chip, 2011, 11(23), 3970–3978 RSC.
- H. A. Stone, A. D. Stroock and A. Ajdari, Engineering flows in small devices, Annu. Rev. Fluid Mech., 2004, 36(1), 381–411 CrossRef.
- C. Lochovsky, S. Yasotharan and A. Gunther, Bubbles no more: in-plane trapping and removal of bubbles in microfluidic devices, Lab Chip, 2012, 12(3), 595–601 RSC.
- P. Cicuta and A. M. Donald, Microrheology: a review of the method and applications, Soft Matter, 2007, 3(12), 1449–1455 RSC.
- T. M. Squires and T. G. Mason, Fluid Mechanics of Microrheology, Annu. Rev. Fluid Mech., 2010, 42(1), 413–438 CrossRef.
- K. M. Schultz and E. M. Furst, High-throughput rheology in a microfluidic device, Lab Chip, 2011, 11(22), 3802–3809 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
