Elastomer based tunable optofluidic devices†
Wuzhou
Song
,
Andreas E.
Vasdekis
and
Demetri
Psaltis
School of Engineering, Ecole Polytechnique Fédérale de Lausanne (EPFL), CH-1015, Lausanne, Switzerland
First published on 19th June 2012
Abstract
The synergetic integration of photonics and microfluidics has enabled a wide range of optofluidic devices that can be tuned based on various physical mechanisms. One such tuning mechanism can be realized based on the elasticity of polydimethylsiloxane (PDMS). The mechanical tuning of these optofluidic devices was achieved by modifying the geometry of the device upon applying internal or external forces. External or internal forces can deform the elastomeric components that in turn can alter the optical properties of the device or directly induce flow. In this review, we discuss recent progress in tunable optofluidic devices, where tunability is enabled by the elasticity of the construction material. Different subtypes of such tuning methods will be summarized, namely tuning based on bulk or membrane deformations, and pneumatic actuation.
Wuzhou recently obtained his doctoral degree in photonics under the supervision of Prof. Demetri Psaltis in EPFL (Switzerland, 2012). He previously obtained his bachelor’s degree in physics from Wuhan University (China, 2005) and master’s degree in photonics from Nanyang Technology University (Singapore, 2007). He is now working on optofluidics, micro-optics, nanofluidics, nano-plasmonics and spectroscopy. |
Andreas received his college and graduate degrees in physics from Athens University (Greece, 2002) and the University of St. Andrews (UK, 2008), respectively. During this period, he developed an interest in optics and soft materials. He subsequently joined the Optics Laboratory, exploring optofluidics under the direction of Prof. Psaltis until 2012, when he moved to Pacific Northwest National Laboratories (USA) as a staff member. His current research focuses on developing and applying precision measurements using optofluidics in biophysics and energy. |
Demetri Psaltis is a Professor of Optics at the Ecole Polytechnique Fédérale de Lausanne (EPFL) in Switzerland. He was educated at Carnegie-Mellon University and started his academic career at Caltech, where he was the Thomas G. Myers Professor of Electrical Engineering until 2007, when he moved to Lausanne. His research is currently focused on optofluidics, imaging methods, and biophotonics. |
1. Introduction
During the last few years, as a multidisciplinary research field, optofluidics has been gaining significant progress by combining microfluidics with optical components and methods.1–5 Although the idea of using fluids to construct optical devices can be traced back hundreds of years6 to when the liquid mirror and waveguide were invented, the emergence of optofluidics was preceded by the development of microfluidics that has led to the miniaturization of the fluidic circuit and its control. Similar to the development of microfluidics which has enabled the integration of multiple fluidic tasks on a chip, optofluidic integration provides a broad spectrum of optical toolkits by combining optics and microfluidics.Integration and reconfigurability are the two major advantages associated with optofluidics. Optofluidic devices show exceptional capability for adaptation and tunability resulting from their construction with fluidic materials and also the elastomer materials often used for construction of the microfluidic channels. For example, liquids hold a wide choice of different refractive indices and optical nonlinear properties. A specific refractive index can be easily realized by fluidic mixing. Liquids can also be doped with different gain or absorbing media, such as fluorescent dyes or plasmonic particles to achieve various properties.7,8 In addition, liquids can naturally form smooth boundaries of optical quality.9,10 Moreover, due to the inherent property of the liquid state, the shape of the fluid can be variable. For instance, through the electrowetting effect, several tunable optofluidic devices have been developed and commercialized successfully, most notably the tunable liquid lens and electronic paper.11,12
In this review, we focus on a different group of tunable optofluidic devices, based on the elasticity of the solid-state material that defines the devices themselves. While similar efforts have been reported for mechanically tunable optical devices based on crystalline materials,13–15 herein our focus is the employment of soft materials, such as polydimethylsiloxane (PDMS), and specifically how the elastomer deformation can give rise to optical tunability. The latter can take place in two ways. The first is by physical deformations of embedded optical microstructures (e.g. a photonic crystal, Fig. 1a) or membranes (Fig. 1b) that in turn modify the optical boundary conditions. The second is light affecting the shape or geometry of the elastomers giving rise to flow, but also the opposite tunability emerges due to flow (Fig. 1c).
 | ||
| Fig. 1 A schematic summary of the different possibilities for inducing optical tunability in optofluidics by applying external or internal forces on elastomers. | ||
As an elastomer, PDMS is one of the most versatile materials in optofluidics, exhibiting excellent elasticity and optical properties. PDMS is also biocompatible,16 inexpensive, transparent (240 nm–1100 nm) with low autofluorescence,17 and it can be molded with resolution of several nanometers using a modified chemical composition of PDMS.18 PDMS has a refractive index of around 1.41 with a visible and negative thermo-optic coefficient corresponding to dn/dT = −4.5 × 10−4 °C−1.17,19 A PDMS replica can be covalently attached to a glass substrate using a simple plasma treatment to form a sealed microfluidic device. The deformability of PDMS allows the realization of leak proof fluidic connections and easy integration of fluidic membrane valves that can be also included in an optofluidic chip. Large-scale integration of monolithic microfluidic valves and pumps on PDMS chip has been realized by multilayer soft lithography,20,21 novel trapping methods22 and also tunable microstructure separation.23 Many pioneering optofluidic efforts were made using PDMS microfluidic chips.24,25
There are two principal methods to alter the geometry of the optical elements in PDMS based tunable optofluidic devices. The tuning can be actuated by external or internal forces. For example, in the mechanical tunable optofluidic distributed feedback (DFB) dye laser,26 the tuning is implemented by compressing or stretching the PDMS based dye laser chip with an external mechanical actuator. Internal forces in a PDMS based tunable optofluidic devices are normally generated by the pressure of fluids which fill the inside of the PDMS structure. For example, in a pneumatically tunable dye laser the lasing wavelength can be remotely tuned by inserting compressed air in an internal air-gap etalon in the PDMS laser chip.27 Several such elastomer based optofluidic devices have been demonstrated in the past few years.28–30
2. Tunable optofluidic devices with bulk deformation
The use of bulk deformation as a tuning mechanism has been frequently reported with polymer based micro-optical devices in the past.31–34 Such tuning was realized by varying certain dimensions or the volume of the whole device, thereby changing critical dimensions of the optical components, such as the periodicity of a grating or a photonics crystal. For example, several elastomeric optical elements with deformable surface topographies have been demonstrated by Whitesides' group for tunable light transmission and light focusing, as well as force sensing.31 As another example, periodic structures are also known to exhibit form birefringence which can be tuned by strain in elastomers. This was demonstrated by Wilson et al. using electrostatic forces to change the size of a PDMS microstructure.32 The change of the physical dimensions of a polymer photonic crystal due to strain has also been achieved by a chemical method where the device is subjected to swelling in the presence of an organic solvent.33 Unlike in polymer based micro-optical devices, in optofluidic devices the liquid and solid co-exist, and the liquid shape is normally adapted to the solid (elastomer) structure. The bulk deformation of the device induces the simultaneous change in its solid and liquid shape.Different types of optofluidic dye lasers have been demonstrated,35–37 and for the majority of them the lasing wavelength tuning was realized by changing the index of the fluid in the dye solution. The mechanically tunable optofluidic distributed feedback dye laser shown in Fig. 2 was demonstrated by Psaltis' group26 and it was fabricated on a monolithic replica molded PDMS chip. The optical feedback was provided by a phase-shifted higher order Bragg grating embedded in the liquid core of a single mode buried micro-channel waveguide. The laser wavelength could be tuned by mechanically stretching the grating period. For the PDMS composition used in this experiment, it was found that the elastic deformation of PDMS could be over 120%. With such large deformation, the limit on the tunability of the laser was the gain bandwidth of the laser dyes. Only ∼5% deformation was used to achieve the ∼60 nm tuning range demonstrated in this experiment based on the combination of two common dye molecules, Rhodamine 6G and Rhodamine 101. The tuning was continuous and completely reversible, and no noticeable degradation of the chip was observed during a 5-cycle full range tuning test. The advantage of such a tunable dye laser lies in the simplicity and low cost which could be attractive for inexpensive imaging and spectroscopy instruments.
 | ||
| Fig. 2 Diagram of a mechanically tunable optofluidic DFB dye laser chip. The upper inset shows an actual monolithic PDMS laser chip. The lower inset is an optical micrograph of the central phase-shifted region of the laser cavity. | ||
Another type of tunable optofluidic dye laser with deformable micro-droplet was demonstrated by Saito et al.38 This laser cavity is a liquid micro-droplet resonator embedded within an elastomeric material. As shown in Fig. 3, the droplet was generated by an inkjet method. The dye solution was then covered by the prepolymer of PDMS. After curing of PDMS, a spherical micro-droplet was encapsulated by the solid transparent elastomer. Under application of external pressure on the chip, the micro-droplet shape changed and as a result its resonant frequency was modified.
 | ||
| Fig. 3 Steps in the fabrication of a droplet cavity embedded in deformable PDMS: (a) inkjet of the dye solution, (b) injection of the PDMS prepolymer, (c) droplet deformation, and (d) solidification of the elastomer. | ||
3. Tunable optofluidic devices with flexible membrane
An alternative method for tuning elastomer based optofluidic devices is through the internal gas or liquid pressure. The local deformation of the elastomer structure induced by pressure implements the optofluidic tuning. There are a large number of such tunable optofluidic devices with a PDMS thin membrane structure which are mostly actuated by pressure.39–43 For example, the focusing of tunable fluidic lenses was reported with thin elastic membranes.39–41,44–47Fig. 4 shows a tunable liquid-filled microlens array on top of a microfluidic network which was fabricated using soft lithographic techniques.39 The microlens array consists of circular chambers 200 μm in diameter and 100 μm thick, covered with a 40 μm thick flexible membrane made of PDMS elastomer. The chambers were filled with highly refractive liquid (n = 1.51). The simultaneous control of the focal length of all the microlenses composing the elastomeric array was accomplished by pneumatically regulating the pressure of the microfluidic network. The experimental results revealed an almost linear dependence of the maximum membrane deflection on the applied pressure (≈0.16 μm kPa−1), while a maximum deflection of 11 μm was achieved. By varying the pressure from 0 to 35 kPa, the focal length changed by more than 70%. Such an array can potentially be used in dynamic imaging systems and adaptive optics. | ||
| Fig. 4 Schematic representation of a microlens array. Upon pressurizing the elastomeric liquid filled chamber, the membrane deforms forming a plano-convex lens array. | ||
It is worth noting that the flexible PDMS membrane is extensively utilized in microfluidics. Developed by Quake's group, the integrated membrane valve has been used for flow control as well as for trapping micro-objects (cells, molecules).48 Based on these integrated membrane valve arrays, Quake's group demonstrated highly parallel measurements of the bimolecular interaction kinetic constants on a microfabricated optomechanical device.49 A schematic representation of the working principle is shown in Fig. 5. During the association phase of the experiment, the membrane button (valve) is raised for a specific amount of time, during which proteins (P) in solution can associate with a bound protein (B) on the surface. As the protein P is fluorescence tagged, a fluorescent image of the button array is taken when the button is brought down, and the process is repeated. From the collected data, a time-dependent molecule association curve can be created. For the dissociation phase measurement, the process is reversed.
 | ||
| Fig. 5 Cartoon of button functionality in the measurement of the bimolecular interactions. | ||
With a similar membrane valve design, we recently presented an optofluidic differential method for carrying out absorption spectroscopy of sub-nanoliter volumes of liquid samples on a chip.50 At low liquid volumes, absorption spectroscopy in microfluidics is often hindered by either low sensitivity or complex fabrication. To address this, we realized an optofluidic modulator which can be integrated on to a standard PDMS based microfluidic chip. It contains a micro-scale chamber which is divided into two symmetric sections by a thin membrane. When the fluid pressure of the upper section is higher than the lower section, the membrane is pushed down. In this mode, the reference liquid occupies most of the chamber, hence only the reference liquid contributes to the signal for absorbance spectroscopy. When the fluidic pressure of the lower section is higher than the upper section, only the analyte liquid contributes to the absorbance. With the aid of this modulator, we measured the spectra in each mode and then subtracted them. As the absorbance of the reference liquid is known, the remaining signal corresponds to the analyte absorption spectrum. Due to the method used, the measurement has a high sensitivity and is insensitive to background light. Another advantage is that this method doesn't need a complicated fabrication step, and is compatible with conventional microfluidics, while measurements can be carried out on a conventional microscope.
The elastomer membrane based tuning mechanism was also employed in an optofluidic maskless lithography (OFML) by Lee et al.51 They demonstrated a method for high-throughput generation of 3D microstructures using a membrane mounted microfluidic channel. By utilizing an OFML system, photopolymerized 3D microstructures were fabricated in a layer-by-layer fashion with the thickness of each layer controlled by the deformation of the membrane. A schematic representation of the working principle is shown in Fig. 6. The OFML is fabricated in a two-layered PDMS microfluidic channel with a UV curable resin filling at the bottom. The resin in the UV exposure area polymerizes throughout the cross-section of the channel except in the oxygen inhibited region at the surface of the PDMS. The height of the bottom channel is controlled via deformation of the PDMS membrane under the pneumatic pressure of the top chamber. Thus, the thickness of the polymerized particles can be controlled by the air pressure. As a result, in this 3D-OFML, the height of the channel was modulated in order to generate multilayered structures. High vertical resolution can be achieved with low numerical aperture (NA) optics, which allows a large field-of-view for high-throughput projection lithography.
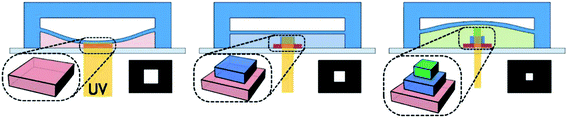 | ||
| Fig. 6 Schematic illustration of working principle of the 3D-OFML. | ||
As can be seen from the above examples, normally the membranes in these tunable optofluidic devices are actuated by pressure. Alternative mechanisms involve the application of electrical fields52 or membrane deformation driven by laser-induced cavitation bubbles. With regard to the latter, Chiou's group presented a pulsed laser driven peristaltic pump for driving fluids in a PDMS microchannel.53 A schematic representation of the device is shown in Fig. 7. It is a two-layer PDMS microfluidic device consisting of a top sample channel and a bottom control channel containing dye solution for enabling laser absorption. A tightly focused laser pulse can induce cavitation bubbles which provide large mechanical forces to act on the surrounding fluid and structures. By synchronization, the laser induced cavitation bubbles deform the thin membranes in a certain sequence and hence propel the fluid in the channel. Compared to conventional pneumatic driven processes, this new light pumping process eliminates the tubing required for fluid control and replaces it with an optical scanner that does not have to physically interface with the microfluidic chips.
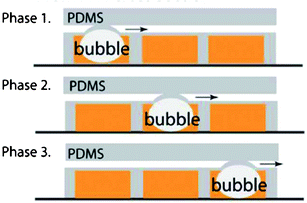 | ||
| Fig. 7 Schematic representation of a pulsed laser driven peristaltic membrane pump. The synchronized laser induced cavitation bubbles deform the thin membranes in sequence to push the fluid in the sample channel forward. | ||
In addition to the pressure mediated active tunable optofluidic device presented above, passive membrane based tunable optofluidic devices have also been reported for pressure sensing applications.54–58 Calixto et al. demonstrated an imaging method for measuring the pressure in liquids or gases by means of a flexible lens.56 This method relies on the deformation of a flexible lens by the pressure applied on its surface. Images given by the lens are analyzed and a calibration curve, in terms of the visibility of the images and pressure, can be determined. To obtain high sensitivity for pressure sensing, the Psaltis group presented an alternative imaging method which is based on a chip-scale optofluidic membrane interferometer.57,58Fig. 8 shows the schematic of the pressure sensing apparatus, including the optofluidic pressure sensor chip and a camera. The chip was made of PDMS by multilayer soft lithography. The image of the air-gap cavity was projected into the camera on a microscope. Upon illumination by monochromatic light, the reflected rays from the air-gap boundaries interfere either constructively or destructively, depending on the air-gap thickness. During measurement, the pressure from the fluid causes deformation of the flexible membrane, hence the variation of the air-gap thickness and the interference pattern from the cavity. The pressure was measured by imaging and analyzing the interference patterns. Since the fabrication of the device is fully compatible with conventional PDMS based optofluidic chips, the sensing unit can be integrated with other microfluidic elements for in situ pressure and flow rate measurement.
 | ||
| Fig. 8 Schematic representation of the pressure sensing apparatus including the chip and an imaging device. | ||
4. Tunable optofluidic devices with pneumatic actuation
In the flexible membrane based tunable optofluidic devices, the applied internal pressure is expected to deform the membrane in the out-of-plane direction. This is beneficial for many applications, such as lenses with variable focal lengths.59 However, for a more complex tuning or a situation where the membrane is not suitable, integration of the pneumatic actuators requires a different approach to achieve control of tunable optofluidic devices. Such a method was first demonstrated by Campbell et al. who presented a pressure-driven stretcher and rotator on a composite PDMS device.28Fig. 9 shows a schematic drawing of the stretcher and rotator respectively (Fig. 9a and b are the top view, while c is the cross-section view). When the interior of the device is pressurized and the membrane is inflated, the flaps are lifted by turning about the edges of the frame. As a result, the flaps are lifted and a torque is applied to the circle. In this experiment, the amount of stretching and rotation was studied by measuring the diffraction of light in the gratings stamped onto the surface of PDMS in the center. | ||
| Fig. 9 Composite membrane devices with integrated pneumatically actuated stretcher (a) and rotator (b) respectively. Dark areas correspond to epoxy parts inside the membranes. (c) Schematic drawing of a cross-section of the devices. | ||
As another example of an integrated pneumatic actuator, the Psaltis group introduced a micro-air-bag which is embedded in a PDMS chip and can generate local deformations.29Fig. 10a and b show the working principle of a micro-air-bag enabled tunable diffraction grating. A uniform phase grating is located in the center of the four micro-air-bags, and each of them can be independently controlled. By selectively filling the different sections with compressed air, both shrinking and expanding in the grating area can be achieved. The device is shown in Fig. 10c. The chip was characterized by illuminating a green laser pointer and monitoring the diffraction patterns. From the diffraction pattern in Fig. 10d and e, we found a maximum tuning ratio of 20% for the grating.
 | ||
| Fig. 10 The top-view drawing illustrates the micro-air-bag actuated tuning (a, b); the PDMS chip was illuminated by a green laser beam (c). Its diffraction patterns with/without compressed air are shown in (d) and (e), respectively. | ||
A micro-air-bag can also take the role of optical component by itself. A pneumatically tunable optofluidic dye laser in which the lasing wavelength is determined by an integrated air-gap etalon was recently demonstrated.27 The schematic of the laser cavity structure is illustrated in Fig. 11. The chip was entirely made of PDMS. It is composed of a liquid core waveguide, at the ends of which two micro-scale air chamber structures are integrated which act as the mirrors providing the feedback. The beam reflections occur at the PDMS/air interfaces due the refractive index contrast. The air chamber on the left acts as a simple end mirror in the laser cavity. The air chamber on the right behaves as a low finesse Fabry–Perot (F–P) etalon which determines the lasing wavelength. Since the PDMS is elastic, the air-gap size of the etalon can be varied by pumping the gas into the air chamber with different pressure. Fig. 11c shows the cross-section view of the air-gap etalon; upon increase of the air pressure, the air chamber expands, thereby increasing the effective air gap size.
 | ||
| Fig. 11 Schematic representation of the laser cavity structure. Inset (a) shows the detail of the angled mirror, only one interface of which is effective for the laser cavity. Inset (b) shows the air-gap etalon and (c) illustrates the tuning process by air pressure. | ||
5. Microchannel volume modulation for tunable optofluidics
Light–matter interactions coupled with mechanical systems have received renewed interest recently in fields such as cavity optomechanics or photoacoustic imaging,60,61 as well as more traditional application in optics, such as in liquid crystal elastomers,62 where strain and orientation order of the liquid crystal molecules are strongly coupled. Similar examples can also involve a variable phase retarder realized by modifying the optical path length within an elastomeric dielectric, or an optical attenuator achieved by applying pressure on an elastomeric cladding and thus modulating the radiation losses of an embedded waveguide.63 In fluid mechanics, mechanical waves propagating in an elastomer can induce changes to the walls of an integrated microchannel. As a result the channel volume is modulated, causing mass transport and flow. This type of flow is referred to as peristaltic due to its similarity to many physiological ways that fluids are propelled or mixed in living organisms.64 In PDMS microfluidics, peristaltic pumping was one of the first demonstrations of how soft lithography enables the integration of multiple functions on-a-chip, such as pumps and mixers.65 Similar applications have more recently appeared by integrating MEMs actuators with microfluidics.Such highly integrated flow methods have only recently started to be used in optofluidics. In general the optical properties of a fluid do not change as the velocity with which it flows changes. Therefore fluid transport does not directly give rise to optical functionalities, apart from when fluids interact with solids as mentioned before, or with other fluids.60–61 However, by replacing common buffers with anisotropic fluids, optical effects can arise from simple flow. Such fluids are materials that exhibit order, with typical examples involving colloidal suspensions and liquid crystals.66 Liquid crystal microflows have been recently employed in optofluidics, where their direct peristalsis resulted in optical tuning and modulation in the kHz range.67 In this paper, the authors demonstrate that the surface energy of PDMS and the channel's structural dimensions are adequate to align the liquid crystals in a microfluidic channel inducing a long range structural order. Under flow, birefringence emerges in the channel. The emerging birefringence is dependent on the flow velocity, thus providing a way of tuning these optofluidic filters. In addition, it is known that birefringence exhibits strong wavelength dependence. This has been recently employed in developing tunable color filters and on-chip optofluidic spectroscopy.68
6. Summary
Various PDMS based tunable optofluidic devices have been developed in the past few years for different applications. Meanwhile, PDMS microfluidic chips are extensively utilized in biological and chemical studies. Due to similarities in materials and fabrication methods, optofluidic devices can be easily integrated with other PDMS based microfluidic devices to enable more complex and multiplexed functions. For example, tunable dye laser sources can be used in compact micro-chip scale flow cytometry for high throughput cell screening. Multiple optofluidic interferometers can be integrated into a single microfluidic chip for multi-spot pressure and flow sensing. Optofluidic differential absorbance spectroscopy can be integrated with cell-culture on a single chip for measuring the concentration of protein or DNA in situ. Optofluidic light filters can be used in optofluidic microscopy and spectroscopy.66,69 Being compatible with conventional microfluidics is the key advantage of elastomer based tunable optofluidic devices. We can foresee more integration of such optofluidic elements towards complete systems for lab-on-a-chip applications, for example, studying mechanical properties at the single cell level.70 In parallel, elastomer tunable optofluidic devices can also be extended into the nano-optics or nanofluidic fields. Several tunable plasmon nano devices fabricated on a stretchable PDMS substrate have been demonstrated recently.71,72 Combining elastomer based micro/nano devices with nanoplasmonic elements can be interesting for molecule level imaging and spectroscopy.73 A tunable elastic nanofluidic channel was demonstrated recently on a PDMS chip for nanoparticle separation and molecule trapping.74 One of the challenges of PDMS based tunable nano-devices is the high accuracy in control. We expect that precision control of PDMS based tunable structures can be realized by very fine pneumatic actuation or connection to a piezo-actuator. We see great potential in further developing tunable optofluidic devices by taking advantage of the elastic deformability of the construction material and the synergetic coupling between optics and fluidics. The operational stability of such devices under operating conditions can be enhanced by adapting lifetime enhancement techniques as in microfluidics; for example the mechanical stability of PDMS can be enhanced by further doping the polymer with glass nanoparticles,75,76 while fluidic evaporation can be addressed by doping the PDMS with evaporation inhibitors, such as parylene.77References
- D. Psaltis, S. R. Quake and C. H. Yang, Developing optofluidic technology through the fusion of microfluidics and optics, Nature, 2006, 442(7101), 381–386 CrossRef CAS.
- C. Monat, P. Domachuk and B. J. Eggleton, Integrated optofluidics: A new river of light, Nat. Photonics, 2007, 1(2), 106–114 CrossRef CAS.
- D. Erickson, D. Sinton and D. Psaltis, Optofluidics for energy applications, Nat. Photonics, 2011, 5(10), 583–590 CrossRef CAS.
- X. D. Fan and I. M. White, Optofluidic microsystems for chemical and biological analysis, Nat. Photonics, 2011, 5(10), 591–597 CrossRef CAS.
- H. Schmidt and A. R. Hawkins, The photonic integration of non-solid media using optofluidics, Nat. Photonics, 2011, 5(10), 598–604 CrossRef CAS.
- B. K. Gibson, Liquid mirror telescopes: History, J. Roy. Astron. Soc. Can., 1991, 85, 158–171 Search PubMed.
- W. Z. Song, et al., Low-order distributed feedback optofluidic dye laser with reduced threshold, Applied Physics Letters, 2009, 94(5) Search PubMed.
- G. L. Liu, et al., Optofluidic control using photothermal nanoparticles, Nat. Mater., 2006, 5(1), 27–32 CrossRef CAS.
- Y. Yang, et al., Optofluidic waveguide as a transformation optics device for lightwave bending and manipulation, Nature Communications, 2012, 3 Search PubMed.
- H. Huang, et al., Tunable two-dimensional liquid gradient refractive index (L-GRIN) lens for variable light focusing, Lab Chip, 2010, 10(18), 2387–2393 RSC.
- S. Kuiper and B. H. W. Hendriks, Variable-focus liquid lens for miniature cameras, Appl. Phys. Lett., 2004, 85(7), 1128–1130 CrossRef CAS.
- R. A. Hayes and B .J. Feenstra, Video-speed electronic paper based on electrowetting, Nature, 2003, 425(6956), 383–385 CrossRef CAS.
- O. Manzardo, et al., Miniaturized time-scanning Fourier transform spectrometer based on silicon technology, Opt. Lett., 1999, 24(23), 1705–1707 CrossRef CAS.
- M. Li, W. H. P. Pernice and H. X. Tang, Broadband all-photonic transduction of nanocantilevers, Nat. Nanotechnol., 2009, 4(6), 377–382 CrossRef CAS.
- M. C. Wu, A. Solgaard and J. E. Ford, Optical MEMS for lightwave communication, J. Lightwave Technol., 2006, 24(12), 4433–4454 CrossRef.
- M. C. Belanger and Y. Marois, Hemocompatibility, biocompatibility, inflammatory and in vivo studies of primary reference materials low-density polyethylene and polydimethylsiloxane: A review, J. Biomed. Mater. Res., 2001, 58(5), 467–477 CrossRef CAS.
- A. Piruska, et al., The autofluorescence of plastic materials and chips measured under laser irradiation, Lab Chip, 2005, 5(12), 1348–1354 RSC.
- F. Hua, et al., Polymer imprint lithography with molecular-scale resolution, Nano Lett., 2004, 4(12), 2467–2471 CrossRef CAS.
- C. Markos, K. Vlachos and G. Kakarantzas, Thermo-optic effect of an index guiding photonic crystal fiber with elastomer inclusions, 21st International Conference on Optical Fiber Sensors, 2011, 7753 Search PubMed.
- M. A. Unger, et al., Monolithic microfabricated valves and pumps by multilayer soft lithography, Science, 2000, 288(5463), 113–116 CrossRef CAS.
- T. Thorsen, S. J. Maerkl and S. R. Quake, Microfluidic large-scale integration, Science, 2002, 298(5593), 580–584 CrossRef CAS.
- S. J. Maerkl and S. R. Quake, A systems approach to measuring the binding energy landscapes of transcription factors, Science, 2007, 315(5809), 233–237 CrossRef CAS.
- J. P. Beech and J. O. Tegenfeldt, Tuneable separation in elastomeric microfluidics devices, Lab Chip, 2008, 8(5), 657–659 RSC.
- D. V. Vezenov, Integrated fluorescent light source for optofluidic applications, Appl. Phys. Lett., 2005, 86(4) CrossRef CAS.
- X. Heng, et al., Optofluidic microscopy - a method for implementing a high resolution optical microscope on a chip, Lab Chip, 2006, 6(10), 1274–1276 RSC.
- Z. Y. Li, et al., Mechanically tunable optofluidic distributed feedback dye laser, Opt. Express, 2006, 14(22), 10494–10499 CrossRef CAS.
- W. Z. Song and D. Psaltis, Pneumatically tunable optofluidic dye laser, Applied Physics Letters, 2010, 96(8) CAS.
- K. Campbell, et al., Pressure-driven devices with lithographically fabricated composite epoxy-elastomer membranes, Appl. Phys. Lett., 2006, 89(15) CrossRef CAS.
- D. Psaltis, et al. , Conference on Lasers and Electro-Optics, 2010 Search PubMed OSA Technical Digest,: p. paper CTuY1.
- M. Gersborg-Hansen and A. Kristensen, Optofluidic third order distributed feedback dye laser, Appl. Phys. Lett., 2006, 89(10) CrossRef CAS.
- B. Grzybowski, et al., Elastomeric optical elements with deformable surface topographies: applications to force measurements, tunable light transmission and light focusing, Sens. Actuators, A, 2000, 86(1–2), 81–85 CrossRef.
- B. K. Wilson and L. Y. Lin, Variable wave plate via tunable form-birefringent structures, J. Microelectromech. Syst., 2008, 17(4), 1039–1046 CrossRef CAS.
- Y. J. Wang, et al., Tunable photonic crystals on a freestanding polymer membrane, J. Micromech. Microeng., 2010, 20(1) CrossRef.
- B. D. Viers and J. E. Mark, Elastomeric properties of polysiloxane networks: Birefringence measurements on bimodal elastomers that are presumed to be spatially inhomogeneous, J. Inorg. Organomet. Polym. Mater., 2007, 17(1), 283–288 CrossRef CAS.
- W. Z. Song, et al., Optofluidic evanescent dye laser based on a distributed feedback circular grating, Appl. Phys. Lett., 2009, 94(16) CrossRef.
- A. E. Vasdekis, et al., Fluidic fibre dye lasers, Opt. Express, 2007, 15(7), 3962–3967 CrossRef CAS.
- W. Lee, Tunable single mode lasing from an on-chip optofluidic ring resonator laser, Appl. Phys. Lett., 2011, 98(6) CrossRef.
- M. Saito and K. Koyama, Deformable Microdroplet Cavity Fabricated by an Inkjet Method, Jpn. J. Appl. Phys., 2010, 49(9) CrossRef.
- N. Chronis, et al., Tunable liquid-filled microlens array integrated with microfluidic network, Opt. Express, 2003, 11(19), 2370–2378 CrossRef.
- G. Y. Zhou, Liquid tunable diffractive/refractive hybrid lens, Opt. Lett., 2009, 34(18), 2793–2795 CrossRef.
- G. H. Feng and Y. C. Chou, Fabrication and characterization of optofluidic flexible meniscus-biconvex lens system, Sens. Actuators, A, 2009, 156(2), 342–349 CrossRef.
- W. Z. Song and D. Psaltis, Pneumatically tunable optofluidic 2 × 2 switch for reconfigurable optical circuit, Lab Chip, 2011, 11(14), 2397–2402 RSC.
- A. Werber and H. Zappe, Tunable Pneumatic Microoptics., J. Microelectromech. Syst., 2008, 17(5), 1218–1227 CrossRef.
- M. Agarwall, Polymer-based variable focal length microlens system, J. Micromech. Microeng., 2004, 14(12), 1665–1673 CrossRef.
- H. W. Ren and S. T. Wu, Variable-focus liquid lens, Opt. Express, 2007, 15(10), 5931–5936 CrossRef.
- H. B. Yu, et al., A liquid-filled tunable double-focus microlens, Opt. Express, 2009, 17(6), 4782–4790 CrossRef CAS.
- P. Fei, Discretely tunable optofluidic compound microlenses, Lab Chip, 2011, 11(17), 2835–2841 RSC.
- J. Melin and S. R. Quake, Microfluidic large-scale integration: The evolution of design rules for biological automation, Annu. Rev. Biophys. Biomol. Struct., 2007, 213–231 CrossRef CAS.
- S. R. Bates and S.R. Quake, Highly parallel measurements of interaction kinetic constants with a microfabricated optomechanical device, Appl. Phys. Lett., 2009, 95(7) CrossRef CAS.
- W. Song and J. Yang, Optofluidic differential spectroscopy for absorbance detection of sub-nanoliter liquid sample, Lab Chip, 2012, 12, 1251–1254 RSC.
- S. A. Lee, et al., Three-dimensional fabrication of heterogeneous microstructures using soft membrane deformation and optofluidic maskless lithography, Lab Chip, 2009, 9(12), 1670–1675 RSC.
- S. Rosset, et al., Metal Ion Implantation for the Fabrication of Stretchable Electrodes on Elastomers, Adv. Funct. Mater., 2009, 19(3), 470–478 CrossRef CAS.
- T. H. Wu, et al., Pulsed laser triggered high speed microfluidic fluorescence activated cell sorter, Lab Chip, 2012, 12(7), 1378–1383 RSC.
- W. Z. Song and D. Psaltis, Imaging based optofluidic air flow meter with polymer interferometers defined by soft lithography, Opt. Express, 2010, 18(16), 16561–16566 CrossRef CAS.
- A. Orth, E. Schonbrun and K. B. Crozier, Multiplexed pressure sensing with elastomer membranes, Lab Chip, 2011, 11(22), 3810–3815 RSC.
- S. Calixto, F. J. Sanchez-Marin and M. E. Sanchez-Morales, Pressure measurements through image analysis, Opt. Express, 2009, 17(20), 17996–18002 CrossRef.
- W. Z. Song and D. Psaltis, Optofluidic pressure sensor based on interferometric imaging, Opt. Lett., 2010, 35(21), 3604–3606 CrossRef CAS.
- W. Z. Song and D. Psaltis, Optofluidic membrane interferometer: An imaging method for measuring microfluidic pressure and flow rate simultaneously on a chip., Biomicrofluidics, 2011, 5(4) CrossRef.
- S. H. Ahn and Y.K. Kim, Proposal of human eye's crystalline lens-like variable focusing lens, Sens. Actuators, A, 1999, 78(1), 48–53 CrossRef.
- T. J. Kippenberg and K. J. Vahala, Cavity optomechanics: Back-action at the mesoscale, Science, 2008, 321(5893), 1172–1176 CrossRef CAS.
- L. V. Wang, Multiscale photoacoustic microscopy and computed tomography, Nat. Photonics, 2009, 3(9), 503–509 CrossRef CAS.
- M. Camacho-Lopez, et al., Fast liquid-crystal elastomer swims into the dark, Nat. Mater., 2004, 3(5), 307–310 CrossRef CAS.
- N. Galler, et al., Integrated optical attenuator based on mechanical deformation of an elastomer layer, Appl. Phys. B: Lasers Opt., 2011, 104(4), 931–934 CrossRef CAS.
- M. Y. Jaffrin and A. H. Shapiro, Peristaltic Pumping, Annu. Rev. Fluid Mech., 1971, 3, 13–36 CrossRef.
- A. Y. Fu, et al., A microfabricated fluorescence-activated cell sorter, Nat. Biotechnol., 1999, 17(11), 1109–1111 CrossRef CAS.
- A. Sengupta, S. Herminghaus and C. Bahr, Nematic Liquid Crystals and Nematic Colloids in Microfluidic Environment, Mol. Cryst. Liq. Cryst., 2011, 547, 203–212 CAS.
- J. G. Cuennet, et al., Optofluidic modulator based on peristaltic nematogen microflows, Nat. Photonics, 2011, 5(4), 234–238 CrossRef CAS.
- J. G. Cuennet, A. E. Vasdekis and D. Psaltis, Pressure dependent liquid-crystal microflows for tunable optofluidic colour filters and spectroscopy., submitted, 2012 Search PubMed.
- C. Denniston, E. Orlandini and J. M. Yeomans, Simulations of liquid crystals in Poiseuille flow, Comput. Theor. Polym. Sci., 2001, 11(5), 389–395 CrossRef CAS.
- J. P. Shelby, et al., A microfluidic model for single-cell capillary obstruction by Plasmodium falciparum infected erythrocytes, Proc. Natl. Acad. Sci. U. S. A., 2003, 100(25), 14618–14622 CrossRef CAS.
- F. M. Huang and J. J. Baumberg, Actively Tuned Plasmons on Elastomerically Driven Au Nanoparticle Dimers, Nano Lett., 2010, 10(5), 1787–1792 CrossRef CAS.
- I. M. Pryce, et al., Highly Strained Compliant Optical Metamaterials with Large Frequency Tunability, Nano Lett., 2010, 10(10), 4222–4227 CrossRef CAS.
- G. Lu, H. Li and H. Zhang, Nanoparticle-coated PDMS elastomers for enhancement of Raman scattering, Chem. Commun., 2011, 47(30), 8560–8562 RSC.
- D. Huh, et al., Tuneable elastomeric nanochannels for nanofluidic manipulation, Nat. Mater., 2007, 6(6), 424–428 CrossRef CAS.
- H. G. Elias, Makromoleküle, Industrielle Polymere und Synthesen, 2001, Vol. 3, Wiley-VCH. 505, Weinheim Search PubMed.
- R. D. Lovchik, H. Wolf and E. Delamarche, High-grade optical polydimethylsiloxane for microfluidic applications, Biomed. Microdevices, 2011, 13(6), 1027–1032 CrossRef CAS.
- Y.-S Heo et al., Osmolarity control of microfluidic embryo cell culture using hybrid PDMS-parylene membranes, Microdevices in Biology and Medicine, ed. Y. Nahmias and S. Bhatia, 2009, Artech House. 110, Norwood MA Search PubMed.
Footnote |
| † Published as part of a themed issue on optofluidics |
| This journal is © The Royal Society of Chemistry 2012 |
