Surfactant-stabilized graphene/polyaniline nanofiber composites for high performance supercapacitor electrode†
Lu
Mao
,
Kai
Zhang
,
Hardy Sze
On Chan
* and
Jishan
Wu
*
Department of Chemistry, National University of Singapore, 3 Science Drive 3, Singapore, 117543. E-mail: chmcsoh@nus.edu.sg; Fax: (+65) 6779 1691; chmwuj@nus.edu.sg
First published on 12th October 2011
Abstract
Polyaniline nanofibers were prepared by in situ polymerization of aniline in the presence of surfactants such as tetrabutylammonium hydroxide and sodium dodecyl benzenesulfonate stabilized graphene under acidic condition. A homogeneous dispersion of individual graphene sheets within the polymer matrix was achieved due to the good dispersibility of surfactant-stabilized graphene in aqueous phase. The morphology and electrochemical properties of both components were well preserved due to the mild reaction conditions. The composite materials were used for supercapacitor electrode and a high specific capacitance of 526 F g−1 was obtained at a current density of 0.2 A g−1 with good cycling stability.
1. Introduction
Graphene, a two-dimensional all-sp2-hybridized carbon with unique electronic and mechanical properties, has received rapidly growing research interest in recent years. Graphene and chemically modified graphene sheets possess high conductivity, high surface area, and good mechanical properties, comparable with other carbon materials.1–3 Moreover, graphene offers superior chemical stability, large surface-to-volume ratio, and a broad electrochemical window, which render it as an attractive electrode material and also substrate material when compounded with polymers and inorganic particles for lithium ion batteries and electrochemical capacitors.4–13 However, large scale production of high quality graphene still remains challenging using current technologies, including mechanical exfoliation, epitaxial growth on silicon carbide or metal surface, and reduction of graphene oxide (GO), etc.14 Meanwhile, as for graphene based composites, it is important to prevent the agglomeration of graphene sheets and have them homogeneously dispersed in the matrix in order to improve their electrochemical properties. Our group recently developed a method to prepare a series of surfactant-stabilized graphene (SSG) materials with good dispersibility in aqueous solvents and high specific capacitances,15 which opens a new way for the fabrication of graphene-based materials for the above applications with enhanced performance.Conducting polymers are well known for their high flexibility and relatively high specific capacitance. Among these materials, polyaniline (PANI) has been considered as one of the most promising electrode materials because of its low cost, easy synthesis and relatively high conductivity.16 Huang et al. proposed a rapid mixing method for preparing 1D polyaniline nanofibers (PANI-F) with diameters <100 nm, possessing superior electrochemical properties due to the combination of the properties of low-dimensional organic conductors with high surface area materials.17,18 A major problem of using polyaniline materials for supercapacitors is that they exhibit poor stabilities during the charge/discharge process.19–21 Therefore, composites of graphene and polyaniline have been prepared and used for supercapacitor electrodes with improved performance by several research groups including us.22–31,35
Wang et al.25 and Han et al.29 investigated the synergistic effect of PANI-GO composites. However, the insulating property of GO limits further improvement on their electrochemical performance. Wang et al.,24 and Zhao et al.35 prepared PANI-graphene composites through electropolymerization with improved capacitor performance. Meanwhile, Eftekhari et al.31 proposed convincing mechanisms upon similar systems. Yan et al.26 and Murugan et al.27 reported the fabrication of PANI-graphene composites through in situ polymerization method using graphene directly reduced from GO, which may involve the agglomerated form of graphene sheets and hinder the performance of as-prepared composites for supercapacitor electrodes. Wu et al.23 and Liu et al.30 prepared graphene-polyaniline film sheets through vacuum filtration of mixed dispersions of both components. Wang et al. synthesized graphene/polyaniline hybrid material by an in situ polymerization-reduction/dedoping-redoping process with improved electrochemical behavior.28 Our group also reported that homogeneous graphene/polyaniline nanofiber composites can be prepared by in situ polymerization of aniline in the presence of well-dispersed GO in acid solution followed by reduction of GO with hydrazine and re-oxidation of polyaniline by strong oxidant.22 However, during the reduction and re-oxidation process, the chemical and electronic structure of the polyaniline was unavoidably changed, leading to degradation of capacitor performance. Therefore, the ability to prepare homogeneous composites while keeping the intrinsic structure and property of both graphene and polyaniline is crucial for preparing high performance supercapacitor electrode materials.
Based on this background, our aim is to utilize the well-dispersed SSG for the preparation of homogeneously dispersed graphene/PANI-F composites with improved electrochemical performance. The morphology and intrinsic properties of both components should be well preserved in order to achieve a better synergetic effect.
In this work, we report for the first time an easy one-step in situ polymerization method for the preparation of surfactant-stabilized graphene/PANI-F composites, which exhibit very good performance as supercapacitor electrode. Tetrabutylammonium hydroxide (TBAOH) and sodium dodecylbenzene sulfonate (SDBS) stabilized graphene materials were prepared by reduction of GO in the presence of surfactants and the obtained SSG can form a stable dispersion in aqueous solutions and exhibit good capacitance performance based on our recent results.15 Herein, such SSG materials were used for the preparation of homogeneous graphene/PANI-F composites by simple in situ polymerization of aniline in the presence of SSG without the need of post-reduction and re-oxidation. Therefore, the intrinsic structures and properties of graphene and polyaniline remained under this process. Different weight feed ratios of SSG and aniline in the polymerization were also investigated to select the SSG/PANI-F composite with best performance. SSG/PANI-F composites were fabricated through the polymerization of aniline using rapid mixing method in the presence of SSG. Due to the good dispersibility of SSG and the mild reaction condition, a homogeneous dispersion of individual graphene sheets within the polymer matrix was achieved and the morphology as well as intrinsic properties of both components was well preserved, which has an important impact on the improvement of the specific capacitances of the graphene/PANI-F composites. SSG/PANI-F composites prepared in this easy one-step polymerization method show improved performance as electrode materials of supercapacitors compared with the results reported previously.22
2. Experimental
2.1. Material synthesis
Graphene oxide (GO) was synthesized from natural graphite by a modified Hummers method.22 TBAOH and SDBS stabilized graphene materials, termed as GTR and GSR, respectively, were prepared by chemical reduction of GO in the presence of surfactants in water followed by filtration and washing.15 As illustrated in Fig. 1, SSG/PANI-F composites were prepared by in situ polymerization of aniline in a suspension of GTR or GSR in acidic solution. The weight feed ratio of aniline to SSG was varied as 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, 50
10, 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50, and 20
50, and 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 80, and the resulting composites were named as PAGT9:1, PAGT5:5, PAGT2:8, and PAGS9:1, respectively. Typically the purified aniline was dissolved in 1 M HCl at a concentration of 0.3 M and the SSG sample was dispersed in the resulting solution with the aid of ultrasonication for 15 min. While maintaining vigorous stirring, an equal volume of 0.08 M ammonium peroxydisulfate in 1 M HCl was rapidly poured into the mixture. The mixture was allowed to stir at room temperature overnight. The solid was collected by vacuum filtration washed with D.I. water and ethanol and dried in a vacuum oven at 80 °C for 12 h. The real mass percentages of the polyaniline in the composites are estimated as 65% for PAGT9:1, 21% for PAGT5:5, 9% for PAGT2:8 and 67% for PAGS91 by weighing the powder before and after polymerization. For comparison, pure PANI-F was synthesized in the absence of SSG using a similar procedure.17
80, and the resulting composites were named as PAGT9:1, PAGT5:5, PAGT2:8, and PAGS9:1, respectively. Typically the purified aniline was dissolved in 1 M HCl at a concentration of 0.3 M and the SSG sample was dispersed in the resulting solution with the aid of ultrasonication for 15 min. While maintaining vigorous stirring, an equal volume of 0.08 M ammonium peroxydisulfate in 1 M HCl was rapidly poured into the mixture. The mixture was allowed to stir at room temperature overnight. The solid was collected by vacuum filtration washed with D.I. water and ethanol and dried in a vacuum oven at 80 °C for 12 h. The real mass percentages of the polyaniline in the composites are estimated as 65% for PAGT9:1, 21% for PAGT5:5, 9% for PAGT2:8 and 67% for PAGS91 by weighing the powder before and after polymerization. For comparison, pure PANI-F was synthesized in the absence of SSG using a similar procedure.17
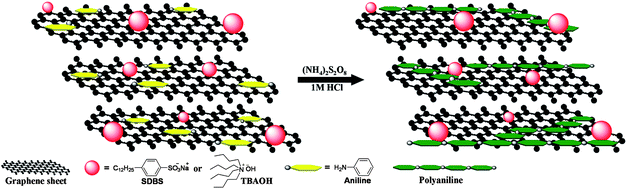 | ||
| Fig. 1 Illustration of the process for the preparation of SSG/PANI-F composites. | ||
2.2. Material characterization
The structure and morphology of the products were characterized by transmission electron microscope (TEM; JEOL 2010 FEG, 200 keV), scanning electron microscope (SEM; JEOL-6300F, 5 kV), X-ray diffraction (XRD; Bruker-AXS D8 DISCOVER, GADDS Powder X-ray diffractometer, copper K alpha line, λ = 1.5406 Å). The thermal data of the products were determined by thermogravimetric analysis (TGA; TA Instruments 2960) at a heating rate of 10 °C min−1 under nitrogen flow. Conductivity measurements of the composites were made on pressed pellets (1.3 cm diameter, < 1 mm thickness) measured by a conventional four-probe technique with an SD-600 Sheet Resistivity Meter. A three-electrode cell system was used to evaluate the electrochemical performance by electrochemical impedance spectroscopy (EIS), cyclic voltammetry (CV), and galvanostatic charge-discharge techniques on an Autolab PGSTAT302N at room temperature. The working electrode was prepared by casting a Nafion-impregnated sample onto a glassy carbon electrode with a diameter of 5 mm. Typically, 5 mg of a composite was dispersed in 1 mL of an ethanol solution containing 5 μL of a Nafion solution (5 wt% in water) by sonication for 30 min. 20 μL of this sample was then dropped onto the glassy carbon electrode and dried in an oven before the electrochemical test. The electrolyte used is a 2 M H2SO4 aqueous solution. A platinum sheet and a AgCl/Ag electrode were used as the counter and the reference electrodes, respectively.3. Results and discussion
3.1. Microstructure characterizations
Fig. 2a shows the XRD patterns of GTR, GSR, pure PANI-F and various SSG/PANI-F composites. For GTR and GSR, the diffraction peaks at 2θ = 24.5 and 42.8° can be attributed to the graphite-like structure (0 0 2) and (1 0 0), respectively. As is commonly known, polyaniline has three ideal oxidation states, as shown in Fig. 3a. Leucoemeraldine with n = 1, m = 0 is the fully reduced state. Pernigraniline is the fully oxidized state (n = 0, m = 1) with imine links instead of amine links. The emeraldine (n = m = 0.5) form of polyaniline, often referred to as emeraldine base (EB), is neutral; if doped it is called emeraldine salt (ES), with the imine nitrogens protonated by an acid.32 The crystalline peaks of as-formed PANI-F show the highly pronounced oscillation structure of PANI in its ES form.33,34 The peak of SSG somewhat overlaps with the diffraction from the PANI nanofibers at 2θ = 25.2°. With the increasing ratio of PANI/SSG, peaks from polyaniline gradually appear and the decrease in the intensity of diffraction peaks of SSG at 2θ = 42.8° was easily observed. The XRD data of SSG/PANI-F composites reveal that SSG is well mixed with PANI-F with no other crystalline structural changes.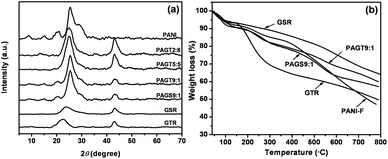 | ||
| Fig. 2 XRD patterns (a) and TGA curves (b) of GTR, GSR, pure PANI-F and various SSG/PANI-F composites. | ||
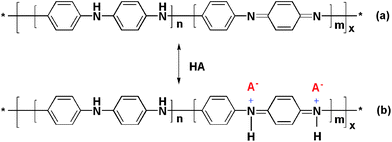 | ||
| Fig. 3 Reversible faradic reaction of electrochemical doping-dedoping of polyaniline in acidic aqueous solution, n + m = 1, x = degree of polymerization. | ||
Fig. 2b illustrates the representative results of TGA. It can be seen that all the materials show a little mass loss around 100 °C due to the deintercalation of H2O. GTR shows steep weight loss at around 200 °C due to the destruction of TBAOH in the interlayer of graphene and 50% weight loss at 800 °C was observed. The poor stability of GTR is attributed to the release of TBAOH at lower temperature. In contrast, GSR shows a smaller weight loss at around 500 °C, and 36% total weight loss at 800 °C. At the same time, the PANI-F sample displays a 47% mass loss from 100 to 800 °C. GTR/PANI-F composites show less weight loss compared with GTR and PANI-F, which may be ascribed to the neutralization and release of TBAOH that combined on the graphene sheet during the acidic condition reaction. It was also found that the composites with higher GTR loading show higher thermal stability and with less weight loss (Fig. S1 in ESI†). Meanwhile, PAGS9:1 shows similar TGA features to PANI-F and GSR with a 43% mass loss at 800 °C.
The morphology and structure of SSG/PANI-F composites were further studied by SEM and TEM (Fig. 4). For PAGT9:1 and PAGS9:1 composites, PANI fibers with similar size coat homogeneously on the surface of GTR and GSR, and the graphene sheet retains its original layer-like structure. For PAGT5:5 and PAGT2:8, where PANIs are not in majority, fibrous PANIs mainly adsorb on the surface of GTR.
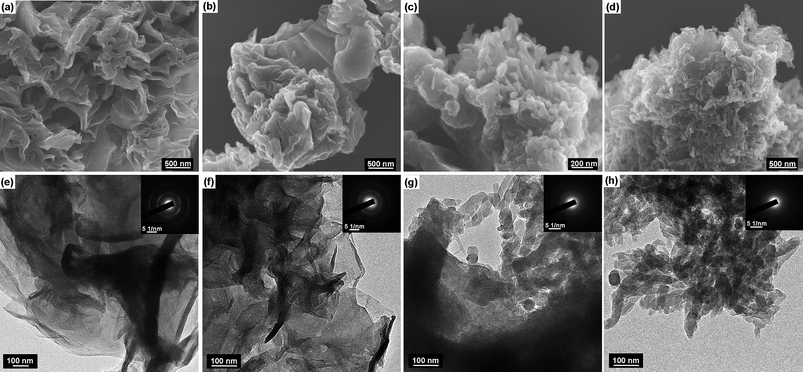 | ||
| Fig. 4 SEM, TEM images and selected area electron diffraction patterns of different SSG/PANI composites: (a) SEM-PAGT2:8; (b) SEM-PAGT5:5; (c) SEM-PAGT9:1; (d) SEM-PAGS9:1; (e) TEM-PAGT2:8; (f) TEM-PAGT5:5; (g) TEM-PAGT9:1; (h) TEM-PAGS9:1. | ||
The mechanism involved during the preparation of SSG/PANI-F composites can be depicted as follows. Since SSG can be easily dispersed into single or few layers in aqueous solution under ultrasonication,15 it is beneficial for the adsorption of aniline monomer to the surface of graphene sheets under stirring. When added into the SSG suspension, the aniline monomer as electron donor can immediately absorb onto the surfaces of SSG, which serves as electron acceptor, due to electrostatic attraction.26,35 Residual carboxylic groups at the edges of SSG may also contribute to this kind of electrostatic interaction.23 When oxidant is added, the aniline molecules absorbed on the sheets and edges are initiated to polymerize just from the absorbed sites, and the strong π–π stacking between the backbones of PANI-F and the graphene basal planes also makes PANI-F homogeneously coat onto the graphene sheets.36,37 It can be concluded that this kind of morphology resulted from the interaction between graphene sheets and PANI-F may facilitate the electron transfer and bring a synergistic effect on electrochemical properties of the hybrid material.
Meanwhile, the typically curved, layer-like structure of GTR is reserved. Since SSG has good crystalline character3 and PANI is poorly crystallized, it can be seen from SAED patterns that the composites show a more crystalline character with increasing of SSG loadings.
3.2. Electrochemical behavior
The performance of SSG/PANI-F composites as electrode materials for supercapacitor was investigated by standard cyclic voltommetry (CV) and galvanostatic charge-discharge technique. The as-formed PANI-F exists in ES form, as confirmed by XRD, and is quite stable at room temperature and electrically conductive upon doping with acid. The pseudo-capacitance of PANI comes from the redox reaction involving counterion influx and outflux from the polymer (Fig. 3). Upon doping or oxidation, electron delocalisation occurs along the polymer chains to enable high conductivity and pseudo-capacitance. This doping charge is reclaimed after the polymer is dedoped by reduction. Thus, acid aqueous solution is commonly chosen as electrolyte for evaluating the electrochemical behavior of PANI, while in a neutral aqueous solution, protonic doping-dedoping of PANI almost could not happen due to a low concentration of H+.38,39The cyclic voltammograms of the different composite electrodes within a potential window from −0.2 to 0.8 V (vs AgCl/Ag) in 2 M H2SO4 (aq.) at 100 mV s−1 are shown in Fig. 5a. GTR and GSR exhibit a relatively rectangular shape which is characteristic of an electric double layer capacitance (EDLC) with specific capacitances of 200 and 160 F g−1, respectively (Fig. S2 in ESI†). The CV curve of PANI-F demonstrates the typical CV characteristic of PANI with two pairs of redox peaks, attributed to the redox transition of PANI between a semiconducting state (leucoemeraldine form) and a conducting state (polaronic emeraldine form) and transformation of emeraldine–pernigraniline.40 As for various SSG/PANI-F composites, two pairs of redox peaks can be seen as a result of the redox transition of PANI. Meanwhile, this kind of feature for PANI in their CV curves decrease with the decreasing of the PANI/SSG ratio in the composites. It can also be inferred from these CV curves that the specific capacitances of these hybrid composites increase with increasing PANI loading, and PAGT9:1 has a larger specific capacitance than PAGS9:1. Fig. 5b shows the CV curves of PAGT9:1 at different scan rates. With increasing scan rate, the redox current increases clearly, indicating its good rate ability. It can also be noted that with an increase in scan rate, a positive shift of oxidation peaks and a negative shift of reduction peaks are observed, which is mainly due to the resistance of the electrode.41
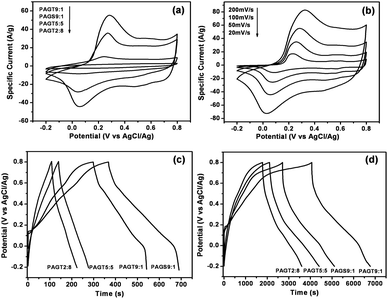 | ||
| Fig. 5 (a) Cyclic voltammograms of the different composite electrodes; (b) CV curves of PAGT9:1 at different scan rates; galvanostatic charge-discharge curves of the SSG/PANI-F composites at (c) 1 and (d) 0.2 A g−1. | ||
Fig. 5c and 5d illustrate the galvanostatic charge-discharge curves of the SSG/PANI-F composites at two representative current densities of 1 and 0.2 A g−1 within a potential window of −0.2 to 0.8 V (vs. AgCl/Ag). PAGT2:8 and PAGT5:5 exhibit more symmetric triangular-shape curves at 1 A g−1, implying the capacitances are mainly attributed to pure electric double layer (EDL) capacitance. The derivation to linearity for curves of PAGT9:1 and PAGS9:1 in 1 A g−1 and charge-discharge curves in 0.2 A g−1 is typical of a pseudocapacitive contribution, indicating that the capacitances of PAGT9:1 and PAGS9:1 mainly originate from pseudocapacitance and the pseudocapacitive behavior is more obvious at lower current density. It can be seen easily that the discharging curves show two voltage stages, from 0.8 to 0.55 V and from 0.55 to −0.2 V, respectively. For the former stage, the relatively short discharging duration is ascribed to the EDL capacitance. For the latter one, the much longer discharging duration is associated with the combination of EDL capacitance and faradic capacitance of PANI-F. Furthermore, during the negative potential region, decreased current in CV curves and relatively short discharging duration can be seen, which are due to the decreased conductivity arising from the undoped PANI in this potential region. The average specific capacitance values, Cavg (F g−1) of the samples were calculated according to the following equation
| Cavg = I × Δt/(ΔV × m) | (1) |
| Samples | Conductivity (S m−1) | Specific capacitance (F g−1) | ||
|---|---|---|---|---|
| 1 A g−1 | 0.5 A g−1 | 0.2 A g−1 | ||
| PAGT2:8 | 54.2 | 115 | 193 | 363 |
| PAGT5:5 | 40.8 | 136 | 240 | 460 |
| PAGT9:1 | 40.3 | 321 | 388 | 526 |
| PAGS9:1 | 61.0 | 243 | 304 | 480 |
| PANI-F | 10.6 | 130 | 140 | 264 |
| GTR | 2.7 | 194 | — | — |
| GSR | 83 | 159 | — | — |
Meanwhile, PAGS9:1 has slightly lower specific capacitances at all current densities compared with PAGT9:1, which is consistent with the CV curves discussed above. For PAGT5:5 and PAGT2:8, no obvious specific capacitance changes can be seen compared with pure PANI-F, GTR and GSR at a current density of 1 A g−1. However, their specific capacitances increase more obviously at lower current densities in contrast to pure PANI-F. These data suggest that the specific capacitances of SSG/PANI-F composites are significantly enhanced due to synergic effects from both pristine components, and the pseudocapacitance of PANI-F plays a more important role in the improvement of total specific capacitance. On the one hand, due to the high conductivity and large surface area, SSG forms a conducting network and greatly improves the redox activity of PANI-F. From Table 1, it can be found that the conductivities of SSG/PANI-F composites are much higher than that of pure PANI-F. On the other hand, the fibrous structure of PANIs can inhibit the re-aggregation of graphene sheets. Meanwhile, the incorporation of SSG can effectively enhance the stability of the hybrid materials.
The cycling electrochemical stabilities of PAGT9:1 based electrode was tested by galvanostatic charge-discharge at a current density of 2 A g−1 (Fig. S3 in ESI†): 74% of the original capacitance retained after 500 cycles, much higher than that of pure PANI (43% retained), which suggests the greatly improved cycling stability ascribed to the addition of SSG. Due to the presence of graphene sheets as substrate, the swelling and shrinkage of PANI during doping–dedoping processes can be efficiently restrained, thus hybrid material becomes more adaptable to volumetric changes during redox reaction and a significant improvement on cycling stability is achieved.21,28
The high-performance electrodes consisting of SSG/PANI-F were further examined by the electrochemical impedance spectroscopic (EIS) analysis in a frequency range of 0.1–10 kHz, and results are shown in Fig. 6. Equivalent series resistances of all the materials are around 2 Ω, which can be determined from the x intercept of the Nyquist plots. The Nyquist plots of PANI-F and SSG/PANI-F composites exhibit a semicircle over the high frequency region, indicating the interfacial charge-transfer resistance of the material. Meanwhile, no semicircles can be detected for GTR and GSR, which suggests their low interfacial charge transfer resistances. Similarly, PAGT2:8 shows a more negligible semicircle because of the low content of PANI-F and the high conductivity of GTR sheets. For PAGT5:5, PAGT9:1 and PAGS9:1, a straight 45° sloped line can be seen in the low frequency region which is Warburg resistance resulting from the frequency dependence of ion diffusion/transport in the electrolyte. PAGT2:8 also shows negligible Warburg region on the Nyquist plots, which can be ascribed to the short and equal diffusion path length of the ions in the electrolyte. Furthermore, GTR, GSR and PAGT2:8 show nearly vertical Nyquist plots at the low frequency part, indicating a nearly ideal capacitor response.
 | ||
| Fig. 6 Nyquist plots of different composites electrodes. Insets are the amplified images of Nyquist plots in the high frequency region. | ||
The specific capacitances of SSG/PANI-F composites are higher than the previous reported graphene/PANI composite materials,22 which can be ascribed to the unchanged original morphology and homogeneous mixing of both components under the mild reaction conditions. In this study, due to the well-preserved properties of PANI-F, the significant improvement of total specific capacitance of SSG/PANI-F composites is mainly attributed to the pseudocapacitance of PANI-F, and the SSG may act as a template and conducting material. Thus, a modified PANI/SSG ratio of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with higher capacitance can be concluded. This explains the highest specific capacitance of 526 F g−1 obtained from PAGT9:1 at a current density of 0.2 A g−1 and even at a higher current density of 1 A g−1, a quite excellent value of 321 F g−1 can be obtained. Meanwhile, the cycling stability of PAGT9:1 was greatly enhanced due to the addition of GTR.
1 with higher capacitance can be concluded. This explains the highest specific capacitance of 526 F g−1 obtained from PAGT9:1 at a current density of 0.2 A g−1 and even at a higher current density of 1 A g−1, a quite excellent value of 321 F g−1 can be obtained. Meanwhile, the cycling stability of PAGT9:1 was greatly enhanced due to the addition of GTR.
4. Conclusions
A series of homogeneous surfactant-stabilized graphene/PANI-F composites was prepared through a simple one-step in situ polymerization method. The morphology and electrochemical properties of both components were well preserved. High specific capacitances and good cycling stability were achieved for most composites with the highest specific capacitance of 526 F g−1 at a current density of 0.2 A g−1 for PAGT9:1. More studies based on the surfactant-stabilized graphene materials and conducting polymers or inorganic particles as electrode materials are undergoing in our lab.Acknowledgements
This work was financially supported by Singapore A*Star SERC Thematic Strategic Research Program – Sustainable Materials: Composites & Lightweights (no. 092 137 0011).Notes and references
- A. K. Geim and K. S. Novoselov, Nat. Mater., 2007, 6, 183 CrossRef CAS.
- D. R. Dreyer, R. S. Ruoff and C. W. Bielawski, Angew. Chem., Int. Ed., 2010, 49, 9336 CrossRef CAS.
- C. N. R. Rao, A. K. Sood, K. S. Subrahmanyam and A. Govindaraj, Angew. Chem., Int. Ed., 2009, 48, 7752 CrossRef CAS.
- E. Yoo, J. Kim, E. Hosono, H. Zhou, T. Kudo and I. Honma, Nano Lett., 2008, 8, 2277 CrossRef CAS.
- M. D. Stoller, S. Park, Y. Zhu, J. An and R. S. Ruoff, Nano Lett., 2008, 8, 3498 CrossRef CAS.
- T. Kuilla, S. Bhadra, D. Yao, N. H. Kim, S. Bose and J. H. Lee, Prog. Polym. Sci., 2010, 35, 1350 CrossRef CAS.
- H. Kim, A. A. Abdala and C. W. Macosko, Macromolecules, 2010, 43, 6515 CrossRef CAS.
- J. Zhang, J. Jiang, H. Li and X. S. Zhao, Energy Environ. Sci., 2011, 4, 4009 RSC.
- M. Pumera, Energy Environ. Sci., 2011, 4, 668 RSC.
- Y. Sun, Q. Wu and G. Shi, Energy Environ. Sci., 2011, 4, 1113 RSC.
- L. L. Zhang, R. Zhou and X. S. Zhao, J. Mater. Chem., 2010, 20, 5983 RSC.
- P. J. Hall, M. Mirzaeian, S. I. Fletcher, F. B. Sillars, A. J. R. Rennie, G. O. Shitta-Bey, G. Wilson, A. Cruden and R. Carter, Energy Environ. Sci., 2010, 3, 1238 RSC.
- R. Liu, J. Duay and S. B. Lee, Chem. Commun., 2011, 47, 1384 RSC.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2009, 110, 132.
- K. Zhang, L. Mao, L. L. Zhang, H. S. On Chan, X. S. Zhao and J. Wu, J. Mater. Chem., 2011, 21, 7302 RSC.
- Y. G. Wang, H. Q. Li and Y. Y. Xia, Adv. Mater., 2006, 18, 2619 CrossRef CAS.
- J. X. Huang and R. B. Kaner, Angew. Chem., Int. Ed., 2004, 43, 5817 CrossRef CAS.
- D. Li, J. X. Huang and R. B. Kaner, Acc. Chem. Res., 2009, 42, 135 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2011 10.1039/C1CS15060J.
- S. Jiao, J. Tu, C. Fan, J. Hou and D. J. Fray, J. Mater. Chem., 2011, 21, 9027 RSC.
- G. Lota, K. Fic and E. Frackowiak, Energy Environ. Sci., 2011, 4, 1592 RSC.
- K. Zhang, L. L. Zhang, X. S. Zhao and J. Wu, Chem. Mater., 2010, 22, 1392 CrossRef CAS.
- Q. Wu, Y. Xu, Z. Yao, A. Liu and G. Shi, ACS Nano, 2010, 4, 1963 CrossRef CAS.
- D.-W. Wang, F. Li, J. Zhao, W. Ren, Z.-G. Chen, J. Tan, Z.-S. Wu, I. Gentle, G. Q. Lu and H.-M. Cheng, ACS Nano, 2009, 3, 1745 CrossRef CAS.
- H. Wang, Q. Hao, X. Yang, L. Lu and X. Wang, Electrochem. Commun., 2009, 11, 1158 CrossRef CAS.
- J. Yan, T. Wei, B. Shao, Z. Fan, W. Qian, M. Zhang and F. Wei, Carbon, 2010, 48, 487 CrossRef CAS.
- A. V. Murugan, T. Muraliganth and A. Manthiram, Chem. Mater., 2009, 21, 5004 CrossRef CAS.
- H. L. Wang, Q. L. Hao, X. J. Yang, L. D. Lu and X. Wang, Nanoscale, 2010, 2, 2164 RSC.
- B. H. Han, J. J. Xu, K. Wang, S. Z. Zu and Z. X. Wei, ACS Nano, 2010, 4, 5019 CrossRef CAS.
- S. Liu, X. Liu, Z. Li, S. Yang and J. Wang, New J. Chem., 2011, 35, 369 RSC.
- A. Eftekhari and B. Yazdani, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 2204 CrossRef CAS.
- W. J. Feast, J. Tsibouklis, K. L. Pouwer, L. Groenendaal and E. W. Meijer, Polymer, 1996, 37, 5017 CrossRef CAS.
- J. P. Pouget, M. E. Jozefowicz, A. J. Epstein, X. Tang and A. G. MacDiarmid, Macromolecules, 1991, 24, 779 CrossRef CAS.
- M. Cochet, W. K. Maser, A. M. Benito, M. A. Callejas, M. T. Martinez, J.-M. Benoit, J. Schreiber and O. Chauvet, Chem. Commun., 2001, 1450 RSC.
- Y. Zhao, H. Bai, Y. Hu, Y. Li, L. Qu, S. Zhang and G. Shi, J. Mater. Chem., 2011, 21, 13978 RSC.
- K.-S. Kim and S.-J. Park, Electrochim. Acta, 2011, 56, 1629 CrossRef CAS.
- X. Zhou, T. Wu, B. Hu, G. Yang and B. Han, Chem. Commun., 2010, 46, 3663 RSC.
- C. Peng, D. Hu and G. Z. Chen, Chem. Commun., 2011, 47, 4105 RSC.
- K. Wang, J. Huang and Z. Wei, J. Phys. Chem. C, 2010, 114, 8062 CrossRef CAS.
- S. Ding, D. Chao, M. Zhang and W. Zhang, J. Appl. Polym. Sci., 2008, 107, 3408 CrossRef CAS.
- Y. G. Wang, H. Q. Li and Y. Y. Xia, Adv. Mater., 2006, 18, 2619 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Fig. S1–S3. See DOI: 10.1039/c1jm12869h |
| This journal is © The Royal Society of Chemistry 2012 |
