Fundamental questions and concepts about photoreception and the case of Euglena gracilis
Laura
Barsanti
,
Valtere
Evangelista
,
Vincenzo
Passarelli
,
Anna Maria
Frassanito
and
Paolo
Gualtieri
*
Istituto di Biofisica, CNR, via Moruzzi 1, 56124 Pisa, Italy. E-mail: paolo.gualtieri@pi.ibf.cnr.it; Fax: +39 050 315 2760; Tel: +39 050 315 3026
First published on 14th November 2011
Abstract
The ability to sense light can be considered the most fundamental and presumably the most ancient property of visual systems. This ability is the basis of phototaxis, one of the most striking behavioral responses of motile photosynthetic microorganisms (i.e. microalgae) to light stimuli, which allows them to move toward or away directional light. In order to fully exploit the information content of light (intensity, direction, distribution) microorganisms need proper perceiving devices, termed photoreceptors, which must act as sensors, to perceive wavelength and direction of light, as transducers, to convert the light signal into chemical and/or electrical information, but also as amplifiers and eventually as transmitters. This review describes the universal structural, behavioral and physiological features necessary for the proper functioning of these devices in algae, and how these features have been investigated by means of different analytical techniques such as for example microspectroscopy, digital fluorescence microscopy, two photons FLIM. The insight of the photoreceptive response mechanism is explained using the unicellular alga Euglena gracilis, in which the different structural, behavioral and physiological features combine to achieve a concerted, efficient response to light stimuli.
Insight, innovation, integrationThis review describes the universal structural, behavioral and physiological features necessary for the proper functioning of photoreceptor systems in algae, and how they have been investigated by means of different analytical techniques such as for example electron microscopy and Fourier analysis, microspectroscopy, digital fluorescence microscopy, two photons FLIM. The insight of the photoreceptive response mechanism is explained using the unicellular alga Euglena gracilis, in which the different structural, behavioral and physiological features combine to achieve a concerted, efficient response to light stimuli. |
Fundamental structural features
In aquatic ecosystems, light is a physical factor of fundamental importance to both photosynthetic and (non-photosynthetic) heterotrophic organisms. They are able to sense and respond to light stimuli, ability essential to optimize physiological processes, and to time their lives to feeding, reproduction, defense, and virtually all their functions. One of the most striking responses is phototaxis, in which motile photosynthetic microorganisms adjust their swimming path with respect to incident light in a finely tuned manner. Many are the advantages of phototaxis: it allows photosynthetic organisms to position themselves for optimal light capture and efficient photosynthesis, and facilitates avoidance responses in those situations where light is intense enough to damage pigments and chloroplasts. In the case of many sexually reproducing species, such as Ulva sp.1 both female and male gametes show positive phototaxis, which may cause the co-localization of both gametes near the surface of seawater improving the possibility of encountering. On the other hand, zygotes usually become negatively phototactic, enabling them to swim towards the bottom of the seashore where they can find a suitable substratum.2 Also, non-photosynthetic organisms (heterotrophic) have shown to benefit the ecological advantages of phototaxis. For example, the colorless marine dinoflagellate, Oxyrrhis marina, can orient to light and is able to use photosensory response to locate patches of phytoplankton prey by detection of chlorophyll a autofluorescence.3The full exploitation of light information necessitates proper perceiving devices able to change the small signal represented by the light falling upon them, into a larger signal and response of an entirely different physical nature. These devices, termed photoreceptors, must act as sensors, to perceive wavelength and direction of light, as transducers, to convert the light signal into chemical and/or electrical information, but also as amplifiers and eventually as transmitters. The proper functioning of these basic visual systems in phototactic eukaryotes (algae) relies on some necessary and universal structural, behavioral and physiological features, such as the following:
• Shading device (structural)
• Photoreceptor and photoreceptive proteins (structural)
• Sampling strategies (behavioral)
• Trajectory control (behavioral)
• Signal transmission (physiological).
The shading device and the photoreceptor are the structural elements used to screen the environment and provide information about the intensity and directionality of the incident light. These two elements are always in close association.4,5 The shading device is usually an absorbing element that prevents light coming from certain direction from reaching the detector.
In flagellate algae, the eyespot position is usually strictly defined with respect to the plane of beat of the flagella, though variation exists, however, along the longitudinal cell axis, the eyespot being located either more anteriorly or more posteriorly. The position of the eyespot gives the algae a distinctive left–right asymmetry.6 The typical organization of the eyespot consists of a single layer of closely packed globules containing mainly carotenoids that can play the shading role thanks to their strong absorbance in the 400–500 nm range.7
The most common (and primitive) type of photoreceptor consists of extensive two-dimensional patches of photosensitive proteins, embedded in the plasma membrane in close association with the eyespot; the proteins have a ordered disposition in order to maximize light absorption.5 For detecting the direction of light of a specific spectral range, a photoreceptor demands a high packing density of chromophore molecules organized in a lattice structure, with high absorption cross section of the chromophore, i.e. high probability of photon absorption by the chromophore, and very low dark noise. The dark noise is the noise inherent in a receptor, constant and independent of the light level, which arises from the random thermal motions of the molecules.5,8 For detecting patterns of light, the number and location of photoreceptors having fixed size and exposure time must be viewed according to the pattern of motion of algae; therefore the design of the photoreceptive apparatus in conjunction with the helical movement of the cell produces a highly directional optical device allowing effective tracking of the light direction.9,10 The function of photoreception is inseparable from the presence of photoreceptive proteins but not necessarily from the presence of an eyespot; hence, when the eyespot is absent its function must be performed by the whole algal body.11
Only few photoreceptors can be identified by optical microscopy (Fig. 1a), whereas the eyespot is always easily recognizable because of its size and color, usually orange-red (Fig. 1b).
 | ||
| Fig. 1 (a) Euglena graciliscell. The photoreceptor is the oval body close to the red eyespot in the apical part of the cell. Scale bar: 5 μm. (b) Trachelomonas sp. cell. The red eyespot is clearly visible in the upper part of the cell. Scale bar: 15 μm. | ||
Types of photoreceptive systems
Depending on their organization and structure, photoreceptive systems can be grouped into three main types.4,5In Chlamydomonas reinhardtii (Chlorophyta) the eyespot is about 1 μm in length, located at the equator of the cell, usually composed of two organized rows of carotenoid-filled lipid globules separated by thylakoid membranes, and packed under the chloroplast envelope.7 The layered structure of the shading organelle in this type of photoreceptor reflects orthogonal light toward the photoreceptor and blocks light originating from other directions.10 The photoreceptor of the green alga Chlamydomonas should consist of an extensive two-dimensional patch of photosensitive proteins in the plasma membrane that lies directly over the closely apposed chloroplast envelope at the level of the eyespot.12 This photoreceptor has been recently visualized by immunofluorescence microscopy (see Fig. 2 in the study of Mittelmeier et al., 2011).
Other two examples of Type I system are Leptolyngbya sp. (Cyanophyta)13 and Silvetia compressa (Heterokontophyta).11 Further on in the review we will use these two algae also as examples of phototactic behavior. The deep red cyanobacteriumLeptolyngbya sp. lives in Roman hypogea at extremely low light intensity (1013photons m−2 s−1). It possesses an orange eyespot at the tip of the apical cell of the trichome, which is characterized by osmiophilic globules of about 100 nm in diameter. The globules are arranged in a peripheral cap extending 2–3 μm from the apex and with a possible layered pattern (Fig. 2).13
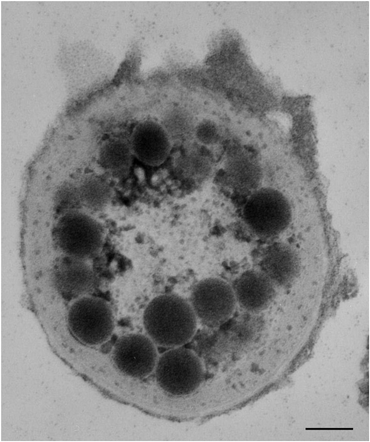 | ||
| Fig. 2 Transmission electron micrograph of a transverse section of the apical portion of a Leptolyngbyatrichome. The micrograph clearly shows osmiophilic globules that resemble the eyespot of eukaryotic algae. Scale bar: 0.15 μm. | ||
The photoreceptor consists of photoreceptive molecules packed in the plasma membrane of the tip of the apical cell, which were identified by means of microspectroscopy (Fig. 3).13
 | ||
| Fig. 3 Absorption spectrum of the tip of the apical cell of a Leptolyngbyatrichome performed by microspectroscopy (black line). The decomposition of the spectra in Gaussian components is shown: carotenoids (blue line), rhodopsin-like proteins (red line). | ||
A different topology is found in the egg of the fucoid brown algae, Silvetia compressa (Heterokontophyta). Immunofluorescence microscopy indicated that the photoreceptor molecules are present inside the whole cell membrane (Fig. 4). The shading function is assigned to the whole cell body, since no eyespot can be detected in these algal stages.11
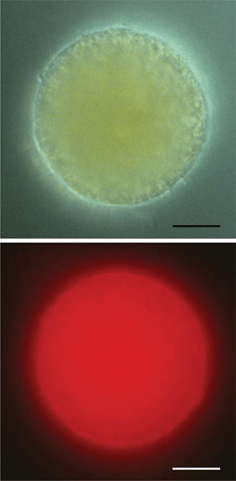 | ||
| Fig. 4 Upper part: bright field micrograph of an egg cell of Silvetia compressa. Lower part: immunofluorescence image of the same cell; the labeling of opsin-like protein(s) within the plasma membrane is impressive. The exposure time is 30 s. Scale bar: 20 μm. | ||
This type of photoreceptive system is present in Heterokontophyta and Euglenophyta. An example of the first division is Ochromonas danica: the eyespot located at the anterior end of the cell consists of a single layer of carotenoid globules contained by the outermost thylakoid of the chloroplast. The cell membrane above the eyespot forms a slight invagination; this depression accommodates the photoreceptor consisting of a 3D assemblage of proteins located inside the base of the smooth flagellum (Fig. 5).14
 | ||
| Fig. 5 Transmission electron micrograph of a transverse section of the apical portion of Ochromonas danica. The micrograph shows the photoreceptor (P) inside the membrane of the short flagellum in juxtaposition of the chloroplastic eyespot (E). Scale bar: 0.3 μm. | ||
An example of the photoreceptive system of Euglenophyta is present in Euglena gracilis. The eyespot consists of a loose collection of globules situated on the dorsal side of the reservoir, which vary in size (from 240 to 1200 nm) and number, can lie in a single layer, or are bunched together. Individual globules may be membrane-bound, but there is never a membrane surrounding the whole complex, and no association with any chloroplast component is present. The position of the eyespot within the reservoir region can vary among the species, but it is always in front of the photoreceptor situated on the long or emergent flagellum (Fig. 6).15 The photoreceptor is a 3D ordered assemblage of stacked membranes formed by 2D crystals of photoreceptive proteins. The organelle is located inside the membrane of the locomotory flagellum, connected to its axoneme by the paraflagellar rod.16 The photoreceptive system of Euglena will be described and discussed in detail in the second part of the review.
 | ||
| Fig. 6 Transmission electron micrograph of a transverse section of the apical portion of Euglena gracilis. The section through the reservoir shows the photoreceptor (P) inside the membrane of the emergent flagellum facing the extrachloroplastic eyespot (E), and some uncoiled filaments of the paraxial rod (arrowheads). Scale bar: 0.3 μm. | ||
 | ||
| Fig. 7 Transmission electron micrograph of a longitudinal section of the ocellus of Erythropsidinium sp. showing the ocelloid chamber (OC), the retinoid (R) and the droplets of the vesicular layer (D), resembling the eyespot. The inset shows a higher magnification of the multilayered structure of the retinoid. Scale bar: 0.5 μm. (Courtesy of C. Greuet). | ||
Photoreceptive proteins
Let's now examine which proteins are the possible candidates for the photoreceptive role.Nature has been highly conservative in choosing the range of molecules for photoreceptive functions and evolved a very limited number of them in the different evolutionary branches of the tree of life because of the closely similar needs of organisms for the detection of the external world.20 In the past, almost every molecule capable of absorbing light was considered a possible candidate for photoreceptive functions.21,22 This assumption proved to be erroneous and we can narrow the group to two molecules: proteins in which the prosthetic group is a polyene as retinal, and related molecules derived from β-carotene, and proteins in which the prosthetic group is a multicyclic compound as flavins.
The first light-sensing, retinal-based receptor was discovered in the haloarchaeal prokaryote Halobacterium salinarum about 30 years ago.23 This first photoreceptor, named Sensory rhodopsin I, was a phototaxis-receptor. It turned out to be a membrane-embedded protein using a photoisomerization of a retinal chromophore as the photoreception mechanism, as visual pigments in human retina do. It consists of seven transmembrane helices forming a pocket in which retinal is attached to a mid-membrane lysyl residue in the seventh helix by means of a protonated Schiff base linkage, like in mammalian visual pigments. A wealth of information from genetics, crystallography, time-resolved spectroscopy and chemical methods have made SRI and the closely related sensory rhodopsin II among the best-understood membrane embedded sensory receptors in terms of atomic structure and function.24
Now, more than three decades since the discovery of rhodopsins in H. salinarum, genome sequencing and sequence comparison tools have revealed that genes encoding rhodopsin-like photoreceptive proteins are shared among distant taxa in all three domains of life: Archaea, Eubacteria, and Eukarya.25 Hence, rhodopsins can be considered photoreceptor proteins universally used from archaebacteria to humans. All rhodopsins consist of a proteic part, the opsin, organized in seven transmembrane α-helices, and a light absorbing group, the retinal (i.e. the chromophore). The retinal is located inside a pocket of the opsin, approximately in its center. As far as algae are concerned, the earlier predictions based on photobiology and biochemistry are in many cases corroborated by contemporary genomic data on Type I rhodopsin sequences in different taxa.26–28 Spectroscopical and biochemical evidence of the rhodopsin-based photoreceptor is available for algae belonging to Euglenophyta29–31 and Heterokontophyta.14,32Genes encoding functional protein probably related to photoreception have been detected both in prokaryotic (Cyanophyta)33 and eukaryotic genera (Cryptophyta, Chlorophyta, Dinophyta),34–37 though examples of transporters (proton or chloride transport) are also present.38 Very recent reports have shown the presence of rhodopsin also in Glaucophyta39 and have confirmed the ubiquity of rhodopsin genes in Dinophyta.40,41 Two different opsins, OPS1 (Genbank accession number GQ402542) and OPS2 (Genbank accession number GU138075), have been sequenced in Cyanophora paradoxa (Glaucophyta), but their function has still to be determined. Immunofluorescence microscopy showed the even localization of OPS1 on the entire surface of the muroplast membrane (Fig. 8).
 | ||
| Fig. 8 Immunofluorescence images of Cyanophora paradoxamuroplasts labeled with the anti-OPS1 antibody. The labeling shows the localization of the OPS1 protein within the muroplast membrane. Scale bar: 5 μm. | ||
According to Frassanito et al. (2010)39 the presence of an integral membrane protein in the membrane of the muroplast, the most primitive of all plastids, should indicate that the common primordial rhodopsin existed in pre-eukaryotic cells before the appearance of the first photosynthetic eukaryotic cell about 1.55 × 109 years ago.42 As to dinoflagellates, rhodopsin-coding genes have been recently identified in natural dinoflagellate assemblage in Long Island Sound (Connecticut, USA) by metatranscriptomic analysis,40 and in the marine predator Oxyrrhis marina species previously believed to be strictly heterotrophic.41 In this dinoflagellate the gene is the most abundantly expressed nuclear gene, and its high level of expression is consistent with the bright pink color of O. marinacells. The functionality of the protein has been proved by Hartz et al. (2011),3 whose results demonstrated that O. marina can orient to light based on rhodopsins present in the outer cell membrane. Moreover, the dinoflagellate uses photosensory response to detect algal prey based on chlorophyll autofluorescence. These reports provide examples of the broad distribution of type I rhodopsin-based photoreceptors among different algal divisions.
Rhodopsins can be considered special for many reasons.26,43 First, the retinal–opsin complex has an intense absorption band whose maximum can be shifted into the visible region of the spectrum, over the entire range from 380 nm to 640 nm. Second, light isomerizes the retinal inside the protein very efficiently and rapidly. This one-molecule isomerization, i.e. the event initiating the vision reaction cascade, can be triggered almost exclusively by light. In the dark it occurs only about once in a thousand years.44 Third, remarkable structural changes (movements of single α-helix) are produced by isomerization of retinal. Light is converted into atomic motion of sufficient magnitude to trigger a signal reliably and reproducibly.45 Fourth, the photocycle (the photoreceptive protein upon light excitation undergoes a series of conformational changes which can be driven back to the original conformational state) is very fast, hence the intracellular photoreceptive machinery is immediately reset for a new response. Fifth, retinal is derived from β-carotene, a precursor with a widespread biological distribution.46
All these supporting data are not available in the case of flavoproteins, which are considered the photoreceptive molecules in few organisms. Flavoproteins, or yellow enzymes, are a diverse group of more than 70 oxidoreductases found in animals, plants and microorganisms. These proteins have a flavin as a prosthetic group covalently attached. The proposition that these proteins could function as a near-UV-visible-light detector dates back to more than forty years.47 Despite the ubiquity and ancient origins of flavoproteins, their role in acquiring information from the radiation environment still remains a complex area of study. Apart from difficulties in their identification, much of the reason for the lack of understanding lies in their diversity of function. Typical absorption spectra of flavoproteins show a dominant protein peak at 280 nm, and major peaks at 380 nm and 460 nm.47 The overall similarity of many blue-light action spectra with flavoprotein absorption spectra is one of the main reasons for the belief that flavoproteins can function as blue-light photoreceptors.48 So far, the only biochemical identification of a presumptive flavin-based photoreceptor has been carried out in Euglena gracilis.49 Despite this paucity of evidence, and the enormous difference among the amount of literature supporting the universal presence of rhodopsin-based photoreceptor in photosynthetic organisms and that supporting the role of flavoproteins, the hypothesis of a flavin-based photoreceptor in algae has withstood time and still remains an accepted working hypothesis.
Fundamental behavioral and physiological features
The structural features above described should be accompanied by necessary behavioral and physiological characteristics (sampling strategies, trajectory control, and signal transmission).Sampling strategies
To determine the orientation of a stimulus field, an organism has to measure the stimulus intensity at different positions, i.e. it should detect either spatial or temporal patterns of light. Femtoplankton (0.02–0.2 μm) is too small compared to the wavelength of light to create differential light intensity; hence, it cannot determine the direction of a light source. Still, femtoplankton microorganisms can use light, but can only measure its intensity and move in a light-intensity gradient. In contrast, phytoplankton dimensions are large enough to determine the light direction and allow the scanning of the environment by means of their directionally sensitive receptor.Two fundamental and alternative strategies exist for obtaining information on the light direction: parallel sampling and sequential sampling. In parallel sampling, the stimulus is detected by multiple separated receptors positioned on different parts of the organism surface. In this case the organism measures directly the spatial gradient by simultaneous comparison of light intensities at two different parts of its body (one instant mechanism). This strategy is present in the zygote of the brown alga Silvetia compressa; the photoreceptive system of the zygote is presumably the same of the egg before fertilization, which has been described in the previous section.11 The zygote absorbs or scatters more than 95% of the effective blue wavelengths within one cell diameter, thus creating a steep light gradient across the cells. Photoreceptors in or near the plasma membrane are activated differentially, and the cellular response to this gradient of photoreceptor activation is to organize an axis and germinate from the darkest point. Photopolarization is an early event, which has been observed 4 hours after fertilization.50 The zygote produces a rhizoidal bulge at about 10 h after fertilization. Initially, any point on the cell surface is capable of becoming the germination site. The bulge elongates by tip growth (i.e. addition of new cell membrane and wall material by localized vesicle secretion), and about 18 h after fertilization the first cell division occurs. The plane of division is perpendicular to the growth axis, resulting in the formation of two highly asymmetrical cells with different developmental fates. The cell bearing the bulge will form the rhizoid, which will show negative phototaxis, while the other cell will form the thallus. Fig. 9 shows the first steps of development of a Silvetiazygote from fertilization to rhizoid formation: changing of the light direction causes a change in the rhizoid growth axis.
 | ||
| Fig. 9 Photobehavior of Silvetia compressazygote: refer to text for details. | ||
In sequential sampling, the stimulus is detected by a single receptor as the cell changes its position in relation to the light source. In this situation the organism measures directly a temporal gradient, and then infers the spatial gradient from the information on the movements of the receptor (two-instant mechanism). This strategy is present in all the algae with a single photoreceptor. These algae have only a limited vision of the three-dimensional world in which they navigate and cannot detect light directions by measuring light intensity at two different positions in the cell body. An example is Leptolyngbya sp., whose photoreceptor system has been described above.13 This cyanobacterium characterized by quite inflexible trichomes uses light to grow towards optimal light intensities, with a sort of oriented movement with respect to the stimulus direction. It changes the direction of the trichome by turning towards the light source at the level of the apical cell (Fig. 10a and b), where both the photoreceptor and the eyespot are located (Fig. 2). When the photoreceptor system is impaired, the movement becomes unguided, and the trichomes appear disordered (Fig. 10c).
 | ||
| Fig. 10 Photobehavior of Leptolyngbya: refer to text for details. Scale bars: 60 μm. | ||
The simultaneous comparison of signals requires widely spaced receptors to detect the intensity gradient, which makes large body size advantageous. On the other hand, sequential sampling requires a coherent pattern of movement. Many algae perform sequential sampling by swimming on helical paths along which their photoreceptor acts as a light antenna continuously searching space for bright spots. Sequential sampling also requires some form of memory to allow the comparison with previously recorded intensities.
Trajectory control
Another fundamental distinction is based on whether an organism is able to make turns in its motion path, which will direct it toward its destination. Depending on the characteristic of the stimulus and the abilities of the microorganism, guiding may be either direct in the sense of taking a straight-line path to the destination or indirect, as in the case of a biased random walk, to reach the vicinity of the destination.Trajectory control characteristics, and thus behavioral peculiarities, are connected with both the shape of the cell and the functioning of the propelling structure of the algae, i.e. the flagellum. If the cell is asymmetric, i.e. it possesses recognizable dorsal and ventral sides, it advances spinning along its axis. It can correct its trajectory by either sudden steering obtained by changing the insertion angle of flagella, for example Ochromonas danica, or stiffening of the flagellumvia the accessory structure of the axoneme, for example Euglena gracilis. This behavior can be attributed to all heterokont and uniflagellate algae. In the case of a symmetric cell, it can accomplish a gradual smooth correction of its trajectory going forward without spinning (or rotating with a very long period), and displacing the barycenter of the motor couple, for example Dunaliella salina. This behavior can be attributed to all isokont cells.
Signal transmission
Signal transmission in algae is still a poorly investigated topic. Algae are aneural organisms, lacking any system for the transmission of the stimuli received from the outside. The information carried by light has to be translated into an organism-specific swimming control mechanism that allows orientation to the light with high fidelity. Hence, the light signal will be first transduced in an electric signal by means of electron or ion flux, and this electric signal will be transmit to the algae motor apparatus, i.e. the flagellum/a. The group of Spudich (2009)51 showed that in Chlamydomonas reinhardtii light absorbed by sensory rhodopsins initiates local photocurrents (a fast photocurrent and a slow photocurrent) in the eyespot region, presumably in the plasma membrane right above the eyespot, where the photosensitive proteins are located. At low intensities sensory rhodopsins trigger a highly efficient biochemical amplification reaction which involves activation of yet unknown downstream elements and control of some diffusible messenger, a process analogous to vision in animals. At high light intensities the second function of sensory rhodopsins, namely direct channel activity, begins to contribute to depolarization of the plasma membrane, which eventually allows motility responses. Biphasic photocurrents have been shown to exist also in other algae, such as for example Volvox carteri (Chlorophyta)52 and Cryptomonas sp. (Cryptophyta).36The example: photoreceptor and photoreception in Euglena
As we have described in the previous section, in order for phototaxis (photoreception) to occur, proper perceiving devices (structural features) satisfying essential requirements are necessary together with specific behavioral and physiological features. In this section we will use Euglena as example to analyze how these structural, behavioral and physiological aspects combine to achieve a concerted response to light stimuli. Euglena dwells in natural shallow ponds, and uses sunlight as source of energy and information. Its chloroplasts are the energy supplying devices, whereas the simple but sophisticated photoreceptive system already described is used as a light detector.According to Eakin’s theory (1968, 1972)53,54 the photoreceptor ciliary line of evolution, which had its climax in the elaborate and remarkably complex vertebrate photoreceptor, originated from the photoreceptor of Euglena. Gehring (2005)55 described the eye as an organ consisting of at least two different cell types, photoreceptor cells and pigment cells. Photoreceptor cells are located close to the effector, and transmit the information conveyed by the light directly to it (without an intervening information processing organelle). In the case of Euglena, the “eye” is formed within a single cell by the assembly of pigmented and photoreceptive molecules within that cell in two distinct organelles, namely the eyespot and the photoreceptor. In the following we will describe and analyze the different components of this primitive eye to demonstrate how its simple design fits Gehring's definition.
The eyespot consists of a loose collection of globules situated on the dorsal side of the reservoir, always in front of the photoreceptor. The globules vary in size and number, can lie in a single layer (Fig. 6), or are bunched together. Individual globules may be membrane-bound, but there is never a membrane surrounding the whole complex. The pigments extracted by the eyespot globules by Heelis et al. (1979)56 were identified by HPLC as carotenoids such as β-carotene, diatoxanthin and diadinoxanthin. The absorption spectrum of the eyespot recorded in vivo by Gualtieri et al. (1989)57 shows a unique and large band centered at 460 nm (Fig. 11a).
 | ||
| Fig. 11 Absorption spectra of the different components of Euglena gracilis photoreceptive system: (a) absorption spectrum of the eyespot; (b) absorption spectra of the two stable states of the photoreceptor, A (green) and B (blue); (c) superimposition of the eyespot absorption spectrum (orange) with the absorption spectra of A and B; (d) emission spectrum of state B (red) superimposed to the absorption difference spectrum (light green) between A and B. | ||
As we have seen in the previous section a photoreceptor must have a regular structure. Let us examine the structure of Euglenaphotoreceptor, (Fig. 12), i.e. the organelle located near the base of the locomotory flagellum.
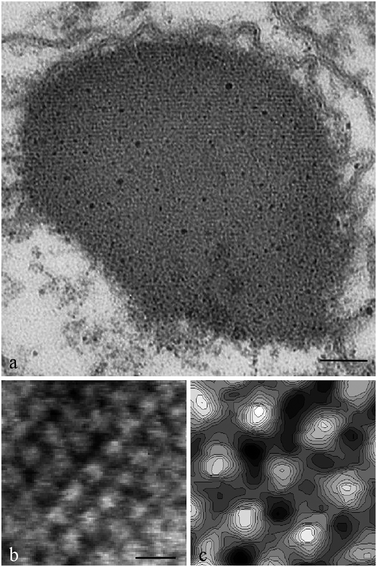 | ||
| Fig. 12 (a) Transmission electron micrograph of a cross section of the Euglena gracilisphotoreceptor showing the fine structure of the organelle. Scale bar: 150 Å. (b) Surface of a lamella showing the ordered pattern of protein oligomers. Scale bar: 100 Å. (c) Grey scale contour plot of the central hexagon of B. | ||
The first ultrastructural investigations on this organelle were performed by Wolken (1977)58 and Piccinni and Mammi (1978).59 These authors analyzed TEM images of thin sections of Euglenaorganelle, but failed to resolve the real pattern. Wolken suggested a fibrillar structure of packed rods, Piccinni and Mami a single crystal with a defined orientation. The detailed structure was revealed by Barsanti et al. (2008),30 and this was the first structural characterization of an algal photoreceptor. So far no other algal photoreceptors have been structurally described in detail. Fig. 12a shows a portion of the photoreceptor at high magnification: the ordered disposition of the membrane layers is evident, made easily recognizable by osmium tetroxide staining of the phospholipids head groups. The authors calculated a periodicity of about 50 Å comparable to the distance between the hydrophilic head layers of a typical membrane bilayer. About 50 membrane layers are present in the photoreceptor. Electron micrograph of a negatively stained 2D lamellae, obtained by ionically induced uncoupling of the 3D compact structure of the photoreceptor, reveals stain excluding units protruding from the surface of the layer, arranged into a regular mesh (Fig. 12b). After Fourier analysis, this mesh shows the ordered patches formed by the oligomers of the photoreceptive membrane spanning protein assembled in a hexagonal lattice as visible in the grey scale contour plot (Fig. 12c), that is a magnification of the central zone of Fig. 12b.
We can conclude that the membrane layers composing the photoreceptor are characterized by in-plane hydrophobic interactions, while their closely stacked disposition is due to the interlayer interactions between charged protein extramembrane domains and the membrane in adjacent layers through charge density matching. About 106 photoreceptive proteins assembled in a hexagonal lattice span the membrane layers.30 Because of these characteristics, the photoreceptor of Euglena as a whole can be defined a “Type I” crystal,60i.e. an ordered assemblage of stacked membranes formed by 2D crystals of membrane proteins. The Euglenaphotoreceptor structure is an example of a perfect device to absorb light with a maximal cross section (Fig. 13).
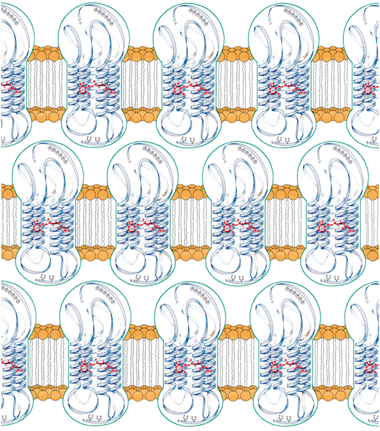 | ||
| Fig. 13 Disposition of the photoreceptive proteins within the membrane layers of the Euglena gracilisphotoreceptor. | ||
Interestingly, the 2D and 3D structural data are very similar to those recently published on artificially induced crystals of proteorhodopsin,61 a membrane protein used by marine bacterioplankton and marine eukaryotes mainly as a light-driven proton pump. Proteorhodopsin artificially assembles as Type I crystal, and the structural similarities of this crystal with Euglenaphotoreceptor structure are impressive.
The spectral properties of the photoreceptor are quite more complex than those of the eyespot. The absorption spectrum of this organelle was first recorded by Gualtieri et al. (1989),57 and after three years by James et al. (1992).62 Their spectra had similar shape and absorbance maximum. Due to the great similarity between these spectra and the absorption curve of the rhodopsin α-band centered at 500 nm, both research groups suggested the presence of rhodopsin-like proteins in the photoreceptor of Euglena. From 1989 to now, the group of Gualtieri performed further investigations on the biochemical and spectroscopic features of the Euglenaphotoreceptor, which provided data to further support the rhodopsin-like proteins hypothesis. The main finding was that the proteins in the photoreceptor are characterized by optical bistability, i.e. they possess two isomeric forms A and B, which interconvert along a photocycling path photochemically but not thermally.63 The absorption spectrum of A has a band centered at 498 nm (from now addressed as A498) (Fig. 12b); this is the dominant form in the photoreceptor under physiological conditions, and it is on this form that the first absorption spectrum was recorded in 1989. The absorption spectrum of B has a band centered at 462 nm (from now addressed as B462) (Fig. 11b). If the absorption spectrum of the eyespot is plotted together with the absorption spectra of the two isomeric forms of the photoreceptor proteins, it will perfectly match the absorption spectrum of the B462 form (Fig. 11c).
These spectroscopic characteristics were assessed also by digital microscopy,64 which provided a direct visualization of the different absorbance and the shift between the spectra of the two forms of the photoreceptor proteins.
Two well defined regions are present in the difference spectrum between the two isomers: a negative region from 400 to 484 nm, and a positive region from 484 to 600 nm. The wavelengths in the positive part of the spectrum drive the transition of A498 to B462, while the wavelengths in the negative part drive the opposite transition.64 Another positive region exists in the UV, below 400 nm, as reported by Barsanti et al. (1997),63 which these authors couldn’t measure by microspectroscopy, because of the extremely complex instrumental setup necessary for this technique.
A498 is the non-fluorescent form, while B462 is the fluorescent form, energetically lower, which can be considered the signaling state of the protein. The fluorescence emission spectrum of the B462 isomer shows a band centered at 550 nm (Fig. 11d), and it is almost superimposable to the positive region of the difference spectrum. Fig. 14 shows the photocycle recorded on a single cell (upper part) and on a microscopic field of isolated photoreceptors (lower part). The null effect of blue light excitation on the isomeric form A498 is evident in Fig. 15: no emission, hence the isomeric form B does not form.
 | ||
| Fig. 14 Photocycle recorded on a single Euglena graciliscell (upper part), and on isolated photoreceptors (lower part). Scale bar: 6 μm. | ||
 | ||
| Fig. 15 The null effect of 436 nm excitation on a single Euglena graciliscell (upper part), and on isolated photoreceptors (lower part). Scale bar: 6 μm. | ||
The presence of optical bistability together with a fluorescent form is a common feature of euglenoid photoreceptors, present also in Phacus and Trachelomonas.64
Though the extraction of photoreceptor protein has not yet been reported, the extraction of retinal from Euglenaphotoreceptor sample,65 together with the matching between the spectral properties and structural peculiarities of Euglenaphotoreceptor protein and those of proteorhodopsin,61,66 allowed the authors to confirm that this organelle is a three-dimensional assemblage of one photoreceptive protein belonging to the superfamily of rhodopsin-like proteins.
Moreover, the spectroscopic characteristics of the two stable states of Euglenaphotoreceptor proteins allow the analogy with the couple metarhodopsin–rhodopsin present in the photocycle of rhodopsins of both invertebrates and vertebrates. A well-fitting example is that of the moth Galleria sp.:67 the absorption spectra of rhodopsin and metarhodopsin, the difference spectrum between them, and the thermal stability of the metarhodopsin are dramatically similar to those of Euglena. The in vivo green emission present in the photoreceptor of Euglena is identical to that of rhodopsin inside the rhabdomeres of Calliphora (Fig. 2 in the study of Schmitt et al., 2005).68
The spectroscopic behavior of the Euglenaphotoreceptor is not consistent with the spectroscopic features of BLUF domain of PAC (photoactivated adenylyl cyclase) proposed as light-sensory protein of Euglena by other research groups.49 Detailed evidences of this discrepancy were described by Barsanti et al. (2009).64 We would like to stress one main and unquestionable point: no emission or only a feeble fluorescent emission is reported in the BLUF protein found in Euglena.49 In order to see a fluorescent signal the authors had to denaturate the protein by boiling it. On the contrary, the emission intensity of the isomeric form B462 of Euglenaphotoreceptor proteins is clearly visible and undisputable.64 To fully appreciate the intensity of the photoreceptor emission, refer to a video in the Supplementary Material of the paper by Mercatelli et al. (2009).31
The question about rhodopsins fluorescence emission was definitely clarified by the group of Garavelli.69 It is well known that cis/transphotoisomerization is the key photochemical event of retinal chromophores triggering proteins activity (i.e.A498 and B462 correspond to two cis/trans isomers of retinal which can be converted only photochemically since the energy barrier on the electronic ground state is too high). Accurate CASPT2/CASSCF calculations on different models of the protonated Schiff base of retinal (PSBR)70 have shown that this process invariably involves two main nuclear motions. After photo-excitation on the first-excited S1 state in the Franck–Condon region, an initial alternate stretching of the carbon backbone triggers the subsequent torsion around the isomerising bond, leading to a conical intersection with the ground electronic state S0. In the rhodopsin cavity, the torsion is actually coupled to other degrees of freedom to give rise to a more complex space-saving crankshaft motion.71 By means of accurate CASPT2/CASSCF calculations on different models of the protonated Schiff base of retinal (PSBR), the authors further stated that the quenching of the counterion by the rhodopsin-like-protein is a prerequisite to have an efficient and ultrafast photoisomerization, since it recovers a barrierless path and a twisted Conical Intersection funnel. Thus, counterion quenching is the fundamental mechanism that provides for both the spectral tuning regulating absorption energies and the photoisomerization, since it promotes catalysis that would be otherwise depressed if the counterion were unshielded. The most interesting consequence of the above said calculations is that a rhodopsin-like-protein with a smaller counterion shielding power possesses a photoreactivity impaired and the fluorescence from the excited-state intermediate becomes a highly competitive process. That is, changing the protein electrostatic field would be the way to switch from photoisomerizable devices to fluorescent dyes.
We can extend the conclusions of Garavelli to the Euglenaphotoreceptor, saying that the non-fluorescent A498 isomeric form has a high counterion shielding power, and is responsible for the very high photoreactivity efficiency and the fluorescent B632 isomeric form has a small counterion shielding power, and is characterized by a so high fluorescent quantum yield to be defined a fluorescent dye. The meaning and the importance of this statement in the functioning of Euglenaphotoreceptor will become clearer in the following.
Under physiological conditions, the available kinetic processes reach a photodynamic equilibrium in which the photoreceptive protein pools consist mainly of A498 isomeric form, and consequently photoreceptor fluorescence is not observed. What happens upon photon absorption?
The primary events of Euglena photoreception were studied by means of fluorescence lifetime imaging microscopy (FLIM) using a two-photon confocal microscope by Mercatelli et al. (2009).31
These authors analyzed intact photoreceptors and photoreceptors treated with the detergent Triton X-100, which increases the distance between the proteins by intercalating the original lipid component of the membrane. In the case of intact photoreceptors, the decay profile is almost perfectly described by a double-exponential curve, with a preponderant fast component (τ1 = 154.8 ps, a1 = 0.915) and a minor slow component (τ2 = 3233.5 ps, a2 = 0.085) (Fig. 16a). The explanation of this decay curve is that the photoisomerizable isomeric form A498 undergoes an intramolecular photoswitch, i.e.S0A498 becomes S0B462 through S1 excited state, and contemporaneously an intermolecular and unidirectional Forster-type energy transfer occurs, i.e. the newly formed fluorescent dye B462 acts as a donor for the nearby protein in the A498 form, which acts as an acceptor. The FRET process (Forster Resonance Energy Transfer), within the couple B462 donor–A498 acceptor, is unidirectional due to the almost perfect match of the fluorescence spectrum of B462 and the spectral region driving the transition of A498 to B462, i.e. it can only drive the transition of A498 to B462 and not that of B462 to A498. This process is also very efficient since about 92% of proteins interact with a timescale of few ps, due to the close distance between the FRET pairs in the regular topography of the photoreceptive proteins and the optimized orientation in space between the transition dipoles of donor and acceptor. The remaining 8% proteins perform physiological emission with a timescale of about 3223.7 ps.
 | ||
| Fig. 16 Two-photon lifetime image of Euglena gracilis: refer to text for details. Scale bars: (a) 16 μm, 8 μm; (b) 16 μm, 8 μm; (c) 16 μm, 8 μm. | ||
In the case of detergent treated photoreceptors, the number of proteins interacting (i.e. the efficiency of energy transfer) decreases as the distance between the FRET pair increases due to the detergent action. The effect on the structure depends upon incubation time: after 12 hours the effect of detergent determined a 50% decrease of FRET efficiency, and the decay profile is almost perfectly described by a double-exponential curve, with two components having almost the same percentage, i.e. a fast component (τ1 = 221.9 ps, a1 = 0.489) and a slow component (τ2 = 3189.7 ps, a2 = 0.511) (Fig. 16b). After 24 hours incubation the effect of the detergent is complete, almost no interactions occur among the proteins, and the FRET process is totally hindered: the decay profile can be described by a single-exponential curve, with only the slow component (τ1 = 3330.3 ps and a1 = 1) (Fig. 16c).
In conclusion, the model for the functioning of Euglenaphotoreceptor in nature can be described as in the following: the cells normally swim by rotating along a helicoidally path; during this motion the photoreceptor proteins are in a photodynamic equilibrium in which S0A498 is the dominant isomer. The equilibrium is interrupted when the eyespot comes between the incoming light and the photoreceptor, thus screening the organelle (Fig. 17). Due to the superimposition of the absorption spectra of the eyespot and the isomer form B462, only UV and green light illuminate the photoreceptor during the screening period, and the only possible transition is that of A498 to B462. Under these conditions, the intramolecular switch and the FRET follow each other with a domino progression along the orderly organized and closely packed proteins in the layer(s), modulating the isomeric composition of the photoreceptive protein pool. As a consequence, S0B462, the signaling state of the protein energetically lower than S0A498, becomes the dominant isomer with a different electrostatic field. This mechanism guarantees that the photoreceptor produces a signal detectable by the cell even when few photons are absorbed with no need of chemical amplification, differently from all the photoreceptors so far analyzed.72–74
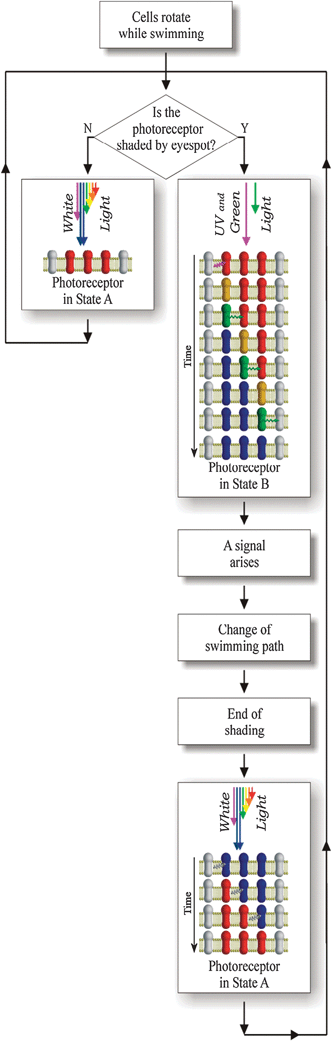 | ||
| Fig. 17 Euglena gracilis phototaxis flow chart. | ||
The authors stressed that the energy needed for the transition of the isomeric form A498 to B462 and the energy transferred from one protein to the nearby protein derive not only from the photons absorbed during the photoreceptor screening by the eyespot but also from the photons absorbed by the photoreceptor in the no-screening period. These photons restore the photoreceptor isomeric composition with S0A498 as dominant isomer, which has a higher energy level than S0B462. Once the screening by the eyespot is over, the transition of B462 to A498 can again occur due to the full spectrum light (natural light) impinging on the photoreceptor, and the isomeric composition with A498 as dominant isomer is again restored.
The Euglenaphotoreceptor is a compact 3D structure of one rhodopsin-like protein with no transduction proteins in its membrane layers. Therefore, this organelle is poorly suited for chemical amplification, but exceptionally suited for electronic amplification; this amplification is necessary since the amount of light absorbed by the organelle is in the range of hundreds of photons at most.
These results indicate that the functioning of Euglenaphotoreceptor differs from that of all the photoreceptors so far analyzed. In vertebrate rods and cones, the information is relayed via transducin (Gt), and cGMP;72,73,75 in rhabdomeric-type visual cells of higher invertebrates Gq-type G protein passes the light information to the phosphoinositol signaling cascade;73 in algae a cation influx across the eyespot that partly overlays the plasma membrane follows light excitation.74
How the signal generated by the photoreceptor drives the Euglena movement? As above described, changes in the motion trajectory by bending of the locomotory flagellum are due to the screening of the photoreceptor by the eyespot that changes the electrostatic field. The locomotory flagellum carries along the entire length of its axoneme a hollow rod-like structure with a diameter of 90 nm, the paraxial rod (PAR), i.e. the true effector. The PAR is composed of seven 22-nm filaments coiled into a 7-start left-handed helix.76 The pitch of the helix has been reported to be 45°, with a lattice periodicity of 54 nm.77 Goblet-like projections have been observed to link the PAR with one of the microtubule doublets in the flagellar axoneme,78 probably microtubule doublet no. l, or no. 2 or no. 3.78 When the photoreceptor is screened by the eyespot, S0B462, the signaling state of the protein, becomes the dominant isomer, and a change of the electrostatic field occurs.
Since the photoreceptor and the paraxial rod are a structural unit (Fig. 18), we can hypothesize that the photoelectric signal could be propagated through the paraxial rod filaments via charge transfer between rod proteins, modifying the pitch of its helix, which in turn modifies the distribution of the mass of the rod along the axoneme. This leads to a change in the motion wave running along the flagellum, and eventually to a change in the swimming direction.
 | ||
| Fig. 18 Two-photon lifetime image of Euglena gracilis: refer to text for details. Scale bars: (a) 16 μm, 8 μm; (b) 16 μm, 8 μm; (c) 16 μm, 8 μm. | ||
Conclusions
The attempt of this review was to categorize into structural, physiological and behavioral the universal features upon which the photoreception systems of algae rely for light perception and exploitation. They are: shading device, photoreceptor and photoreceptive proteins, sampling strategies, trajectory control, and signal transmission. Each of these features was analyzed in detail in the unicellular flagellate Euglena gracilis, which can be considered a model organism with a simple still very sophisticated visual system.The paths by which photoreceptive systems in different phyla have evolved are steadily becoming clearer, although uncertainties still abound. What is undeniable is that despite a remarkable variation in size and complexity, the common indispensable basis of the molecular machinery underlying these systems is the presence of photosensitive molecules such as rhodopsin. Since the first step in both non-imaging and imaging vision is light sensing, rhodopsin is the molecule that absorbs light and thus ‘senses’ light.
Many aspects of the functioning of algal photoreceptive devices require further investigations to clarify the different steps of the photoresponses, and to gain insight into the evolution of signal transmission and transduction, which in many cases remain unknown.
References
- S. Miyamura, T. Hori and T. Nagumo, Phycol. Res., 2003, 51, 143–146 CrossRef.
- S. Miyamura, S. Sakaushi, T. Hori and T. Nagumo, Phycological Research, 2010, 58, 258–269 CrossRef.
- A. J. Hartz, B. F. Sherr and E. B. Sherr, Journal of Eukaryotic Microbiology, 2011, 58, 171–177 CrossRef.
- J. Dodge, in Evolution of the Eye and Visual System, ed. J. R. Cronly-Dillon and R. L. Gregory, Macmillan Press, London, 1991 Search PubMed.
- P. Gualtieri, Micron, 2001, 32, 411–426 CrossRef CAS.
- H. Robenek and M. Melkonian, J. Cell Sci., 1981, 50, 149–164 CAS.
- G. Kreimer, Curr. Genet., 2009, 55, 19–43 CrossRef CAS.
- R. Birge, R. B. Barlow and J. R. Tallent, in Structures and Functions of Retinal Proteins, ed. J. L. Rigaud, Eurotext, Montrouge, 1992, pp. 283–286 Search PubMed.
- H. S. Jennings, Behavior of the Lower Organisms, Columbia University Press, New York, 1906 Search PubMed.
- K. W. Foster and R. D. Smyth, Microbiol. Rev., 1980, 44, 572–630 CAS.
- P. Gualtieri and K. R. Robinson, Photochem. Photobiol., 2002, 75, 76–78 CrossRef CAS.
- T. M. Mittelmeier, J. S. Boyd, M. R. Lamb and C. L. Dieckmann, J. Cell Biol., 2011, 193, 741–753 CrossRef CAS.
- P. Albertano, L. Barsanti, V. Passarelli and P. Gualtieri, Micron, 2000, 31, 27–34 CrossRef CAS.
- P. L. Walne, V. Passarelli, P. Lenzi, L. Barsanti and P. Gualtieri, J. Eukaryotic Microbiol., 1995, 42, 7–11 CrossRef CAS.
- G. Rosati, F. Verni, L. Barsanti, V. Passarelli and P. Gualtieri, Electron Microsc. Rev., 1991, 4, 319–342 CrossRef CAS.
- F. Verni, G. Rosati, P. Lenzi, L. Barsanti, V. Passarelli and P. Gualtieri, Micron Microsc. Acta, 1992, 23, 37–44 CrossRef.
- D. Francis, J. Exp. Biol., 1967, 47, 495–501 CAS.
- C. Greuet, Protistologica, 1977, 13, 127–143 Search PubMed.
- M. Hoppenrath, T. R. Bachvaroff, S. M. Handy, C. F. Delwiche and B. S. Leander, BMC Evol. Biol., 2009, 9, 116–130 CrossRef.
- M. Delbruck, Carlsberg Res. Commun., 1976, 41, 299–309 CrossRef.
- D. P. Hader, Arch. Microbiol., 1985, 143, 100–104 CrossRef.
- W. Nultsch and A. Mecke, Arch. Microbiol., 1988, 150, 343–347 CrossRef CAS.
- D. Oesterhelt and W. Stoeckenius, Nature New Biol., 1971, 233, 149–152 CAS.
- J. Soppa, A. Baumann, M. Brenneis, M. Dambeck, O. Hering and C. Lange, Arch. Microbiol., 2008, 190, 197–215 CrossRef CAS.
- K.-H. Jung, Photochem. Photobiol., 2007, 83, 63–69 CrossRef CAS.
- J. L. Spudich, C.-S. Yang, K.-H. Jung and E. N. Spudich, Annu. Rev. Cell Dev. Biol., 2000, 16, 365–392 CrossRef CAS.
- A. K. Sharma, J. L. Spudich and W. F. Doolittle, Trends Microbiol., 2006, 14, 463–506 CrossRef CAS.
- J. L. Spudich, Trends Microbiol., 2006, 14, 480–487 CrossRef CAS.
- L. Barsanti, V. Passarelli, P. L. Walne and P. Gualtieri, FEBS Lett., 2000, 482, 247–251 CrossRef CAS.
- L. Barsanti, P. Coltelli, V. Evangelista, V. Passarelli, A. M. Frassanito, N. Vesentini and P. Gualtieri, Biochem. Biophys. Res. Commun., 2008, 375, 471–476 CrossRef CAS.
- R. Mercatelli, F. Quercioli, L. Barsanti, V. Evangelista, P. Coltelli, V. Passarelli, A. M. Frassanito and P. Gualtieri, Biochem. Biophys. Res. Commun., 2009, 385, 176–180 CrossRef CAS.
- K. R. Robinson, R. Lorenzi, N. Ceccarelli and P. Gualtieri, Biochem. Biophys. Res. Commun., 1990, 243, 776–778 CrossRef.
- K.-H. Jung, V. D. Trivedi and J. L. Spudich, Mol. Microbiol., 2003, 47, 1513–1522 CrossRef CAS.
- O. A. Sineshchekov, K.-H. Jung and J. L. Spudich, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 8689–8694 CAS.
- O. K. Okamoto and J. W. Hastings, J. Phycol., 2003, 39, 519–526 CrossRef CAS.
- O. A. Sineshchekov, E. G. Govorunova, K.-H. Jung, S. Zauner, U.-G. Maier and J. L. Spudich, Biophys. J., 2005, 89, 4310–4319 CrossRef CAS.
- S. P. Tsunoda, D. Ewers, S. Gazzarrini, A. Moroni, D. Gradmann and P. Hegemann, Biophys. J., 2006, 91, 1471–1479 CrossRef CAS.
- J. P. Klare, I. Chizhov and M. Engelhard, in Results and Problems in Cell Differentiation: Bioenergetics, ed. G. Schäfer and H. S. Penefsky, Springer-Verlag, Berlin, Heidelberg, 2008, vol. 45, pp. 73–122 Search PubMed.
- A. M. Frassanito, L. Barsanti, V. Passarelli, V. Evangelista and P. Gualtieri, Cell. Mol. Life Sci., 2010, 67, 965–971 CrossRef CAS.
- S. Lin, H. Zhang, Y. Zhuang, B. Tran and J. Gill, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 20033–20038 CrossRef CAS.
- C. H. Slamovits, N. Okamoto, L. Burri, E. R. James and P. J. Keeling, Nat. Commun., 2011, 2, 183 CrossRef.
- S. H. Yoon, Y. D. Hackett, C. Ciniglia, G. Pinto and D. Bhattacharya, Mol. Biol. Evol., 2004, 21, 809–818 CrossRef.
- P. L. Walne and P. Gualtieri, Crit. Rev. Plant Sci., 1994, 13, 185–197 CAS.
- H. B. Barlow, Nature, 1988, 334, 296–297 CrossRef CAS.
- J. Nathans, Biochemistry, 1992, 31, 4923–4931 CrossRef CAS.
- J. M. Berg, J. L. Tymoczko and L. Stryer, Biochemistry, W H Freeman, New York, 2002 Search PubMed.
- P. Galland and H. Senger, J. Photochem. Photobiol., B, 1988, 1, 277–294 CrossRef CAS.
- H. G. Dohlman, J. Thorner, M. G. Caron and R. J. Lefkowitz, Annu. Rev. Biochem., 1991, 60, 653–688 CrossRef CAS.
- M. Iseki, S. Matsunaga, A. Murakami, K. Ohno, K. Shiga, K. Yoshida, M. Sugai, T. Takahashi, T. Hori and M. Watanabe, Nature, 2002, 415, 1047–1051 CrossRef CAS.
- R. Hadley, W. E. Hable and D. L. Kropf, BMC Plant Biol., 2006, 6 Search PubMed.
- O. A. Sineshchekov, E. G. Govorunova and J. L. Spudich, Photochem. Photobiol., 2009, 85, 556–563 CrossRef CAS.
- F. J. Braun and P. Hegemann, Biophys. J., 1999, 76, 1668–1678 CrossRef CAS.
- M. E. Eakin, Evol. Biol., 1968, 2, 194–240 Search PubMed.
- M. E. Eakin, in Handbook of Sensory Physiology, Vol. VII/1, Photochemistry of Vision, ed. H. J. A. Dartnall, Springer, Heidelberg, 1972, pp. 625–684 Search PubMed.
- W. J. Gehring, Journal of Heredity, 2005, 96, 171–184 CrossRef CAS.
- D. V. Heelis, W. Kernick, G. O. Phillips and K. Davies, Arch. Microbiol., 1979, 121, 207–211 CrossRef CAS.
- P. Gualtieri, L. Barsanti and V. Passarelli, Biochim. Biophys. Acta, Gen. Subj., 1989, 993, 293–296 CrossRef CAS.
- J. J. Wolken, J. Protozool., 1977, 24, 518–522 CAS.
- E. Piccinni and M. Mammi, Bolletino di zoologia, 1978, 45, 405–414 CrossRef.
- H. Michel, in Crystallization of Membrane Proteins, ed. H. Michel, CRC Press, Boca Raton, Florida, 1990, pp. 73–88 Search PubMed.
- H. Liang, G. Whited, C. Nguyen and G. D. Stucky, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 8212–8217 CrossRef CAS.
- T. W. James, F. Crescitelli, E. R. Loew and W. N. McFarland, Vision Res., 1992, 32, 1583–1591 CrossRef CAS.
- L. Barsanti, V. Passarelli, P. L. Walne and P. Gualtieri, Biophys. J., 1997, 72, 545–553 CrossRef CAS.
- L. Barsanti, P. Coltelli, V. Evangelista, V. Passarelli, A. M. Frassanito, N. Vesentini, F. Santoro and P. Gualtieri, Photochem. Photobiol., 2009, 85, 304–312 CrossRef CAS.
- P. Gualtieri, P. Pelosi, V. Passarelli and L. Barsanti, Biochim. Biophys. Acta, Gen. Subj., 1992, 1117, 55–59 CrossRef CAS.
- M. O. Lenz, R. Huber, B. Schmidt, P. Gilch, R. Kalmbach, M. Engelhard and J. Wachtveit, Biophys. J., 2006, 91, 255–262 CrossRef CAS.
- L. J. Goldman, S. N. Barnes and T. H. Goldsmith, J. Gen. Physiol., 1975, 66, 383–404 CrossRef CAS.
- A. Schmitt, A. Vogt, K. Friedmann, R. Paulsen and A. Huber, J. Exp. Biol., 2005, 208, 1247–1256 CrossRef CAS.
- G. Tomasello, G. Olaso-Gonzalez, P. Altoè, M. Stenta, L. Serrano-Andrés, M. Merchàn, G. Orlandi, A. Bottoni and M. Garavelli, J. Am. Chem. Soc., 2009, 131, 5172–5186 CrossRef CAS.
- R. Gonzales-Luque, M. Garavelli, F. Bernardi, M. Merchan, M. A. Robb and M. Olivucci, Proc. Natl. Acad. Sci. U. S. A., 2000, 97, 9379–9384 CrossRef.
- L. M. Frutos, T. Andruniów, F. Santoro, N. Ferré and M. Olivucci, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 7764–7769 CrossRef CAS.
- K. W. Yau and D. A. Baylor, Annu. Rev. Neurosci., 1989, 12, 289–327 CrossRef CAS.
- S. Yarfitz and J. B. Hurley, J. Biol. Chem., 1994, 269, 14329–14332 CAS.
- P. Hegemann, Annu. Rev. Plant Biol., 2008, 59, 167–189 CrossRef CAS.
- A. Kishigami, T. Ogasawara, Y. Watanabe, M. Hirata, T. Maeda, F. Hayashi and Y. Tsukahara, J. Exp. Biol., 2001, 204, 487–493 CAS.
- J. S. Hyams, J. Cell Sci., 1982, 55, 199–210 CAS.
- O. Moestrup, Phycologia, 1982, 21, 427–528 CrossRef.
- G. B. Bouck, T. K. Rosiere and P. J. Lewlsscur, in Ciliary and Flagellar Membranes, ed. R. A. Bloodgood, Plenum Press, New York, 1990, pp. 65–90 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
