Magnetic carbon nanostructures in medicine
Sławomir
Boncel
*,
Artur P.
Herman
and
Krzysztof Z.
Walczak
Department of Organic Chemistry, Biochemistry and Biotechnology, Silesian University of Technology, Krzywoustego 4, Gliwice, 44-100, Poland. E-mail: slawomir.boncel@polsl.pl@; Fax: +48 32 237 2094; Tel: +48 32 237 2353
First published on 20th October 2011
Abstract
Magnetic carbon nanostructures are hybrid materials containing magnetic and carbon (mainly sp2) nanoallotrope components conjugated in various configurations. Their potential applications in medicine including drug delivery systems, magnetic particle/fluid hyperthermia anti-cancer therapy and magnetic resonance imaging are reviewed.
 Sławomir Boncel | Sławomir Boncel received his PhD in organic chemistry in 2009 under the supervision of Professor Krzysztof Walczak at the Silesian University of Technology in Gliwice, Poland. His research interest covers carbon nanomaterials, their chemistry and applications in medicine and as nanocomposites. He collaborates in these fields with Professor Alan Windle and Dr Krzysztof Koziol from the University of Cambridge. |
 Artur P. Herman | Artur Herman obtained his BSc Eng in organic chemical technology from Silesian University of Technology in 2011. The same year he started his MSc under the supervision of Dr S. Boncel. His current research interests include functionalisation of carbon-based nanohybrids and their comprehensive applications. |
 Krzysztof Z. Walczak | Krzysztof Walczak obtained his PhD in organic chemistry at the Silesian University of Technology in 1988 under the supervision of Professor Jerzy Suwiński. He continued his scientific education as a postdoctoral fellow (1989–91) at the Odense University with Professor Erik B. Pedersen. In 1996 he was granted a six-month fellowship at the Odense University. In 1999 he received his DSc degree. His current research activity is focused on the chemistry of heterocyclic compounds (also sugar derivatives) and enzymatic reactions. His research group is involved also in the synthesis of intercalating agents and chemical modification of carbon nanotubes. |
Introduction
In the past few decades carbon-based nanoarchitectures due to their unprecedented chemical and physical properties have attracted an exponentially growing number of scientists.1 Since breakthrough syntheses and/or isolation of the triad, fullerenes (1985),2carbon nanotubes (CNTs, 1991)3 and graphene sheets (2004),4 there have been published numerous original papers covering their potential and realistic applications in such different disciplines as electronics, catalysis or nanomedicine.5,6 The latter domain is a new area of medical sciences exploiting nanoparticles mostly in ‘theranostic’ (therapy and diagnostic) systems and carries hope to detect and conquer many human diseases in their early stages of development.7The avalanche-like expansion of nanotechnology resulted in a better understanding of size dependent properties of various materials, revealing substantial differences between bulk materials and their nanosized analogues. Inter alia, bulk (multi-domain) ferromagnetic materials become superparamagnetic (single-domain) when their size is reduced to the nanoscale. Therefore, an amalgamation of carbon nanomaterials, exhibiting unique properties themselves, with nanomagnets leading to high-precision multi-task nanomachines, would be a mile step towards the transition from science to medical practice in a variety of applications. The most promising and achievable in the near future utilisations of these hybrid nanomaterials are targeted (controllable) drug delivery systems, magnetic particle/fluid hyperthermia anti-cancer therapy and magnetic resonance imaging (MRI).
By now, many excellent resumes reporting non-carbon magnetic nanoparticles8–11 as well as carbon nanostructures12–14 in medicine have been published, but they had covered their subjects separately. In this review, we would like to discuss hitherto only potential applications of a broad family of magnetic carbon nanostructures (MCNs) (Fig. 1). Due to the pioneering character of the studies, our reconnaissance focuses on the fundamental requirements for utilising MCNs in the field of medicine and emphasises their crucial magnetic parameters towards specific applications. In our work we have intentionally omitted quantification of toxicology of the title materials as it is, firstly, an obvious criterion of their further applicability and secondly, it remains distant from generality (depends on numerous factors).15 Also, toxicology of MCNs was a subject of many cross-sectional, broadened biological studies.16–19
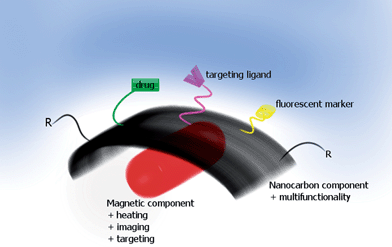 | ||
| Fig. 1 Schematic representation of concept of multimodal carbon nanostructures towards steerable drug delivery and imaging (via fluorescence or nanomagnet) systems; ‘R’ is any arbitrary structure in the functionalised carbon nanomaterials (CNMs). | ||
Classification of magnetic carbon nanostructures
Magnetic carbon nanostructures discussed here are hybrid materials composed of carbon nanostructures (in majority sp2-hybridised) of various dimensionality (0D fullerenoids, 1D carbon nanotubes, 2D graphenoids, etc.) and magnetic nanoparticles. Two general classes of such conjugates can be distinguished (Fig. 2): (1) carbon encapsulated magnetic nanoparticles (CEMNPs) and (2) carbon nanostructures decorated with MNPs. | ||
| Fig. 2 Schematic representation of two general classes of magnetic carbon nanostructures: CEMNPs (left) and carbon nanostructures decorated with MNPs (right); carbon material is shown as black figures whereas magnetic particles are represented by red spheres; for decorated nanostructures—yellow chains correspond to optionally introduced molecular linkers. | ||
Both groups can be represented by a variety of morphologies, chemical compositions, and consequently, different physicochemical properties which lead to different potentials of their application in biomedicine. It should be highlighted here that the fundamental uniqueness and advantages of MCNs compared to other commonly studied magnetic nanomaterials are their enhanced chemical reactivity and, therefore, ‘tunability’ of crucial physicochemical properties like minimal cytotoxicity, controllable content of nanomagnets in hybrids and capability of anchoring drug molecules via covalent bonding.
The primary limitations in a selection of the filling magnetic material are determined by the method of synthesis of nanohybrids and potential to its further enhancement. Some of the reported magnetic core materials were iron,21,31nickel,23cobalt32 and their alloys.33,34 These metallic inners possess excellent magnetic properties and can be simply obtained as ‘non-intentional contamination’ in the catalytic CVD methods of synthesis. Although the above-mentioned magnetic components are the most popular ones, nanohybrids where rare earth metals and their ions were coated with carbon shell have been also successfully synthesised.35
Concerning applications of CEMNPs in medicine, their common lack of solubility in aqueous, in particular biological systems, and tendency to create ‘thick’ aggregates (via van der Waals forces and π–π interactions) is a serious constraint, which however could be overcome with chemical modifications of their outer carbonaceous surfaces. There are two general sp2-carbon surface functionalisation strategies: covalent and non-covalent (Scheme 1).36,37
 | ||
| Scheme 1 The most important examples of covalent and non-covalent functionalisation of carbonaceous vehicles; here shown as reaction pathways of SWNT, but other sp2-allotropes can be also applied e.g. MWNTs, carbon nanohorns, nanoonions, graphenoids, etc. Typically, a higher diameter of the tubes and/or increased number of defects in graphite lattice enhances their susceptibility to organic modifications. | ||
Both strategies can be utilised for water-solubility tuning, and for further introduction of advanced functionalities as drug molecules or targeting agents. Covalent functionalisation of carbon nanostructures covers: (1) defect-site reactions based on the post-treatment of carboxyl (–COOH) groups (amidation, esterification) and (2) addition chemistry including halogenation (predominantly fluorination), 1,3-dipolar cycloaddition, Diels–Alder reaction, nucleophilic/electrophilic or free-radical additions. It must be here emphasised that oxidation of MCNs under harsh acidic conditions, as the only modification in the above series, leads to the introduction of carboxylic and phenolic-like hydroxyl groups accompanied by a partial removal of the magnetic component from MCN hybrids.19 The covalent functionalisation causes from fractional to severe degradation of the nanocarbon unique electronic structure but it usually does not influence their efficiency in medicinal applications. In turn, non-covalent functionalisation of carbon nanoparticles based on (a) hydrophobic interaction, (b) π–π stacking, (c) factual wrapping around nanostructures, or a combination thereof requires use of surfactants38 (e.g. sodium dodecylbenzenesulphonates), hydrophilic moieties (sugar,39 Arabic gum40) or polymer grafting (e.g.polystyrene).41 Obviously this mild route of functionalisation leaves the integrity of MCN hybrids intact. Moreover, so-individualised (‘debundled’) nanostructures may turn from cytotoxic (raw material) into even biodegradable.42
Methods of ‘decoration’ of the carbon component are dominated by step-by-step strategies, and the desired magnetic behaviour is acquired predominantly during functionalisation of the non-modified carbon nanostructures. The rigidity of the final nanoarchitectures is an important factor and can be controlled by choosing interactions between carbon carrier and magnetic nanoparticles—in other words, their anchoring. The most common magnetic components are superparamagnetic iron oxide nanoparticles (SPIONs).43
In contrast to the previously described encapsulates, numerous magnetic NP-decorated carbon nanostructures are relatively well-dispersible in aqueous media,44 because most of the methods of their synthesis involve fundamental functionalisation creating linkers between carbon and magnetic components. However, the available carbon surface becomes reduced, making further functionalisation troublesome.
Therapeutic applications
(i) Magnetic drug delivery systems
A drug delivery system (DDS) provides transport of a drug to target site and then releases it in a controllable manner, optimising the concentration of active molecules at the target site. This therapeutic strategy has many advantages over a free-drug-approach; hence a DDS can minimise the quantity of the drug used in a therapy and thus enhance its efficiency. The benefits are especially evident when drug is poorly soluble, readily degradable in vivo or highly cytotoxic (e.g. anticancer drugs)—in such cases drug delivery could prevent negative side-effects, particularly damage of the healthy tissues.45MCNs can be considered as interesting building blocks for bottom-up drug delivery systems design. The magnetic behaviour they exhibit could be utilised to concentrate drug-loaded structures within target tissue under the influence of external magnetic field, then held there as long as drug is being released. It must be emphasised that due to a rapid magnetic field fall-off (∼r−3) discussed here, the DDS variant is rather restricted to tissues located near to the body surface,46 unless the magnets are located within the target tissue.44,47,48 There are several approaches towards the MCN-mediated drug delivery since drug molecules may be bound to large, carbonaceous surfaces49 or particularly carried in internal cavities of hollow nanocontainers.47 There are no, but possible, technological obstacles with combining these strategies. If a potential drug vehicle meets criteria of fundamental biocompatibility and cytotoxicity, it can be loaded practically with an infinite number of therapeutic agents. Another possibility for utilising magnetic behaviour in the field of drug delivery is magnetically induced heating which can facilitate both diffusion of drug molecules at the target site and their cellular uptake.50
The release mechanism is an essential question in the design of a DDS, and thus drug loading is not just another ‘functionalisation-challenge’. Drug release may be induced with both environmental factors (e.g. enzymatic action) and properties of the carrier (e.g. temperature-induced release involving alternating magnetic fields50). One can envisage that extremely high surface to mass ratio, together with progress made in the chemistry of nanocarbons, open possibilities for incorporation of multimodality—enabling combined targeting (e.g.antibody assisted drug delivery) and therapeutic strategies (with various drugs loaded). Nevertheless, there are only a few reports of examples of magnetic carbon nanostructures (discussed below) tailored for drug delivery although their full potential may be deduced from broadly described properties of corresponding carbon nanostructures and magnetic nanoparticles. This pathway is expected to be particularly explored in the future since the direct use of magnetic nanoparticles such as SPIONs for targeted drug delivery can cause several problems. One of the main experimental challenges is to design a material capable of sufficient drug loading (in a given delivery media) exhibiting no so-called ‘burst effect’ (or ‘burst drug release’).51 The term ‘burst effect’ refers to a large volume of drug being rapidly released into the body. This phenomenon can be dangerous not only due to the economy of the pharmaceutical industry, although expenses of the drugs (research, medical tests, production, etc.) make it unquestionably advantageous to contain them in the delivering vehicle as long as possible and to release them in a sustained manner. At present, MCNs emerge as a sole alternative to address this problem due to a number of possible interactions between carbon and drug molecules accompanied by an excellent magnetic performance.52
Yang and co-workers demonstrated a system composed of multi-walled carbon nanotubes (MWCNTs) grafted with polyacrylic acid (acting as a linker for magnetite NPs) and loaded it with Gemcitabine™ (chemotherapeutic drug) by physical adsorption.53 The authors compared concentrations of Gemcitabine™ in blood and left popliteal lymph nodes for different time intervals after subcutaneous administration (Sprague Dawley rats) to examine whether the synthesised drug vehicle could be preferentially taken into lymphatic vessels just like nanosized activated carbon. Indeed, results revealed that the magnetically guided MWNCT-derived drug vehicle showed enhanced absorption to lymphatic vessels. Although the weak point of the described system is a non-optimised drug release profile, the concentration of Gemcitabine™ in examined lymph nodes was higher than for nanosized activated carbon loaded with the same drug and for free Gemcitabine™ during the whole experiment.
Recently Li and co-workers synthesised folate functionalized Fe@MWCNT nanohybrids and loaded them with Doxorubicin™ (a popular anticancer chemotherapeutic agent) by physical adsorption.48 They performed an in vitro assay (HeLa cells), and demonstrated that delivery effectiveness is 6-times higher than for free Doxorubicin™ (cell viability assay). The release mechanism utilised heating with NIR irradiation. An estimated Doxorubicin™ loading capacity of this system was of 32 μg g−1. Interestingly, graphene oxide (GO) decorated with magnetite nanoparticles hybrids showed even greater Doxorubicin™ loading capacity (1.08 mg mg−1).44 Unfortunately, the GO-based system was neither examined in vitro nor in vivo; consequently its potential as a DDS is disputable.
A remarkable example of an MCN-based drug delivery system was reported by Sherlock and colleagues.50 They developed a system based on Fe/Co nanocrystals coated with graphitic shells. Such nanoparticles (of diameter 4–5 nm) were loaded with Doxorubicin™ (anchored via π–π stacking), and examined in vitro (MCF-7 cells) as a potential drug vehicle. The proposed system showed pH-sensitive drug release. Moreover, photothermally induced heating caused enhanced cellular uptake of Doxorubicin™. It is noteworthy that the magnetic behaviour of synthesised nanoparticles was not utilised to concentrate them at the particular target place, but to decrease relaxation time of water protons; thus the proposed system acted both as drug vehicle and MRI contrast agent. Such a combined approach is very promising as it additionally allows the progress of therapy to be followed.
Since nucleic acids have been successfully conjugated with various carbon nanostructures,54,55 it is clear that the developed methods of synthesis of these conjugates are to a certain extent adaptable to corresponding magnetic nanohybrids. Moreover, some of the synthesised DNA-nanocarbon conjugates were found to be relatively stable in biological systems and were able to cross cell membranes.56 This behaviour implies a possibility of applying MCNs in a gene therapy in the future.
(ii) Magnetic particle/fluid hyperthermia
Magnetic thermotherapy is one of the most interesting prospective medicinal applications of magnetic nanoparticles, particularly magnetic carbon nanostructures. It is based on the enhanced sensitivity of tumour cells to elevated temperatures.57 Penetrating the tumour tissue with magnetic nanoparticles, followed by applying alternating external magnetic fields, leads to controllable heating of the target tissue area, resulting in death of the cancer cells. Generally, the heating mechanism strongly depends on the magnetic behaviour of the material. Although an accurate description of the heat generation process involves numerous factors, heating with ferromagnetic nanomaterials may be described in a simplified way in terms of hysteresis losses, while heating with superparamagnetic single-domain particles involves additional thermal relaxation effects.57The most common parameter used to compare effectiveness of heating performed with different materials is specific loss power (SLP), which expresses mass normalised rate of energy absorption and is measured in watts per gram. The value of the SLP depends on several factors related both to material (shape, size, composition) and external AC magnetic field (frequency, amplitude). Note that in medicinal literature SLP is often described as specific absorption rate (SAR). It has to be emphasised that anticancer thermotherapy performed with MCNs is not restricted to magnetically induced heating, since a variety of carbonaceous components, especially single-walled carbon nanotubes, reveal strong NIR irradiation absorption,58,59 allowing NIR induced additional heat generation.60 Nevertheless, a magnetically induced strategy seems to be advantageous, since NIR light can interact with biological systems to a larger extent than AC magnetic fields and also serve as an imaging probe.61
Optimising the concentration of nanoparticles at the target site could be realised by intratumoural injection, with targeting agents introduced in advance onto the nanoparticles surface or, remotely, with external permanent magnets (meeting the same limitations as mentioned for DDS).
To the best of our knowledge there are no reports on applying magnetic carbon nanostructures as hyperthermia agents neither in vitro nor in vivo, but Krupskaya et al. examined SLP values of Fe@MWCNT hybrids dispersed in water proving their potential in this field.57 Other works, where superparamagnetic iron oxide nanoparticles (SPIONs) were combined with CNTs in nanocomposites also suggest that these hybrids can be applied in this field.62
Diagnostic applications—magnetic resonance imaging
MRI is one of the most valuable non-invasive imaging techniques in the contemporary diagnostic medicine. It can be considered as a variation on 1H NMR spectroscopy, widely used in the field of organic chemistry. In order to improve diagnostic reliability, substances known as contrast agents are used. The essential task of the contrast agent is to decrease relaxation time of water protons. Depending on the nature of the relaxation process, there are T1 and T2 contrast agents decreasing spin-lattice and spin–spin relaxation time, respectively. The most commonly used parameter for characterisation of contrast agents is relaxivity (r1,2), which describes the change in the relaxation rate of water protons per molar concentration of a given contrast agent. Until today, the most common T1 contrast agent used clinically is a strong paramagnetic Gd(III), which has to be sequestrated (e.g. with multidental chelates) because of its toxicity.63 In turn, extensively utilised T2 contrast agents are SPIONs and USPION (ultra-small SPIONs).64 SPIONs and USPIONs are themselves considered to be relatively mild to the body mainly because iron oxide is dissolved under acidic conditions. Creation of a carbonaceous shell for (U)SPIONs not only protects nanomagnet inners from escape (which has in fact a negligible effect to the human body as the released Fe3+ ions can be fed into the natural iron storage) but first of all enables easier surface functionalisation of the hybrid.Although among recently synthesised magnetic carbon nanostructures of different size, shape and composition, many could act as potential contrast agents, there are only a few papers concerning that question in a comprehensive manner. Interestingly, most of them focus on relatively rare Gd@C nanohybrids, despite the fact that remarkable advances in a synthesis of various Fe/Co/Ni/Fe3O4@C nanostructures have been developed and are presented in the following paragraph.
Richard and co-workers reported synthesis of MWNT non-covalently functionalized with amphiphilic Gd(III) chelates.65,66 This strategy seemed to be an extension of contrast agents being in clinical use so far. Anionic surfactant composed of a lipid chain and a DTPA5− (diethylene triamine pentaacetic acid) polar head was chemically adsorbed on the sidewall of purified multi-wall CNTs, making them dispersible in water. Gd(III) ions were subsequently complexed with DTPA5− (strong chelator), thus preventing from undesirable toxic side-effects. Further examination displayed moderately elevated r1 relaxivities as compared to a clinically used gadolinium-based T1 contrast agent (GdDTPA Magnevist®). This relatively low enhancement in relaxivity was ascribed to the effective motion of Gd(III) chelates, being a consequence of a poor rigidity of the studied system. The authors also performed measurements of T2 relaxation times, which revealed that irrespectively from chelated Gd(III) concentrations, T2 values were noticeably lower than in pure water. This fact led them to the hypothesis that the multi-wall CNT electronic structure may be responsible for decreasing T2. Importantly, the authors observed no remarkable toxic side effects during in vivo experiments (mice).
Encapsulation of gadolinium cations within a carbon cage is another convenient method to avoid toxic side-effects, taking an advantage over chelating compounds; hence they could be susceptible to release of free Gd3+ ions.67–69 The first reported superparamagnetic Gd@C hybrids were gadofullerenes.70 Their polyhydroxylated water-soluble derivatives (Gd@C82(OH)x) were studied as MRI contrast agents of better relaxivities when compared to clinical Gd(III)-based contrast agents (5–20 times higher relaxivity). For a long time in this field, the crucial problem remained separation of pristine gadofullerenes (Gd@C60, Gd@C70, Gd@C74, Gd@C82) synthesised simultaneously by the arc-discharge method. Initially only abovementioned Gd@C82 (making up no more than 10% of gadofullerenes produced per cycle) could be extracted from as-obtained carbon soot. These difficulties have been overcome with improved synthesis and purification techniques, and Gd@C60-based T1 contrast agents also revealed superior relaxivities than any Gd3+-based contrast agent in current clinical use. Sitharaman and co-workers reported synthesis of hydrated Gd3+n ion clusters within ultra-short single-wall CNTs and proved their utility as an efficient contrast agent.71 Authors assumed that gadolinium ions occupied defect sites created on the tubes during a cutting step. They studied relaxivities of water protons with obtained materials, dispersing them in aqueous media with biocompatible surfactants, and compared them with those acquired with [Gd(H2O)8]3+ and some other commercially available contrast agents. It appeared that despite low gadolinium content (2.84% w/w—as determined by ICP-OES), relaxivities were far higher than in any contrast agent reported by now. Interestingly, an increase in the relaxivity as compared to conventional gadolinium-based contrast agents was observable particularly at very low fields (90-times greater relaxivity than for conventional contrast agent). Thus, it seems that ‘gadonanotubes’ could face current trends in the development of MR imaging (applying higher and higher magnetic fields). Surprisingly, ‘gadonanotubes’ used as contrast agent showed strong relationship between pH and relaxivity, making possible a design of prospective pH-dependent advanced contrast agents.72 Moreover, contrarily to recently used contrast agents ‘gadonanotubes’ showed relatively constant relaxivities at higher fields.
Another gadolinium(III)-carbon nanohybrid reported was composed of ultra fine (water insoluble) gadolinium oxide Gd2O3 particles (mean diameter 2.3 nm) incorporated into the hollow nanospace of single-walled carbon nanohorns.73 The authors proved the possibility of utilising such structures as MRI contrast agents but did not report values of relaxivities, thus making it incomparable with the previously discussed ‘gadonanostructures’. The same group proposed synthesis of analogous Fe3O4 labelled single-walled carbon nanohorns and performed MR imaging studies in vivo (mice).74
Other promising MWNT-based nanostructures, which could act as T2-contrast agents, may be composed of superparamagnetic iron oxide nanoparticles bound to the outer surface of nanotubes. Wu and co-workers synthesised hydrophilic Fe3O4-decorated-MWNT hybrids (41.3 wt% of Fe3O4) with magnetite nanocrystals of a mean size about 4.5 nm.75 The nanostructures exhibited superparamagnetic behaviour at 150 K and 300 K. The obtained hybrids revealed low cytotoxicity over a broad range of concentrations (up to 200 μg mL−1) and negligible haemolytic activity, thus making them applicable for intravenous administration. Further biodistribution assay displayed that most of the Fe3O4-MWNT hybrids were taken by the liver, spleen and lung (mice), and could be excreted from the liver and kidneys. MRI studies, performed both in vitro and in vivo, displayed potential utility of such structures as T2-weighted contrast agents with comparatively high T2 relaxivity (r2 of 175.5 mM−1 s−1).
Albeit discussing multimodality in the context of therapeutic applications, it is also possible to combine different diagnostic strategies by conjugating nanocarbons with ‘imaging moieties’. Chen and co-workers obtained nanohybrids for multimodal imaging by decorating MWNTs with magnetite nanoparticles and NIR fluorescent CdTe quantum dots by the layer-by-layer approach.76 A layer of magnetite particles acted as a component responsible for decreasing the relaxation time of water protons as well as a spacer between the CNTs surface and CdTe particles, avoiding fluorescence quenching of quantum dots by the CNTs. This system was examined in vitro (293T cells) and proved to be more effective than SPIO-based T2-weighted MRI contrast agents. Moreover, the authors observed enhancement of fluorescent intracellular labelling efficiency, which was ascribed to improved cellular uptake of nanotube-derived hybrids when compared with SPIO-CdTe particles. However, the photoluminescence quantum yield of nanocarbon derived conjugates was significantly lowered owing to the fact that some CdTe particles were directly conjugated with the CNTs surface.
Conclusions and outlook
Magnetic carbon nanostructures are the newest members of the magnetic nanoparticles family. What has to be done to make their transition from science to a technological reality? In chemistry and materials science, particularly if the final product is designed for medicine, the ‘value’ is often strictly related to its purity. Thus it is obvious that there is a need to optimise or to improve synthesis methods; hence uniform particles equal uniform properties, ensuring repetitive effectiveness in particular applications and reliability of toxicological studies. Moreover, a question of great importance is how to develop efficient functionalisation techniques which should provide possibilities for more convenient introduction of desirable functionalities. All the specific applications require also fundamental research, such as appropriate heat transfer models inside the tissues for hyperthermia or drug release mechanisms for DDS.We think that the continuous progress being made in the methods of synthesis, purification techniques, and functionalisation of carbonaceous nanomaterials will put them into widely applied industrial practice in the near foreseeable future.
Notes and references
- M. S. Dresselhaus, G. Dresselhaus and P. Avouris, Carbon Nanotube Synthesis, Structure, Properties and Applications, Springer, New York, 2000 Search PubMed.
- H. W. Kroto, J. R. Heath, S. C. O'brien, R. F. Curl and R. E. Smalley, Nature, 1985, 318, 162 CrossRef CAS.
- S. Iijima, Nature, 1991, 354, 56 CrossRef CAS.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS.
- V. Wagner, A. Dullaart, A. K. Bock and A. Zweck, Nat. Biotechnol., 2006, 24, 1211 CrossRef CAS.
- P. Malhotra and A. Singh, JK Science, 2010, 12, 3 Search PubMed.
- S. Bhaskar, F. Tian, T. Stoeger, W. Kreyling, J. M. de la Fuente, V. Grazú, P. Borm, G. Estrada, V. Ntziachristos and D. Razansky, Part. Fibre Toxicol., 2010, 7, 3 CrossRef.
- T. K. Indira and P. K. Lakshmi, Int. J. Pharm. Sci. Nanotechnol., 2010, 3, 1035 CAS.
- E. Duguet, S. Vasseur, S. Mornet and J.-M. Devoisselle, Nanomedicine, 2006, 1, 157 CrossRef CAS.
- O. V. Salata, J. Nanobiotechnol., 2004, 2, 3 CrossRef.
- S. M. Janib, A. S. Moses and J. A. MacKay, Adv. Drug Delivery Rev., 2010, 62, 1052 CrossRef CAS.
- Z. Liu, S. Tabakman, K. Welsher and H. Dai, Nano Res., 2009, 2, 85 CrossRef CAS.
- K. Kostarelos, A. Bianco and M. Prato, Nat. Nanotechnol., 2009, 4, 627 CrossRef CAS.
- Z. Liu, K. Chen, C. Davis, S. Sherlock, Q. Cao, X. Chen and H. Dai, Cancer Res., 2008, 68, 6652 CrossRef CAS.
- J. Kolosnjaj, H. Szwarc and F. Moussa, Adv. Exp. Med. Biol., 2007, 620, 181 CrossRef.
- H. J. Johnston, G. R. Hutchison, F. M. Christensen, S. Peteres, S. Hankin, K. Aschberger and V. Stone, Nanotoxicology, 2010, 4, 207 CrossRef CAS.
- S. K. Sohaebuddin, P. T. Thevenot, D. Baker, J. W. Eaton and L. Tang, Part. Fibre Toxicol., 2010, 7, 22 CrossRef.
- S. Beg, M. Rizwan, A. M. Sheikh, M. S. Hasnain, K. Anwer and K. Kohli, J. Pharm. Pharmacol., 2011, 63, 141 CrossRef CAS.
- S. Boncel, K. H. Müller, J. N. Skepper, K. Z. Walczak and K. K. K. Koziol, Biomaterials, 2011, 32, 7677 CrossRef CAS.
- S. Liu, S. Boeshore, A. Fernandez, M. J. Sayagués, J. E. Fischer and A. Gedanken, J. Phys. Chem. B, 2001, 105, 7606 CrossRef CAS.
- I. P. Chang, K. C. Hwang and C.-S. Chiang, J. Am. Chem. Soc., 2008, 130, 15476 CrossRef CAS.
- C. N. He, X. W. Du, J. Ding, C. S. Shi, J. J. Li, N. Q. Zhao and L. Cui, Carbon, 2006, 44, 2330 CrossRef CAS.
- E. Terrado, M. Redrado, E. Muñoz, W. K. Maser, A. M. Benito and M. T. Martínez, Mater. Sci. Eng., C, 2006, 26, 1185 CrossRef CAS.
- N. Aguiló-Aguayo, J. L. Castaño-Bernal, J. García-Céspedes and E. Bertran, Diamond Relat. Mater., 2009, 18, 953 CrossRef.
- X. Qi, Ch. Qin, W. Zhong, Ch. Au, X. Ye and Y. Du, Materials, 2010, 3, 4142 CrossRef CAS.
- S. Nepijko, A. Graff, G. Schönhense and C. Schneider, Appl. Phys. A: Mater. Sci. Process., 2009, 94, 543 CrossRef CAS.
- M. Bystrzejewski, A. Huczko and H. Lange, Sens. Actuators, B, 2005, 109, 81 CrossRef.
- G. Korneva, H. Ye, Y. Gogotsi, D. Halverson, G. Friedman, J.-C. Bradley and K. G. Kornev, Nano Lett., 2005, 5, 879 CrossRef CAS.
- H.-Q. Wu, X.-W. Wei, M.-W. Shao, J.-S. Gu and M.-Z. Qu, J. Mater. Chem., 2002, 12, 1919 RSC.
- G. Korneva, H. Ye, Y. Gogotsi, D. Halverson, G. Friedman, J.-C. Bradley and K. G. Kornev, Nano Lett., 2005, 5, 879 CrossRef CAS.
- U. Narkiewicz and M. Podsiadly, Appl. Surf. Sci., 2010, 256, 5249 CrossRef CAS.
- N. Luo, X. Li, Y. Sun and X. Wang, J. Alloys Compd., 2010, 505, 352 CrossRef CAS.
- M. Bystrzejewski, S. Cudzilo, A. Huczko and H. Lange, J. Alloys Compd., 2006, 423, 74 CrossRef CAS.
- A. D. Grief and G. Richardson, J. Magn. Magn. Mater., 2005, 293, 455 CrossRef CAS.
- É. Tóth, R. D. Bolskar, A. Borel, G. González, L. Helm, A. E. Merbach, B. Sitharaman and L. J. Wilson, J. Am. Chem. Soc., 2004, 127, 799 CrossRef.
- D. W. Boukhvalov and M. I. Katsnelson, J. Phys.: Condens. Matter, 2009, 21, 344205 CrossRef CAS.
- N. Karousis, N. Tagmatarchis and D. Tasis, Chem. Rev., 2010, 110, 5366 CrossRef CAS.
- R. Shvartzman-Cohen, Y. Levi-Kalisman, E. Nativ-Roth and R. Yerushalmi-Rozen, Langmuir, 2004, 20, 6085 CrossRef CAS.
- S. Boncel, K. Z. Walczak and K. K. K. Koziol, Beilstein J. Nanotechnol., 2011, 2, 311 CrossRef CAS.
- R. Bandyopadhyaya, E. Nativ-Roth, O. Regev and R. Yerushalmi-Rozen, Nano Lett., 2002, 2, 25 CrossRef CAS.
- F. D'Souza and K. M. Kadish, Handbook of Carbon Nano Materials, World Scientific Publishing Co. Pte. Ltd, Singapore, 2011 Search PubMed.
- L. Tabet, C. Bussy, A. Setyan, A. Simon-Deckers, M. J. Rossi, J. Boczkowski and S. Lanone, Part. Fibre Toxicol., 2011, 8, 3 CrossRef CAS.
- D. L. J. Thorek, A. K. Chen, J. Czupryna and A. Tsourkas, Ann. Biomed. Eng., 2006, 34, 23 CrossRef.
- X. Yang, X. Zhang, Y. Ma, Y. Huang, Y. Wang and Y. Chen, J. Mater. Chem., 2009, 19, 2710 RSC.
- J. D. G. Durán, J. L. Arias, V. Gallardo and A. V. Delgado, J. Pharm. Sci., 2008, 97, 2948 CrossRef.
- Y. Hirota, Y. Akiyama, Y. Izumi and S. Nishijima, Phys. C, 2009, 469, 1853 CrossRef CAS.
- D. Yang, F. Yang, J. Hu, J. Long, C. Wang, D. Fu and Q. Ni, Chem. Commun., 2009, 4447 RSC.
- R. Li, R. Wu, L. Zhao, Z. Hu, S. Guo, X. Pan and H. Zou, Carbon, 2011, 49, 1797 CrossRef CAS.
- C. Tripisciano, K. Kraemer, A. Taylor and E. Borowiak-Palen, Chem. Phys. Lett., 2009, 478, 200 CrossRef CAS.
- S. P. Sherlock, S. M. Tabakman, L. Xie and H. Dai, ACS Nano, 2011, 5, 1505 CrossRef CAS.
- R. B. Patel, A. Carlson, L. Solorio and A. A. Exner, J. Biomed. Mater. Res., Part A, 2010, 94, 476 Search PubMed.
- J. L. Arias, F. Linares-Molinero, V. Gallardo and A. V. Delgado, Eur. J. Pharm. Sci., 2008, 33, 252 CrossRef CAS.
- F. Yang, Ch. Jin, D. Yang, Y. Jiang, J. Li, Y. Di, J. Hu, Ch. Wang, Q. Ni and D. Fu, Eur. J. Cancer, 2011, 47, 1873 CrossRef CAS.
- D. Pantarotto, R. Singh, D. McCarthy, M. Erhardt, J.-P. Briand, M. Prato, K. Kostarelos and A. Bianco, Angew. Chem., Int. Ed., 2004, 43, 5242 CrossRef CAS.
- K. Awasthi, D. P. Singh, S. K. Singh, D. Dash and O. N. Srivastava, New Carbon Mater., 2009, 24, 301 CrossRef CAS.
- L. Gao, L. Nie, T. Wang, Y. Qin, Z. Guo, D. Yang and X. Yan, ChemBioChem, 2006, 7, 239–242 CrossRef CAS.
- Y. Krupskaya, C. Mahn, A. Parameswaran, A. Taylor, K. Krämer, S. Hampel, A. Leonhardt, M. Ritschel, B. Büchner and R. Klingeler, J. Magn. Magn. Mater., 2009, 321, 4067 CrossRef CAS.
- K. Yang, S. A. Zhang, G. X. Zhang, X. M. Sun, S. T. Lee and Z. Liu, Nano Lett., 2010, 10, 3318 CrossRef CAS.
- J. T. Robinson, K. Welsher, S. M. Tabakman, S. P. Sherlock, H. Wang, R. Luong and H. Dai, Nano Res., 2010, 3, 779 CrossRef CAS.
- B. Panchapakesan, S. Lu, K. Sivakumar, K. Taker, G. Cesarone and E. Wickstrom, NanoBiotechnology, 2005, 1, 133 CrossRef CAS.
- R. Klingeler, S. Hampel and B. Büchner, Int. J. Hyperthermia, 2008, 24, 496 CrossRef CAS.
- T. N. Narayanan, A. P. Reena Mary, M. M. Shaijumon, L. Ci, P. M. Ajayan and M. R. Anantharaman, Nanotechnology, 2009, 20, 55607 CrossRef CAS.
- D. R. Broome, M. S. Girguis, P. W. Baron, A. C. Cottrell, I. Kjellin and G. A. Kirk, AJR, Am. J. Roentgenol., 2007, 188, 586 CrossRef.
- E. Amstad, M. Textor and E. Reimhult, Nanoscale, 2011, 3, 2819 RSC.
- C. Richard, B.-T. Doan, J.-C. Beloeil, M. Bessodes, E. Toth and D. Scherman, Nano Lett., 2007, 8, 232 CrossRef.
- S. Laurent, L. V. Elst and R. N. Muller, Contrast Media Mol. Imaging, 2006, 1, 128 CrossRef CAS.
- M. Mikawa, H. Kato, M. Okumura, M. Narazaki, Y. Kanazawa, N. Miwa and H. Shinohara, Bioconjugate Chem., 2001, 12, 510 CrossRef CAS.
- L. J. Wilson, Electrochem. Soc. Interface, 1999, 8, 24 CAS.
- S. Zhang, D. Sun, X. Li, F. Pei and S. Liu, Fullerene Science and Technology, 1997, 5, 1635 CrossRef CAS.
- R. D. Bolskar, A. F. Benedetto, L. O. Husebo, R. E. Price, E. F. Jackson, S. Wallace, L. J. Wilson and J. M. Alford, J. Am. Chem. Soc., 2003, 125, 5471 CrossRef CAS.
- B. Sitharaman, K. R. Kissell, K. B. Hartman, L. A. Tran, A. Baikalov, I. Rusakova, Y. Sun, H. A. Khant, S. J. Ludtke, W. Chiu, S. Laus, E. Toth, L. Helm, A. E. Merbach and L. J. Wilson, Chem. Commun., 2005, 3915 RSC.
- K. B. Hartman, S. Laus, R. D. Bolskar, R. Muthupillai, L. Helm, E. Toth, A. E. Merbach and L. J. Wilson, Nano Lett., 2008, 8, 415 CrossRef CAS.
- J. Miyawaki, M. Yudasaka, H. Imai, H. Yorimitsu, H. Isobe, E. Nakamura and S. Iijima, J. Phys. Chem. B, 2006, 110, 5179 CrossRef CAS.
- J. Miyawaki, M. Yudasaka, H. Imai, H. Yorimitsu, H. Isobe, E. Nakamura and S. Iijima, Adv. Mater., 2006, 18, 1010 CrossRef CAS.
- H. Wu, G. Liu, Y. Zhuang, D. Wu, H. Zhang, H. Yang, H. Hu and S. Yang, Biomaterials, 2011, 32, 4867 CrossRef CAS.
- B. Chen, H. Zhang, C. Zhai, N. Du, C. Sun, J. Xue, D. Yang, H. Huang, B. Zhang, Q. Xie and Y. Wu, J. Mater. Chem., 2010, 20, 9895 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
