Environmentally benign synthesis of heterocyclic compounds by combined microwave-assisted heterogeneous catalytic approaches†
Arif
Daştan
ab,
Aditya
Kulkarni
a and
Béla
Török
*a
aUniversity of Massachusetts Boston, 100 Morrissey Blvd, Boston, MA, USA. E-mail: bela.torok@umb.edu; Fax: (+1)-617-287-6030; Tel: (+1)-617-287-6159
bAtaturk University, Science Faculty, Department of Chemistry, 25240, Erzurum, Turkey
First published on 4th November 2011
Abstract
Recent advances in the application of heterogeneous catalysis combined with microwave irradiation in the synthesis of heterocyclic compounds are reviewed. While a detailed summary of the different catalysts applied in the synthesis of heterocycles is provided, the work mainly focuses on the heterocyclic compounds and their synthesis and not the preparation or characterization of the catalyst. Due to the large number of N-containing heterocycles, the synthesis of these compounds dominates this account, however, the preparation of other heteroatom containing compounds is also covered in detail. The literature data are summarized based on the size of cycles and the number of heteroatoms in the compounds. Since the major goal of the work is to highlight the environmentally benign and sustainable nature of the combined microwave-assisted heterogeneous catalytic methods, the green aspects of the individual synthetic approaches will be emphasized.
1. Introduction
Heterocyclic compounds have always been on the forefront of attention due to their numerous uses in pharmaceutical applications.1 Heterocyclic moieties also serve as an integral part of a broad variety of biologically active natural products and synthetic compounds.2 The overwhelming majority of commercially available synthetic drugs (up to 80%) have a heterocyclic structural component.3 Due to the widespread interest in heterocycles, the synthesis of these compounds has always been among the most important research areas in synthetic chemistry, resulting in the development of several classic named reactions.4 In many cases, the classic syntheses provide reliable access to heterocyclic compounds, however, they are simply no longer acceptable by current environmental and safety standards. Contemporary developments in discovery and process chemistry emphasize new sustainable synthetic routes, requiring rapid and environmentally acceptable alternatives to the classic methods.5 The development of sustainable synthetic procedures to replace the efficient but somewhat outdated classic methods began several decades ago and such methods are still in high demand.Taking into account the basic principles of green synthesis and the production of chemicals outlined by Anastas and Warner,6 various new ecofriendly approaches have been designed. Approaches to address such challenges include the application of non-traditional activation methods, such as microwave irradiation,7 sonochemistry,8 the use of environmentally benign solvents9 and catalysis10 as major tools in green synthesis and engineering.
Due to the focus of this work, only methods that apply heterogeneous catalysis and microwave activation in a combined manner will be discussed. Both methods were broadly applied in the synthesis of heterocycles. Due to the central role of these compounds in a wide range of applications, several recent reviews summarize the latest developments in microwave-assisted11 as well as heterogeneous catalytic12 synthesis of heterocycles. The combined applications of these methods were pioneered by Varma in the early 1990s13 and later expanded upon by others.14 The unique combination of solid catalysts with microwave activation resulted in several beneficial features. (i) First, most solid catalysts absorb microwave irradiation, thus they can serve as an internal heat source for the reactions. As all heterogeneous catalytic reactions occur on the surface of catalysts, it is the most direct and selective heating that one can provide. (ii) The catalyst itself can serve as a heat source and as a medium for reactions, eliminating the need to apply a solvent for these reactions. In fact, the use of solvents has an adverse effect on such reactions; the solvent keeps the reactants in the solution phase, making mass transfer an important, often limiting factor. When the compounds are adsorbed on the surface of the catalysts such limitations do not exist, therefore the reactions are significantly more rapid than in solvents (dry or solvent-free reactions). Such terminology, however, is commonly a target for criticism, as the starting materials can be placed on the surface of a catalyst by simple mortar mixing but the products can only be isolated by the use of a solvent. The usual method is to add a small amount of solvent to dissolve the products and then remove the catalyst by filtration. Therefore, only the reaction and not the entire process is solvent-free. Since the use of most organic solvents, especially at high temperatures, represents a fire-hazard, eliminating the solvent during the reaction step when heating occurs is unambiguously beneficial from a green chemistry as well as a safety point of view. (iii) Based on a broad array of literature data heterogeneous catalytic microwave-assited reactions occur much more rapidly than their conventionally heated counterparts, thus the time factor is another advantage.
Whether the application of microwave irradiation in synthesis in itself, makes a process green is the target of an ongoing debate. Authors often claim that the application of microwave heating makes a process green. This is a commonly believed but rarely proven or even studied “axiom” which came under scrutiny in a recent work by Moseley and Kappe.15 In their critical assessment, the authors pointed out that determining the energy efficiency of microwave-assisted reactions is a complex task and should be evaluated on an individual basis. The authors concluded that microwave heating should not be automatically labeled as green based purely on energy efficiency considerations. While we share this view one cannot fail to notice that all examples, which the authors analyzed were in the solution phase, i.e. homogeneous reactions. Unfortunately, the scope of this work does not allow a detailed discussion of this topic; however, the significant decrease in reaction time, the clearly smaller overall mass heated (no solvent) and the direct energy transformation by the solid catalyst all suggest that the unique combination of heterogeneous catalysis and microwave irradiation would also be more energy efficient than its convectively heated counterpart. A detailed comparative study on this topic is in progress.
Here, we briefly discuss the most common solid catalysts used in microwave-assisted reactions.
1.1. Solid acid–base catalysts
Solid acids are becoming common catalysts for organic synthesis. These materials include a number of Brønsted and Lewis acids and often possess both Brønsted and Lewis acid centers. Solid Brønsted acids include (i) acidic (or cation) ion exchange resins (e.g. Nafion-H, Amberlyst, Dovex, Deloxane etc.), and (ii) heteropoly acids and their derivatives (e.g. H0.5Cs2.5[PW12O40]). Solid Lewis acids are usually based on (i) metal chlorides (e.g. ZnCl2, AlCl3etc.), or (ii) lanthanide triflates and (iii) sulfated metal oxides (e.g. sulfated zirconia). Perhaps the most common group includes catalysts which possess mixed acidity: (i) clays (e.g. K-10 montmorillonite, KSF-montmorillonite and several modified derivatives, such as ClayFen, ClayCop etc.), (ii) zeolites (e.g. H-Y, H-ZSM and other modified and synthetic zeolites) and (iii) metal oxides (e.g. alumina, mixed oxides etc.). Due to the focus of the present work, catalytic aspects such as catalyst preparation and characterization will not be discussed. There are several excellent accounts on this topic where further details can be obtained.16Solid base catalysts are also common, although much less frequently used than solid acids. The most common examples are magnesium oxide, alumina, polyvinyl-pyridine or basic, ion-exchanged clays, hydrotalcites or zeolites.17
1.2. Heterogeneous metal catalysts
Heterogeneous metal catalysts are commercially available in numerous forms. Although the metals used are varied by the target reaction, the majority of these catalysts are prepared for hydrogenation reactions. Platinum group metals and nickel are the most commonly used metals; however, inexpensive metals such as copper are also frequently applied, especially in large scale applications.One particular problem that earlier inhibited the use of solid metal-containing catalysts in microwave reactions is the arcing phenomenon that can create hazardous conditions if flammable solvents are used.18 Arcing, however, is characteristically linked to large metal particles. Most commonly available supported metal catalysts, however, possess metal particles of nanometer size, ensuring that reactions can be safely carried out, even in organic solvents.19
The major groups of metal catalysts include unsupported metals, supported metal catalysts, heterogenized metal complexes and metal nanoparticle-based supramolecular complexes. Unsupported metal catalysts include (i) metal oxide based catalysts, e.g. Adams' platinum (PtO2), (ii) the so-called metal blacks, which are bulk metals,20 and (iii) the group of skeletal metals, which include the RANEY®-type catalysts that are prepared from metal-Al alloys (e.g. Ni–Al alloy).21 Most of these catalysts, including the pyrophoric RANEY®-Ni, are commercially available and routinely used by synthetic chemists. (iv) Amorphous metal alloys are used in the form of simple powders (e.g. NiB or NiP) or metallic glasses. These catalyst precursors usually show low activity and are not commonly used in synthetic applications.22Supported metal catalysts are most frequently used in synthetic chemistry due to their efficacy and economic nature.23 Using a proper support of indifferent or reactive nature, the metal component is deposited on the surface of a support, usually an inorganic solid. They are chemically inert (e.g. charcoal, C or polystyrene) as well as somewhat reactive (SiO2, Al2O3, zeolites etc.) supports.24Heterogenized metal complexes are the very popular metal complexes covalently anchored to solid supports. They mimic the features of soluble complexes, but are not commonly available commercially, and therefore rarely used in synthetic applications.25 The last major group is the recently introduced nanoparticle-based metal catalysts that are based on metal nanoparticles stabilized by certain supramolecular assemblies, such as cyclodextrins or soluble organic polymers.26
2. Heterocycles with nitrogen as the only heteroatom
2.1. 5-Membered rings containing one nitrogen atom
5-Membered N-heterocycles such as pyrroles, indoles and carbazoles are important structural motifs in an extensive number of biologically active compounds.27 They are of exceptional interest in the pharmaceutical industry, as they appear in the core structure of several drugs.A novel three-component reaction of phenacyl and vinyl bromides, pyridine and acetylenes for the synthesis of indolizines was developed (Scheme 1).28 The reaction was catalyzed by basic alumina. It was proposed that an N-alkylpyridinium salt was generated in situ from the condensation of the bromo compound and pyridine, and then converted to a 1,3-dipole species under basic conditions. This dipolar intermediate reacts with the acetylene derivative by a dipolar cycloaddition reaction under microwave-assisted solvent-free conditions to form indolizines in excellent yields (87–94%). For comparison, the reactions were carried out under microwave-assisted conditions in dry toluene. It was observed in the case of all substrates that the solvent-free reactions consistently performed better than those carried out in toluene which gave yields ranging from 60–71%.
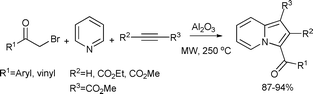 | ||
| Scheme 1 | ||
A Paal–Knorr type method was described for the synthesis of N-substituted pyrroles via the condensation of primary amines and sulfonamides with 2,5-dimethoxytetrahydrofuran (Scheme 2).29 The material used to catalyze the reaction was an environmentally benign, microwave-active solid-acid (K-10 montmorillonite). Various N-substituted pyrroles were synthesized under solvent-free reaction conditions in very good yields in 3–6 min.
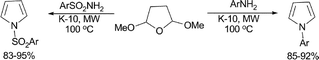 | ||
| Scheme 2 | ||
Hexan-2,5-dione was used as an alkylating agent in a solvent-free solid-acid catalyzed electrophilic annelation reactions for the synthesis of pyrroles, indoles and carbazoles from primary amines, pyrroles and indoles respectively (Scheme 3).30 It was proposed that the transformations occurred by a Paal–Knorr type reaction and the annelation took place by a double alkylation–dehydration sequence. A wide variety of substituted primary amines, pyrroles and indoles were reacted with 2,5-hexandione in solvent-free reactions to obtain the corresponding pyrroles, indoles and carbazoles in excellent yields.
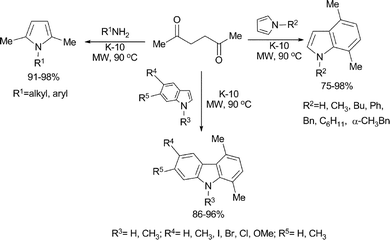 | ||
| Scheme 3 | ||
In a follow up study, Kulkarni et al. investigated the use of alcohols as alkylating agents for the alkylation of indoles using K-10 (Scheme 4).31 A tertiary alcohol, t-butyl alcohol, was used as an alkylating agent to obtain C-3 alkylated products selectively. The study was also extended to investigate the use of hexan-2,5-diol, which underwent C-3 selective Friedel–Crafts alkylation followed by a secondary intramolecular Friedel–Crafts alkylation forming a tetrahydrocarbazole intermediate. Under the experimental conditions, the tetrahydrocarbazole intermediate underwent a K-10-catalyzed oxidative aromatization forming 1,4-dimethylcarbazole.
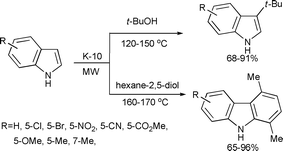 | ||
| Scheme 4 | ||
Varma et al. have synthesized a novel glutathione organocatalyst supported on ferromagnetic nanoparticles.32 The magnetic nanoparticles offer a large surface area to anchor the glutathione organocatalyst. An added advantage of these nanoparticles is that they can be magnetically separated from the reaction mixture with minimal effort and be recycled in subsequent reactions. The novel anchored organocatalyst was synthesized by sonicating Fe2O3 nanoparticles with glutathione in water at room temperature (Scheme 5).
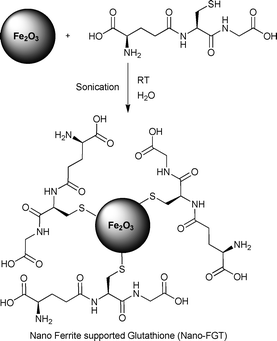 | ||
| Scheme 5 | ||
This newly synthesized material was tested as a nano-organocatalyst in the Paal–Knorr reaction of various substituted primary amines with 2,5-dimethoxytetrahydrofuran (Scheme 6).33 The corresponding pyrroles were obtained in good yields under microwave-assisted, aqueous conditions.
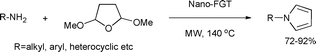 | ||
| Scheme 6 | ||
The same catalyst was also employed in the synthesis of pyrazoles by a double condensation reaction of phenylhydrazine with substituted 2,4-pentanediones under microwave-assisted aqueous conditions (Scheme 7).33
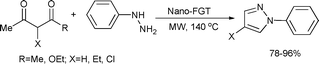 | ||
| Scheme 7 | ||
In both cases (Schemes 6 and 7), the catalyst was magnetically separated from the reaction mixtures and reused up to three times without any loss of catalytic activity.
1,2,3,4-Tetrasubstituted pyrroles were synthesized by a SiO2-catalyzed double domino reaction of conjugated alkynoates and primary amines34 (Scheme 8). Various polysubstituted pyrroles were synthesized in a one-pot fashion. While the reaction takes months to proceed at room temperature and hours under conventional heating conditions, it occurs in 8 min under microwave-assisted conditions. The overall reaction is a solvent-free domino sequence involving the formation of an 1,3-oxazolidine intermediate and its subsequent intramolecular rearrangement to form tetrasubstituted pyrroles. The reported protocol is ideal for diversity-oriented synthesis since a large variety of substituted products can be obtained with the pyrrole backbone under environmentally benign conditions in short reaction times.
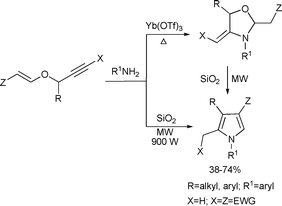 | ||
| Scheme 8 | ||
Lipińska described a microwave-induced heterogeneous catalytic Fischer indolization as the key step in the preparation of 9-methoxyindolo[2,3-a]quinolizine from 2-acetylpyridines (Scheme 9).35 In the key step, the hydrazone of 2-acetylpyridine undergoes the Fischer indole reaction to form 2-pyridoindole. The catalyst of choice was K-10 montmorillonite modified with ZnCl2 (K-10/ZnCl2). The new catalyst is significantly greener than the previously reported catalysts for similar transformations.36 Moderate yields were obtained after a reaction time of 2.5 min under microwave-assisted conditions.
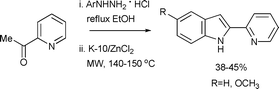 | ||
| Scheme 9 | ||
In an extension of this study, Lipińska and Czarnocki described an improved method for the Fischer indolization of phenyl- and methoxyphenyl-hydrazones of various substituted 2-acetylpyridines as shown in Scheme 10.37 This study compared different catalytic conditions viz. K-10/ZnCl2, K-10/ZnCl2 with triethylene glycol and ZnCl2 in triethylene glycol. It was observed that the homogeneous protocol using ZnCl2 in triethylene glycol was more efficient (up to 52% yield) than the heterogeneous counterpart using K-10 (yields up to 34%)(Scheme 10).
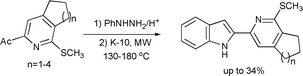 | ||
| Scheme 10 | ||
The Michael addition of electron deficient olefins to pyrroles under solvent-free conditions was reported (Scheme 11).38 The reaction was carried out in presence of silica gel. In the case of hindered olefins, a catalytic amount of BiCl3 (5 mol%) was added to the reaction mixture. The Michael adducts were obtained in 1–4 min in yields up to 95%.
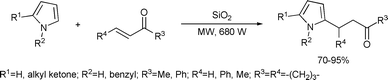 | ||
| Scheme 11 | ||
A K-10-catalyzed ring-closing Friedel–Crafts reaction was used in one of the key steps in the synthesis of (−)-fischerindole G by Baran and Richter (Scheme 12).39
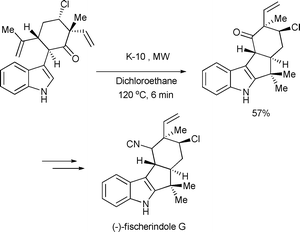 | ||
| Scheme 12 | ||
Laronze-Cochard et al. investigated the synthesis of 2,4-diaryl substituted carbazoles from tetrahydrocarbazole.40 Microwave irradiation of tetrahydrocarbazoles adsorbed on silica gel afforded the corresponding carboxylic acids as a mixture of diastereomers in 71–86% yield. A higher irradiation temperature (200 °C) and longer reaction time permitted a direct transformation of tetrahydrocarbazoles to aromatic carbazoles in moderate yield (41%) by a solvent-free decarboxylation–aromatization domino reaction (Scheme 13).
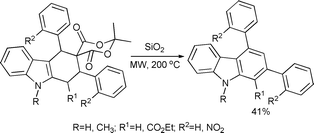 | ||
| Scheme 13 | ||
2.2. 5-Membered rings containing two nitrogen atoms
Substituted pyrazoles and imidazoles constitute the building blocks of a large number of pharmaceutical and agrochemical products. Pyrazoles form the core of several drugs such as Zometapine41 or Sildenafil.42 Compounds containing imidazoles exhibit interesting biological properties such as anti-inflammatory, anti-fungal and analgesic agents.431,3,5-Trisubstituted pyrazoles were synthesized from chalcones and hydrazines catalyzed by a Pd/C/K-10 bifunctional catalyst (Scheme 14).44 The catalyst was a mechanical mixture of commercially available Pd/C and K-10 montmorillonite. The reaction involved a condensation of chalcones with hydrazines and cyclization. These two steps were catalyzed by K-10. Pd/C ensured high rates in the last aromatization step to form the corresponding 1,3,5-trisubstituted pyrazoles in good to excellent yields (80–98%) as represented in Scheme 14.
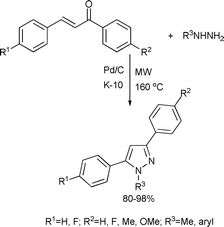 | ||
| Scheme 14 | ||
A four component reaction of benzil, ammonium acetate, benzaldehydes and primary amines was applied to synthesize 1,2,4,5-tetrasubstituted imidazoles. The microwave-assisted reaction was catalyzed by HY zeolite or silica gel (Scheme 15(a)).45 This solvent-free process produced the target compounds in 6 min in yields higher than those achieved by conventional heating.
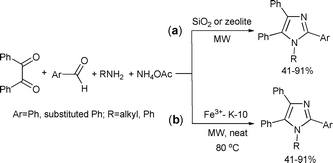 | ||
| Scheme 15 | ||
The four-component cyclocondensation to tetrasubstituted imidazoles can also be carried out using a Fe3+/K-10 catalyst as reported by Raghuvanshi and Singh (Scheme 15(b)).46 The catalyst used in this study is K-10 montmorillonite impregnated with FeCl3. The products were obtained in good yields in 3–4 min of microwave irradiation under solvent-free conditions.
The synthesis of imidazo[1,2-a] annulated pyridines, pyrazines and pyrimidines was reported by a three component microwave-assisted K-10-catalyzed condensation approach (Scheme 16).47 This solvent-free reaction provided the target compounds via the reactions of aldehydes, isocyanides and aromatic amines in a matter of minutes. The products were obtained in yields up to 88%.
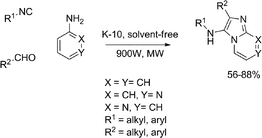 | ||
| Scheme 16 | ||
2.3. 5-Membered rings containing three nitrogen atoms
The azide–alkyne Huisgen [3 + 2]-cycloaddition reaction, better known as the “click” reaction, is a highly efficient reaction for the synthesis of triazoles.48 The reactions can be catalyzed by Cu(I)- or Cu(II)-species and carried out at ambient or slightly elevated temperatures, but it can take several hours to achieve completion. Although limited in number, there are reports of such cycloaddition reactions catalysed by heterogeneous catalysts.Lipschutz et al. reported the use of a charcoal supported copper (Cu/C) as a robust heterogeneous and highly regioselective catalyst for the synthesis of 1,2,3-triazoles (Scheme 17(a)).49 It was observed that under traditional heating conditions the reaction required one equivalent of base such as triethylamine to ensure high reaction rates. Under microwave conditions, however, the reaction could be carried out quantitatively at 150 °C in 3 min without the use of any external ligand or base.
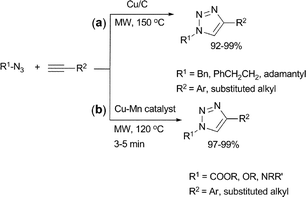 | ||
| Scheme 17 | ||
The Huisgen [3 + 2]-cycloaddition has also been applied under microwave-assisted conditions using a ligand/additive free Cu–Mn bimetallic heterogeneous catalyst (Scheme 17(b)).50 The optimum ratio of Cu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn was found to be 2
Mn was found to be 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25. The click reaction afforded the corresponding 1,4-disubstituted 1,2,3-triazoles from a wide range of substrates in quantitative yields under microwave-assisted conditions. The catalyst was easily removed by filtration after the reaction and reused up to nine times without significant loss of its activity.
0.25. The click reaction afforded the corresponding 1,4-disubstituted 1,2,3-triazoles from a wide range of substrates in quantitative yields under microwave-assisted conditions. The catalyst was easily removed by filtration after the reaction and reused up to nine times without significant loss of its activity.
In another example, Taran et al. have reported the use of Cu(I)-species anchored to functionalized chitosan microspheres (Scheme 18).51 Only a 0.1 mol% catalyst loading was required to obtain 1,4-substitued triazoles in quantitative yields at 150 °C in 15 min. All heterogeneous copper catalysts discussed in this section contain copper in the form of Cu(I).
 | ||
| Scheme 18 | ||
2.4. 6-Membered rings containing one nitrogen atom
Substituted pyridines exhibit a broad range of biological activity. They are used to modulate hypertension, angina pectoris, act as Ca2+-channel blockers and are anti-diabetic, heptaprotective and show anti-tumor properties.52 The fused quinoline moiety is also present in an extensive number of naturally occurring and biologically active compounds and also shows interesting photochemical properties.53 In addition, pyridine derivatives are also used as organic bases and organocatalysts in organic synthesis.A multicomponent reaction of aromatic aldehydes, malononitrile and thiophenols was applied for the one-step synthesis of highly functionalized pyridines (Scheme 19).54 The reaction was catalyzed by KF impregnated alumina. The yields were good in the case of the benzaldehydes, as they have both electron-donating and electron-withdrawing substituents. The authors, however, observed that aliphatic or heterocyclic aldehydes did not undergo the reaction with satisfactory yields. For comparison, the reactions were also carried out under conventionally heated reflux conditions in ethanol using an oil bath. The microwave-assisted reactions consistently gave better results (62–93% yields) when compared to the reactions with conventional heating under ethanol reflux conditions (56–82% yields).
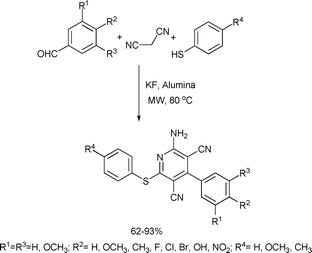 | ||
| Scheme 19 | ||
Syntheses of 2,3-disubstituted-6-aryl pyridines and 7,7-dimethyl-2-aryl-5,6,7,8-tetrahydroquinoline-5-ones were achieved efficiently in a solvent-free reaction catalyzed by a potassium dodecatungstocobaltate trihydrate catalyst (1 mol% catalyst loading) and a strong heteropoly acid (Scheme 20).55 The target compounds were synthesized by the condensation of enaminones and cyclic/acyclic 1,3-diketones in excellent yields in 3–6 min.
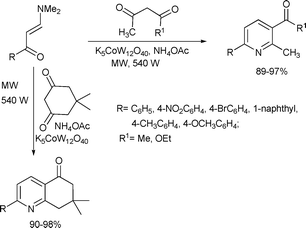 | ||
| Scheme 20 | ||
A metal-free synthesis of pentasubstituted, functionalized polyhydroquinoline derivatives was recently developed via a four component Hantzsch condensation reaction under microwave irradiation (Scheme 21(a)).56 The reaction was catalyzed by a novel heterogeneous organocatalyst, glycine, with only 10 mol% catalyst loading. The reaction gave the products in very short reaction times (1–3 min). For comparison, the authors carried out the reaction under traditional oil bath based reflux conditions (80% yield) and also at room temperature (63% yield). Again, the microwave-assisted reactions performed much better in terms of reaction times and yields than their conventional counterparts.
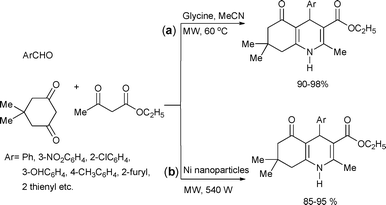 | ||
| Scheme 21 | ||
The Hantzsch condensation reaction has also been carried out with a heterogeneous Ni nanoparticle catalyst (particle size = 80 ± 0.5 nm) under solvent-free reaction conditions (Scheme 21(b)).57 The corresponding polyhydroquinolines were obtained in excellent yields in reaction times of 1–1.5 min. The authors also studied the reuse of the catalyst by recycling it in five subsequent reactions. The Ni nanoparticles showed no signs of deactivation after five runs.
In a unified version of the Hantzsch condensation reaction, the subsequent aromatization and the use of the Pd/C/K-10 catalyst (vide supra) was examined in a condensation–aromatization domino sequence in a one-pot synthesis of substituted pyridines using ethyl acetoacetate, ammonium formate and aliphatic/aromatic aldehydes (Scheme 22).58 The cyclization readily took place on the surface of the K-10 catalyst, whereas Pd/C dehydrogenated the dihydropyridine intermediates to the corresponding aromatic products.
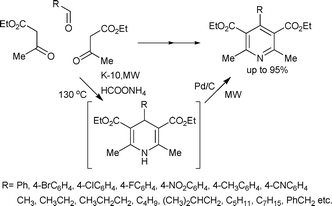 | ||
| Scheme 22 | ||
As an extension to the one-step synthesis of pyridines, the bifunctional Pd/C/K-10 catalyst was also applied in a one-step synthesis of β-carbolines starting with tryptamines and carbonyl compounds such as benzaldehyhdes and aromatic glyoxals,59 respectively (Scheme 23). The final products were formed by a one-pot, three-step domino sequence. In the first step, the aldehyde/glyoxal condensed with the primary amino group of the tryptamine to form an imine. In the second step, the imine underwent a K-10-catalyzed Pictet–Spengler cyclization reaction. In the third and final step, the tetrahydro-β-carboline intermediate underwent a Pd/C-catalyzed dehydrogenation to form β-carbolines in good to excellent yields.
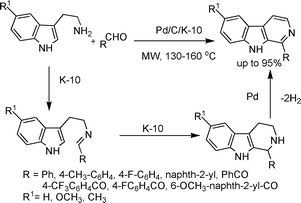 | ||
| Scheme 23 | ||
An efficient route to 2,4-diarylquinolines has been developed by the same group (Scheme 24).60 Various 2,4-disubstituted quinolines were synthesized by a three component reaction of anilines, benzaldehydes and terminal phenylacetylenes in the presence of K-10 montmorillonite under solvent-free conditions. Practically any combination of the three components could be used to synthesize the corresponding 2,4-diarylquinolines. The catalyst could be recycled; the catalytic activity was unaltered even after five cycles.
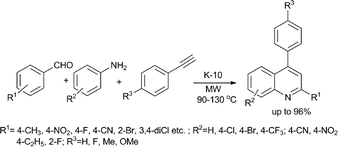 | ||
| Scheme 24 | ||
Recently, the synthesis of 2,4-disubstituted quinolines was achieved by cyclocondensation of 2-aminoarylketones and terminal phenylacetylenes in presence of a K5CoW12O40·3H2O catalyst in a solvent-free reaction (Scheme 25).61 All products were obtained in high yields (75–96%) after 10–20 min of microwave irradiation at 110–120 °C.
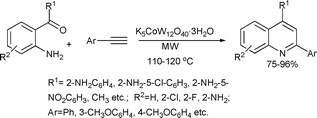 | ||
| Scheme 25 | ||
A modified Doebner–Miller synthesis of quinolines from substituted cinnamaldehydes and anilines was also recently reported.62 In the one-pot cyclization–aromatization domino approach, K-10 ensured the condensation and cyclization of anilines with cinnamaldehydes and also promoted the aromatization to the final product (Scheme 26). The products were obtained in yields ranging from 40–95% in 4–8 min.
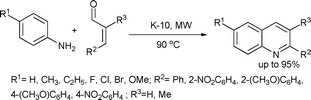 | ||
| Scheme 26 | ||
A highly diastereoselective three component aza–Diels–Alder reaction of benzaldehydes, anilines and cyclohexenone was recently described for the synthesis of azabicyclo[2.2.2]octan-5-ones (Scheme 27).63 The reaction was catalyzed by H4[SiW12O40], a strong heteropoly acid. The corresponding bicyclic products were obtained in moderate yields in excellent diastereoselectivities (up to >99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
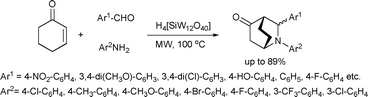 | ||
| Scheme 27 | ||
In a convenient synthesis of symmetrical and unsymmetrical bipyridines by cross coupling reactions Moore et al. illustrated the use of heterogeneous Ni and Pd catalysts under microwave-assisted conditions (Scheme 28).64 In the case of Ni catalysis, the most practical conditions involved Ni/Al2O3–SiO2 (50 mol% loading) with no phosphine ligand. Yields were moderate under Pd-catalysis; however, only 5 mol% catalyst loading was required.
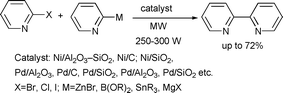 | ||
| Scheme 28 | ||
2.5 6-Membered rings containing two nitrogen atoms
6-Membered aromatic rings containing two nitrogen atoms, such as phthalazinones, quinazolinones, pyrimidines and pyrimidinones, possess a broad spectrum of biological activities and are therefore of interest as target compounds in pharmaceutical and medicinal chemistry.65Phthalaldehydic acid and hydrazines can be coupled to synthesize phthalazinones.66 A simple example of this strategy (Scheme 29), consists of a K-10 montmorillonite-catalyzed solvent-free reaction of phthalaldehydic acid and opianic acid with aliphatic or aromatic hydrazines. The corresponding phthalazinones were obtained in good yields after microwave irradiation for 1–35 min.
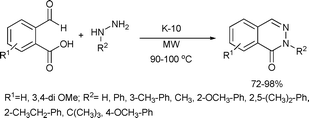 | ||
| Scheme 29 | ||
A one-pot synthesis of 2,3-disubstituted 4-(3H)-quinazolinones was developed by a multicomponent coupling of isatoic anhydride/anthranilic acid, ortho-esters and amines in the presence of Nafion-H as a heterogeneous catalyst (Scheme 30).67 The products were obtained in 2–6 min under solvent-free conditions in good yields. The reaction can tolerate a wide substitution pattern on the ortho-ester and the aniline components.
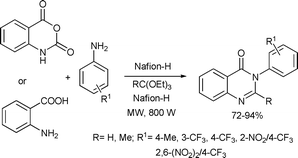 | ||
| Scheme 30 | ||
As shown in Scheme 31, a green approach was developed for the synthesis of morpholinopyrimidines, starting with benzaldehyde, 1-(4-morpholinophenyl)ethanone and guanidine hydrochloride in the presence of catalytic amount of a heterogeneous NaHSO4·SiO2 catalyst.68 In the first step of the mechanism, (E)-1-(4-morpholinophenyl)-3-arylprop-2-en-1-one was formed by the condensation of the three components, which was followed by its rearrangement to morpholinophenyl pyrimidines.
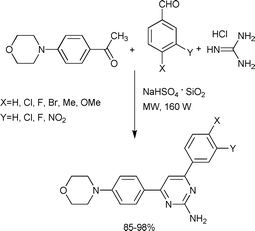 | ||
| Scheme 31 | ||
Sulfated zirconia (ZrO2/SO42−) was used as a solid, reusable acid for catalyzing the three-component cyclocondensation reaction of β-dicarbonyl compounds with urea/thiourea and substituted aromatic aldehydes to synthesize 3,4-dihydropyrimidin-2(1H)-ones and -thiones (Scheme 32(a)).69 The products were obtained in moderate to good yields in a matter of seconds. The catalyst was recycled up to 8 times. Although the catalyst was still active during the 8th run, its activity dropped to almost 50% of the original reactivity.
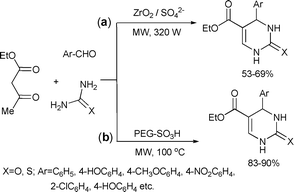 | ||
| Scheme 32 | ||
In a Biginelli reaction, a series of 3,4-dihydropyrimidones were synthesized using poly(ethylene glycol)-bound sulfonic acid (PEG–SO3H) as a catalyst under microwave heating (Scheme 32 (b)).70 The functionalized poly(ethylene glycol) simultaneously acted as a catalyst and as a solvent in the condensation. The products were obtained after a reaction time of 6 min in good yields and high selectivities.
2.6 6-Membered rings containing three nitrogen atoms
1,3,5-Triazines are used as templates in supramolecular chemistry and dendrimer synthesis, due to their C3 symmetric core structure.71Synthesis of symmetrically substituted 1,3,5-triazines was performed by cyclotrimerization of nitriles under solvent-free conditions using silica-supported Lewis acids as catalysts (Scheme 33).72 The catalysts used in these reactions were Lewis acids, such as ZnCl2, AlCl3 and TiCl4, supported on silica gel. Piperidine or morpholine were used as promoters for the cyclotrimerization reactions. Although the microwave-assisted reactions gave good results in short times (1 h), the reactions under conventional conditions over a period of 24 h gave higher isolated yields (up to 84%).
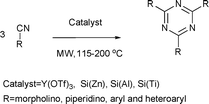 | ||
| Scheme 33 | ||
The preparation of substituted quinazolines was achieved by a microwave-assisted ZnCl2-catalyzed annulation of oxime ethers with aldehydes (Scheme 34). High yields of quinazolines were obtained with aliphatic aldehydes and aromatic aldehydes bearing electron-withdrawing substituents in the 4-position. The yields were lower using aryl aldehydes with electron-donating substituents. Applying ketones as carbonyl compounds dihydroquinazolines were obtained in poor yields.73
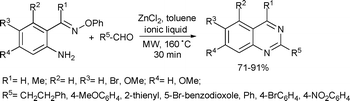 | ||
| Scheme 34 | ||
2.7 7- And higher membered nitrogen containing rings
7-Membered nitrogen containing compounds, e.g. benzodiazepines, show interesting anticancer properties and inhibit HIV-1 reverse transcriptase.741,5-Benzodiazepines can be efficiently synthesized from the condensation reaction of o-phenylenediamines with ketones. This reaction was extensively explored under microwave-assisted heterogeneous catalytic conditions using various acid catalysts. Scheme 35 summarizes the recent developments in this area.
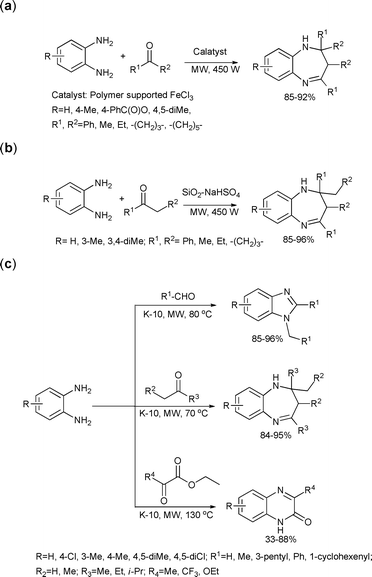 | ||
| Scheme 35 | ||
Chari and Syamasundar used polyvinylpyridine (PVP) supported ferric chloride as a catalyst (Scheme 35(a)).75 Various ketones reacted smoothly with o-phenylenediamines under these reaction conditions to give the corresponding 1,5-benzodiazepine derivatives in excellent yields. Scheme 35(b) shows the synthesis of 1,5-benzodiazepines catalyzed by silica gel supported sodium hydrogen sulfate under solvent-free conditions.76 The products were obtained in excellent yields after microwave irradiation for 0.5–1 min.
Benzodiazepines were also synthesized using the K-10 montmorillonite catalyst in the absence of solvents as shown in Scheme 35(c).77 In addition, the synthesis of benzimidazoles and quinoxalinones was also reported in the same study. The authors explored the condensation reactions of o-phenylenediamines with ketones, aldehydes and α-ketoesters under K-10-catalyzed microwave-assisted solvent-free conditions. In most cases the products were obtained in high yields in good selectivities and short reaction times (1–6 min for benzimidazoles and benzodiazepines, and 3–55 min for quinoxalinones).
Diversity-oriented synthesis of dibenzoazocines and dibenzoazepines was investigated under microwave-assisted conditions by Bariwal et al. (Scheme 36).78 A variety of 6,7-dihydro-5H-dibenzo[c,e]azepines and 5,6,7,8-tetrahydro-dibenzo[c,e]azocines were synthesized by a microwave-assisted copper-catalyzed intramolecular-coupling reaction. Although the reaction occurred more efficiently when a homogeneous CuBr catalyst was used (up to 95% yield), it can also be catalyzed by a heterogeneous Cu/C catalyst after irradiating the reaction mixture for 25 min at 100 °C to obtain the desired product in 82% yield. The use of a heterogeneous Cu/C catalyst enabled the authors to perform the reaction in a continuous flow process at 150 °C to obtain the products in isolated yields up to 79%.
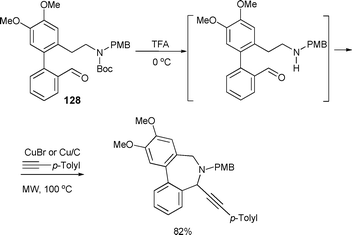 | ||
| Scheme 36 | ||
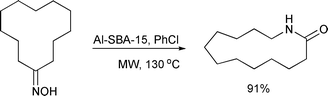
Conesa et al. investigated the Beckmann rearrangement of cyclododecanone oxime for the selective synthesis of ω-laurolactam (Scheme 37) which is a monomer used for the production of nylon-12.79 The authors studied the effect of different heterogeneous catalysts on the outcome of the Beckmann rearrangement. Various solid acids were found to be active and selective, and the desired product was formed in 5 min. The reaction gave a selectivity of 97% and conversion of 94% in the presence of an aluminated mesoporous silica (Al-SBA-15) catalyst in chlorobenzene as a solvent. Under solvent-free conditions, the optimised parameters gave a selectivity of 84% and conversion of 95% towards the desired product.
 | ||
| Scheme 37 | ||
3. Synthesis of oxygen-containing heterocycles
3.1. 3-Membered ring systems
Oxiranes or epoxides are versatile reagents in organic chemistry because these systems can be easily created, and readily react in a highly selective manner.80 This motif is also present in many biologically important compounds.81 Epoxides are easily prepared by the reaction of alkenes with peracids82 or under strongly alkaline conditions using bases such as NaOH, KOH and Na2CO3 , with H2O2 as an oxidant.83 Dimethyldioxirane (DMDO), a mild oxidation reagent, was also used for the epoxidation of alkenes in the last three decades.84 Another particular advantage of using DMDO is that the only by-product of the oxidation is acetone, a fairly innocuous and volatile compound. However, DMDO is not commercially available because of its instability and the known preparation of DMDO is rather inefficient (typical yields <3%). Varma and co-workers investigated the microwave-assisted epoxidation of cyclic and acyclic olefins over hydrotalcite catalysts in acetonitrile in the presence of hydrogen peroxide (Scheme 38).81 The protocol provided a rapid and selective process resulting in excellent yields (up to 100%) and were applicable to a wide variety of substrates. Luque et al. used a solid vanadated mesoporous silica (V-SBA-15) for the epoxidation of alkenes under both microwave and conventional heating conditions.85 Microwave experiments demonstrated that the long reaction times (12–24 h) required under conventional heating could be reduced to a few minutes (15–60 min) with improved activities and selectivities under similar reaction conditions. The same group prepared stable mesoporous organotitanium silicates and found this material to be very active and selective in the oxidation of various alkenes to epoxides under microwave irradiation using hydrogen peroxide as an oxidant.86 The authors also reported the catalyst being highly stable and reusable up to four times preserving over 90% of its initial activity.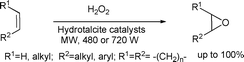 | ||
| Scheme 38 | ||
Luque et al. investigated the selective oxidation of cyclohexene under microwave conditions using an SBA15 immobilized Co–salen-complex as a catalyst (Scheme 39).87 It was reported that depending on the reaction conditions, the epoxide (65% conversion, 75% selectivity), the cyclohex-2-en-1-ol (70% conversion, 80% selectivity) or the cyclohex-2-en-1-one (>99% conversion, 89% selectivity) could be obtained in a short reaction time (1 to 20 min). The reported solvent-free microwave protocol was simple, greener and more efficient than any other reported cyclohexene oxidation.
 | ||
| Scheme 39 | ||
The epoxidation of various cyclic olefins was studied over a carbon templated mesoporous TS-1 under microwave irradiation. The epoxidation of cyclooctene and cyclododecene exhibited good shape-selectivity in contrast to the epoxidation of cyclohexene.88
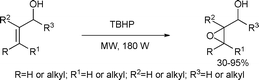
Most procedures for the oxidation of organic substrates use transition metal salts such as Cr(VI), Mn(VII), Mn(IV), Pb(IV) and Ag(I) salts. However, elevated reaction temperatures and basic or acidic conditions can promote undesired side reactions especially in the presence of other sensitive functional groups. Furthermore, a large excess of oxidant is usually required to ensure adequate efficiency and serious environmental problems may arise from the toxicity of heavy metals such as Cr(VI) or Pb(IV). Therefore, many attempts have been made to eliminate these disadvantages, leading to the development of new, catalytic and benign methods. Attempts included the incorporation of transition metal compounds (Ti, V, Cr, Mn, etc.) into inorganic supports, such as montmorillonites and zeolites. Scettri et al. investigated the microwave-assisted oxidation of a series of allylic alcohols with t-butyl hydroperoxide (TBHP) on a zeolite catalyst.89 The authors reported a simple, inexpensive and environmentally safe oxidation, which can be considered of synthetic value due to the short reaction times, solvent-free conditions and the satisfactory chemo-, regio- and diastereoselectivities (Scheme 40).
 | ||
| Scheme 40 | ||
The epoxidation reaction of an allylic diene was investigated with a titanium binaphthyl-bridged Schiff base complex and t-butyl hydroperoxide (TBHP) (Scheme 41). Using only 0.5 mol% of the complex, the epoxyalcohols were obtained in very high regio-, chemo- and diastereoselectivities under a solvent-free microwave-assisted conditions (Scheme 41).90
 | ||
| Scheme 41 | ||
3.2. 5-Membered ring systems
The most widely used approach for the synthesis of furans is the Paal–Knorr method in which 1,4-dicarbonyl compounds are converted to furan derivatives via an acid mediated cyclodehydration. Rao and Jothilingam reported that 2-ene-1,4-diones and 2-yne-1,4-diones can be converted to furan derivatives using formic acid and Pd/C in poly(ethylene glycol)-200 (PEG-200) under microwave irradiation.91 The reaction required less than 5 min, was amenable to scale-up, and worked well for the preparation of both 2,5-diaryl- and 2,3,5-triphenylfuran derivatives. In some cases, catalytic quantity of concentrated H2SO4 was required to promote the cyclodehydration step (Scheme 42).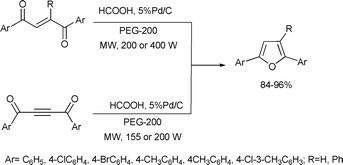 | ||
| Scheme 42 | ||
The catalytic dehydration of fructose, a renewable feedstock, to 5-hydroxymethylfurfural (5-HMF) by microwave heating in acetone–water mixtures in the presence of a cation exchange resin or zeolites as catalysts was reported by Qi et al.92 It was shown that the use of an acetone–water medium resulted in yields of 5-HMF up to 73% with 94% conversion at 150 °C (Scheme 43).
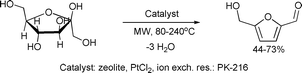 | ||
| Scheme 43 | ||
Benzofurans have attracted considerable interest due to their biological activity and their presence in a variety of natural products.93 The 2-aroylbenzo[b]furans were readily obtained from salicylaldehydes and α-tosyloxyketones in the presence of KF–Al2O3 in excellent yields, up to 96% (Scheme 44).94
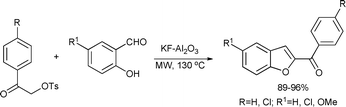 | ||
| Scheme 44 | ||
A one-pot synthesis of functionalized benzofurans was developed via O-alkylation, carbon–carbon coupling/cyclization, and dehydration olefination tandem reactions from phenols and phenacyl bromide. The reactions were carried out under microwave irradiation and solvent-free conditions in the presence of alumina supported inorganic bases. Formation of ethers as by-products were also reported (Scheme 45).95
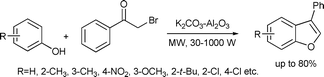 | ||
| Scheme 45 | ||
Kabalka and co-workers reported a microwave-enhanced, solvent-free Mannich condensation–cyclization sequence involving the reaction of 2-ethynylphenol with secondary amines and paraformaldehyde on CuI doped alumina in the absence of solvents. The procedure provided 2-(dialkylaminomethyl)benzo[b]furans in good yields. The synthesis of symmetric bis-benzofuran analogue by using cyclic diamine was also reported.96 (Scheme 46).
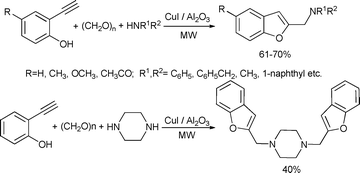 | ||
| Scheme 46 | ||
The same research group investigated the solvent-free microwave-enhanced Sonogashira coupling of iodophenol with terminal alkynes on potassium fluoride doped alumina that yielded benzofurans (Scheme 47). The reaction of o-ethynylphenols with alkyl or aryl iodides resulted in the formation of substituted benzofurans in moderate yields.97
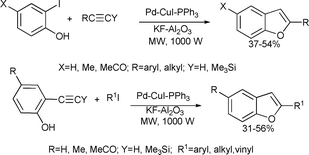 | ||
| Scheme 47 | ||
A microwave-assisted zeolite-catalyzed reaction of enaminone with naphthoquinone afforded the naphthofuran derivative in 76% yield (Scheme 48).98
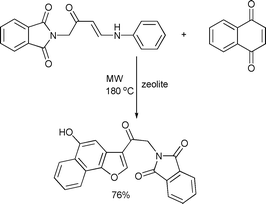 | ||
| Scheme 48 | ||
An optimized procedure for the microwave-assisted synthesis of alkyl D-glucofuranosidurono-6,3-lactones was reported by Richel and co-workers.99 This one-step protocol involves a direct coupling between a completely O-unprotected D-glucuronic acid and methanol in the presence of solid acid catalysts. The method was solvent-free and offered attractive features, such as short reaction times, high yields, easy set-up and work-up procedures (Scheme 49).
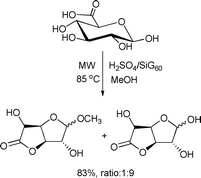 | ||
| Scheme 49 | ||
A simple and efficient procedure for the synthesis of 2-hydroxyimino-3-substituted-tetrahydrobenzofuran derivatives was carried out in the presence of silica gel by Barange et al.100 Cyclic 1,3-dicarbonyl compounds reacted smoothly with various nitroolefins to furnish hydroxyiminotetrahydro-benzofuran derivatives as a mixture of E and Z isomers (Scheme 50).
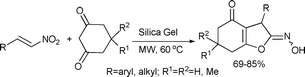 | ||
| Scheme 50 | ||
A solid acid-catalyzed microwave-assisted synthesis of isobenzofuran-1(3H)-ones was performed by Landge et al. (Scheme 51). K-10 montmorillonite appeared to be an excellent catalyst for this condensation and successive lactonization reactions. Reaction of phthalaldehydic acid (2-carboxybenzaldehyde) with methylaryl and cyclic ketones was initiated by microwave irradiation and occurred in one reaction vessel. The reactions were completed in 10–30 min providing excellent yields (90–98%).101
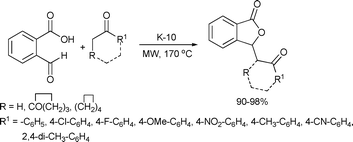 | ||
| Scheme 51 | ||
Microwave-assisted rapid and selective synthesis of cyclic carbonates from phenyl glycidyl ether (PGE) and CO2 was developed using homogeneous and heterogeneous silica supported ionic liquids (SSILs).102 Unlike the conventional reaction, the microwave heating was successful in producing cyclic carbonate, rather than the mixture of cyclic and oligomer products (Scheme 52).
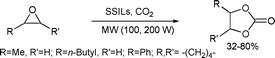 | ||
| Scheme 52 | ||
3.3. 6-Membered ring systems
Research on pyran derivatives is of great interest in organic synthesis as the pyran ring occurs in a number of natural products.103 Pyran derivatives posses a wide range of biological activity and pharmacological properties, such as anti-cancer anti-hypertensive activity.104 In recent years, several studies were published concerning the environmentally benign synthesis of pyran derivatives by microwave-assisted heterogeneous catalysis. Peng and Song developed an efficient and clean approach for the synthesis of 4H-pyrans in ionic liquid (aqueous 1-methyl-3-(2-aminoethyl)imidazolium hexafluorophosphate) [2-aemim][PF6] under microwave conditions.105 The operational simplicity, green reaction medium, reusable catalyst ([2-aemim][PF6]) as well as good yields in short reaction times makes the procedure an economically valuable and environmentally friendly alternative to conventional methods for the synthesis of the target compounds (Scheme 53).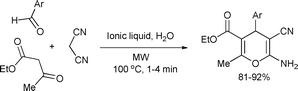 | ||
| Scheme 53 | ||
Two or three-component cyclocondensations are common for the construction of pyrane rings. Dimedon is one of the starting materials for all the reaction designs. Additional reagents and catalysts used to obtain pyran rings (Scheme 54) are as follows; benzaldehyde derivatives with a FeCl3–SiO2 catalyst,106 chalcone units with a InCl3·3H2O catalyst,107 aryl aldehydes and alkyl nitriles with a NaBr catalyst,108 aryl aldehyde and alkyl nitriles with a N,N-diethyl amino-propilated silica (NDEAP) catalyst109 and benzylidenemalononitriles with silica catalyst.110
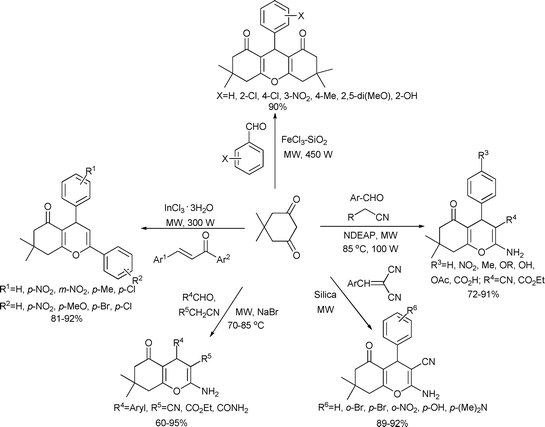 | ||
| Scheme 54 | ||
Nagarapu et al. developed a simple and efficient procedure for the preparation of aryl-14H-dibenzo[a.j]xanthenes by a one-pot condensation of β-naphthol and aryl aldehydes in the presence of potassium dodecatungstocobaltate trihydrate (K5CoW12O40·3H2O), as a heterogeneous catalyst in a solvent-free reaction using microwave irradiation. The method offers several advantages such as excellent yields, simple procedure, short reaction times and milder conditions and the catalyst was found to exhibit remarkable activity111 upon reuse (Scheme 55).
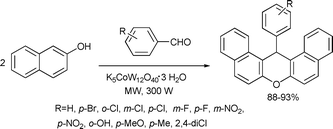 | ||
| Scheme 55 | ||
Mg/Al hydrotalcite, a solid base catalyst was found to be highly efficient in the synthesis of 2-aminochromenes via the multicomponent reaction of aromatic aldehydes, malononitrile and α-naphthol under microwave conditions (Scheme 56). The reaction was rapid, clean, provided products in high yields and the catalyst was reusable.112
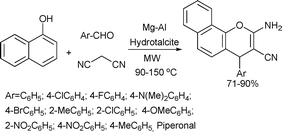 | ||
| Scheme 56 | ||
An efficient microwave-assisted synthesis of 7-aminocoumarins and was performed via the Pechmann reaction of aminophenols with dimethyl oxaloacetate using graphite/K-10 montmorillonite mixture as the catalyst (Scheme 57). The process was driven by the strong microwave absorption of graphite associated with the acid catalytic role of the clay.113
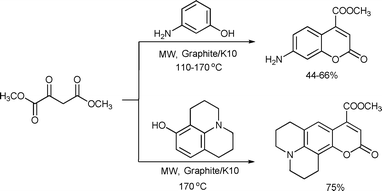 | ||
| Scheme 57 | ||
Another microwave-assisted heterogeneous clay catalyst-based coumarin synthesis was reported by Bandgar and co-workers. A rapid, one-pot synthesis of 3-carboxycoumarins from 2-hydroxy- or 2-methoxybenzaldehydes or acetophenones and Meldrum's acid was described using EPZ10, EPZG or natural kaolinite-based clay under solvent-free conditions using focused microwaves (Scheme 58).114
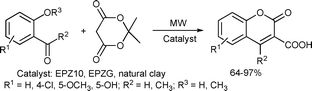 | ||
| Scheme 58 | ||
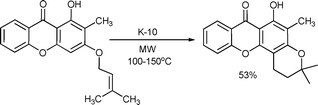
Pinto et al. reported the synthesis of a pyran derivative using K-10 montmorillonite with microwave irradiation in moderate yield. A broad range of derivatives and conditions were investigated.115Scheme 59 only illustrates one model reaction.
 | ||
| Scheme 59 | ||
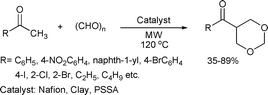
Varma and Polshettiwar developed an efficient approach to attach the 1,3-dioxane functionality to ketones (Scheme 60).116 The use of commercially available and inexpensive polystyrensulfonic acid (PSSA) as a catalyst and water as a reaction medium are additional ecofriendly advantages of this synthetic protocol.
 | ||
| Scheme 60 | ||
4. Synthesis of sulfur containing heterocycles
Cyclic organic sulfur compounds occur naturally in some plant products and are of great importance as components of synthetic pharmaceuticals and dye-stuff.1,117 Since organic sulfur compounds have become increasingly useful, development of convenient and practical synthetic methods for these compounds are highly desirable.Thiiranes are the simplest sulfur containing heterocycles that occur in nature. They have been extensively used in the pharmaceutical, polymer, pesticide and herbicide industries.118 Although there are many classical methods for synthesizing thiiranes, these processes involve long reaction times, the use of highly acidic or oxidizing conditions, high-temperature reactions, expensive reagents, despite which they provide poor yields. Further disadvantages include the formation of several by-products due to the rearrangement or polymerization of the oxiranes and the use of moisture sensitive and foul smelling reagents, which must be handled with care. Kaboudin and Norouzi developed an efficient method for the synthesis of thiiranes from epoxides through a one-pot reaction of epoxides with diethyl phosphite in the presence of ammonium acetate or ammonium hydrogen carbonate/sulfur/and acidic alumina under solvent-free conditions using microwave irradiation.118 This reaction was extended by Zeynizadeh and Yeghaneh using Dowex 50WX8 ion-exchange resin under microwave conditions in 75–98% yield (Scheme 61).119
 | ||
| Scheme 61 | ||
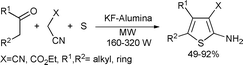
Substituted 2-aminothiophenes are important intermediates in the synthesis of a variety of agrochemicals, dyes and pharmacologically important compounds.120 The most convergent and well-established classical approach for the preparation of 2-aminothiophenes is the Gewald's method, which involves a multicomponent condensation of a ketone with an activated nitrile and elemental sulfur in the presence of morpholine as a catalyst.121 Sridhar and co-workers120 applied KF–alumina as a solid base catalyst for the preparation of 2-aminothiophenes by a microwave accelerated multi-component condensation (Scheme 62). The method is an efficient and convenient modification of the Gewald reaction as it could be carried out in short reaction times under microwave irradiation. Similarly, Huang et al. reported a microwave-assisted synthesis of 2-amino-thiophene-3-carboxylic acid derivatives under solvent-free conditions using the same reagents with silica or alumina as the solid catalyst.122
 | ||
| Scheme 62 | ||
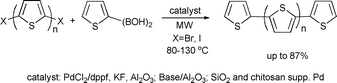
Due to their relevant electronic and optical features, oligothiophenes are among the most important and widely studied organic materials. Barbarella and co-workers developed a synthesis of thiophene oligomers under microwave irradiation in the liquid phase123 and under solvent-free conditions.124 As an extension, a heterogeneous procedure was reported for the preparation of highly pure thiophene oligomers via microwave-assisted Pd catalysis by using silica- and chitosan-supported Pd complexes.125 Their approach was more efficient and greener than the existing homogeneous methodology as it combined a high yield reaction with improved catalyst separation. The microwave-assisted approach afforded the selective preparation of the coupled products in high yields (up to 87% isolated yield, 30–100 min) (Scheme 63).
 | ||
| Scheme 63 | ||
5. Synthesis of heterocycles containing more than one type of heteroatom
Cyclic compounds consisting of more than one type of heteroatom have interesting biological properties and are potentially important in medicinal applications.1,3,4-Oxadiazoles and 1,3,4-thiadiazoles belong to a class of heterocycles, which have attracted significant interest in medicinal chemistry and they have a wide range of pharmaceutical and biological activities including antimicrobial, anti-fungal, anti-inflammatory and anti-hypertensive effects. The widespread use of 1,3,4-oxadiazoles as a scaffold in medicinal chemistry establishes this moiety as an important bioactive class of heterocycles. These molecules are also utilized as pharmacophores due to their favorable metabolic profile and ability to engage in hydrogen bonding.126 Varma and Polshettiwar reported a novel one-pot, solvent-free synthesis of 1,3,4-oxadiazoles and 1,3,4-thiadiazoles by the condensation of acid hydrazide and triethylorthoalkanates under microwave irradiation. It was noted that the solvent-free reaction conditions and the use of Nafion NR50 and P4S10/Al2O3 as the catalyst are particularly ecofriendly attributes of this synthetic protocol (Scheme 64).127
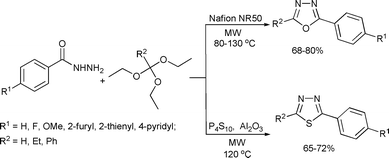 | ||
| Scheme 64 | ||
A series of symmetrical and unsymmetrical 2,5-disubstituted 1,3,4-oxadiazoles were efficiently synthesized via the cyclodehydration of diacylhydrazines by using silica-supported dichlorophosphate as a recoverable cyclizing–dehydrating agent in a solvent-free system under microwave irradiation. This procedure has several advantages, such as being non-corrosive, and having an accelerated rate, and high yields. It has a simple work-up procedure and no waste is generated (Scheme 65).128
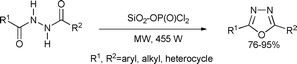 | ||
| Scheme 65 | ||
2,5-Disubstituted 1,3,4-oxadiazoles were prepared by the acidic alumina-catalyzed cyclization of acid hydrazides and benzoic acid (Scheme 66).129
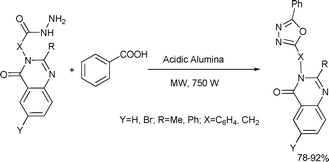 | ||
| Scheme 66 | ||
2-Aryl-5-(coumarin-3′-yl)-1,3,4-oxadiazoles were synthesized by a microwave accelerated solvent-free procedure in high yields via the condensation of coumarin-3-carboxylic acid with benzoic acid hydrazides using poly(ethylene glycol) (PEG) supported dichlorophosphate as the dehydration reagent.130 The authors also noted that the features of the simple work-up procedure; and the utilization of easily prepared and recoverable polymer supported reagents; make this method suitable to high throughput and the combinatorial synthesis of 1,3,4-oxadiazoles (Scheme 67).
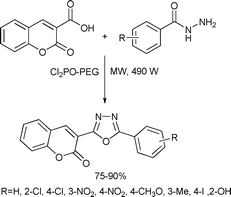 | ||
| Scheme 67 | ||
Oxadiazoles possess important biological properties, such as analgesic or anti-inflammatory activities.131 Kaboudin and co-workers developed two different procedures for the synthesis of 1,2,4-oxadiazole derivatives. Reaction of amidoximes and acyl chlorides with magnesium oxide132 or alumina supported ammonium fluoride133 resulted in the formation of 1,2,4-oxadiazole derivatives. In the other method, the authors used alkyl nitriles, acyl chlorides and magnesia-supported hydroxyl amine hydrochloride134 or the same reagent in the presence of potassium fluoride135 with microwave irradiation (the latter solvent-free), and obtained the target compounds in high yields (Scheme 68).
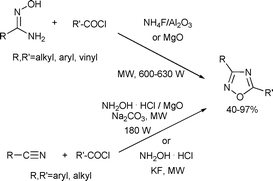 | ||
| Scheme 68 | ||
The oxazole ring system is a basic building block in several biologically important compounds.136 A direct transformation of aromatic ketones into oxazoles in the presence of mercury(II)-acetate under microwave irradiation was described by Lee and Song. It was pointed out that the short reaction time, the high yield, and the simple work-up offer significant advantages over existing methods for the synthesis of multisubstituted oxazole rings (Scheme 69), although the use of a mercury-based catalyst raises questions.137
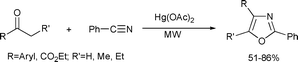 | ||
| Scheme 69 | ||
Thiadiazole derivatives also attracted great attention due to their broad biological activity.138 Li and co-workers developed an efficient solvent-free microwave-assisted protocol for preparation of 2-amino-5-substituted 1,3,4-thiadiazoles by using poly(ethylene glycol)-supported dichlorophosphate (PEG-OP(O)Cl2) as the dehydrating agent (Scheme 70).139
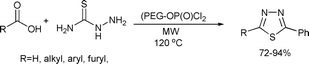 | ||
| Scheme 70 | ||
Thiazoles are also known to posses valuable biological activities.140 Varma et al. developed a new process for the synthesis of 1,3-thiazoles in excellent yields, from thioamides, α-tosyloxyketones and applying K-10 montmorillonite as a solid acid under microwave conditions (Scheme 71).94
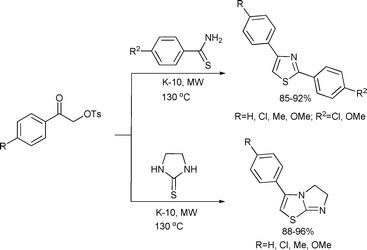 | ||
| Scheme 71 | ||
A new procedure was developed for the synthesis of bithiazole derivatives. It was based on the condensation of thioamides or thiourea with α-bromo ketones under microwave conditions in the presence of K-10 montmorillonite as a solid acid catalyst (Scheme 72).141
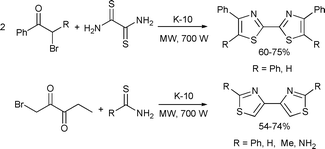 | ||
| Scheme 72 | ||
A mild and efficient method was developed for the preparation of 2-arylbenzothiazoles in the presence of a catalytic amount of Cu1.5PMo12O40/SiO2 under microwave-assisted and solvent-free conditions. The catalyst could be reused several times but loss of activity was observed (Scheme 73).142
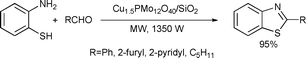 | ||
| Scheme 73 | ||
Substituted benzoxazinone derivatives are important heterocyclic units in numerous biologically active and natural compounds.143 Petricci and co-workers144 developed a cyclohydrocarbonylation process using 2-iodoaniline and acyl chlorides producing benzoxazinones (Scheme 74).
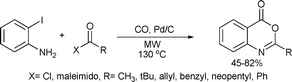 | ||
| Scheme 74 | ||
Benzoxazinone derivatives were synthesized by cyclodehydrazination of salicylaldehyde semicarbazones on K-10 montmorillonite catalyst under microwave-assisted solvent-free conditions145 (Scheme 75).
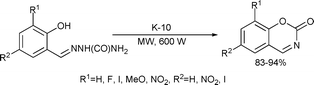 | ||
| Scheme 75 | ||
An environmentally friendly procedure for the synthesis of a series of 8-substituted-2-carboxy-2,3-dihydro-1,5-benzothiazepines under solvent-free microwave conditions was described. The results were compared to those obtained with a classical heating method. The authors noted that a proper choice of the reaction conditions yielded the final products in good yields in a one-step procedure, whereas experiments under conventional heating led to benzothiazepines along with other products in low yields with tedious work-ups (Scheme 76).146
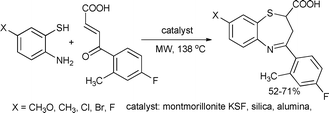 | ||
| Scheme 76 | ||
A solvent-free, microwave-assisted one-pot synthesis of [1,3,4]thiadiazolo[2,3-c][1,2,4]triazinone starting with aromatic aldehydes and triazine derivatives was reported in moderate yields. It was also noted that the reaction was limited to aromatic aldehydes (Scheme 77).147
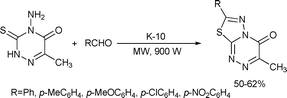 | ||
| Scheme 77 | ||
A convenient approach for the preparation of thiazine and thiazole derivatives was achieved by intramolecular dehydrative ring closure of S-hydroxyalkyl imidazoles using potassium carbonate in DMF using both conventional and microwave methods148 (Scheme 78).
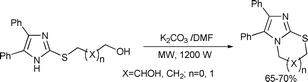 | ||
| Scheme 78 | ||
Yadav and Rai developed a green protocol for an expeditious synthesis of various potentially useful bicyclic hetereocycles starting with readily available substrates employing solvent-free microwave irradiation conditions (Scheme 79).149
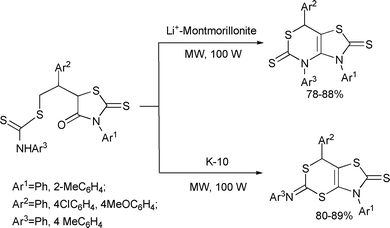 | ||
| Scheme 79 | ||
Yadav and Kapoor reported a clay-catalyzed, atom- and energy-efficient, rapid one-pot synthesis of thiazine derivatives from readily available substrates (chalcones and cyclic thioureas) employing solvent-free microwave activation (Scheme 80).150
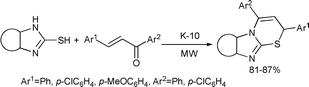 | ||
| Scheme 80 | ||
A novel and ecofriendly method for the synthesis of thiazolo[3,2-b][1,2,4]-triazoles from 3-mercapto-[1,2,4]-triazole and allyl bromide in the presence of acidic silica was also reported (Scheme 81).151
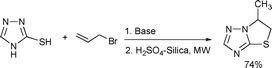 | ||
| Scheme 81 | ||
Yadav et al. developed a diversity-oriented synthetic approach for the preparation of various 1,3-oxazin-2-one-(thione)-fused N- and O-heterocyclic systems using D-glucose (Scheme 82) and D-xylose as renewable resources under solvent-free microwave-assisted conditions (Scheme 82).152
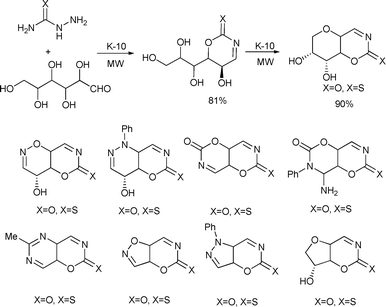 | ||
| Scheme 82 | ||
A heterogeneous catalytic method for the preparation of 2-substituted 1,3-oxazolines, imidazolines and thiazolines was also recently reported. The use of silica-supported 12-tungstophosphoric acid (TPA–SiO2) as non-toxic, recoverable and reusable heterogeneous catalyst makes this procedure environmentally friendly and economically advantageous (Scheme 83).153
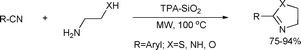 | ||
| Scheme 83 | ||
An intramolecular, domino, Knoevenagel–hetero Diels–Alder reaction sequence was proven to be a useful protocol to prepare polycyclic heterocyclic systems containing coumarin and chromone moieties under mild conditions. Of the various conditions employed, the solvent-free approach using a solid support accelerated by microwaves was found to be the most effective method in achieving high degrees of chemo- and stereoselectivity with a substantial reduction in reaction time (Scheme 84).154
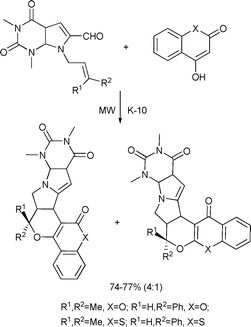 | ||
| Scheme 84 | ||
Conclusions and Outlook
Since the discovery of the microwave heating approach, with the significant developments in the manufacturing of state-of-the-art, commercially available instruments, microwave-assisted synthesis became a mainstream method in organic chemistry. The application of microwave irradiation became a general activation method of primary importance and most synthetic laboratories possess such instruments. In addition, while heterogeneous catalysis had been known for more than a century, the recent developments in material science resulted in an explosion-like development of new practical solid catalysts. The combination of these two well-established approaches appear to lead to the development of effective, rapid, and most of all, environmentally benign synthetic methods. The present work illustrates the advantages of microwave-assisted heterogeneous catalysis in the synthesis of heterocycles; compounds that generate great attention in many applications, particularly in medicinal chemistry and drug development. As the pharmaceutical industry is among the environmentally most problematic branches of the chemical enterprise, it is essential that such green synthesis methods become available for the synthesis of heterocycles. Based on the progress summarized in this work, we feel certain that combined microwave-assisted heterogeneous catalytic approaches will find broad applications and will continue to attract much attention in organic synthesis applications.Acknowledgements
Financial support provided by the University of Massachussetts Boston is gratefully acknowledged. A.D. also thanks The Turkish Council of Higher Education (YÖK) for a fellowship to support his visiting professorship in Boston.Notes and references
- G. W. Gribble, J. Chem. Soc., Perkin Trans. 1, 2000, 1045–1075 RSC; T. A. Gilchrist, J. Chem. Soc., Perkin Trans. 1, 1998, 615–628 RSC; A. Nobuyoshi, O. Akihiko, M. Chikara, T. Tatsuya, O. Masami and S. Hiromitsu, J. Med. Chem., 1999, 42, 2946–2960 CrossRef CAS.
- A. F. Pozharskii, A. T. Soldatenkov, A. R. Katritzky, Heterocycles in Life and Society, Wiley, Chichester, 1997 CrossRef CAS; P. S. Baran, J. M. Richter and D. W. Lin, Angew. Chem., Int. Ed., 2005, 44, 606–609 CrossRef CAS; M. Török, M. Abid, S. C. Mhadgut and B. Török, Biochemistry, 2006, 45, 5377–5383 CrossRef; P. Kohling, A. M. Schmidt and P. Eilbracht, Org. Lett., 2003, 5, 3213–3216 CrossRef; S. J. Yang and W. A. Denny, J. Org. Chem., 2002, 67, 8958–8961 CrossRef; S. Caron and E. Vazquez, J. Org. Chem., 2003, 68, 4104–4107 CrossRef; B. Jiang, C. Yang and J. Wang, J. Org. Chem., 2002, 67, 1396–1398 CrossRef; G. Bringmann, S. Tasler, H. Endress and J. Muhlbacher, Chem. Commun., 2001, 761–762 RSC.
- A. R. Katritzky and A. F. Pozharskii, Handbook of Heterocyclic Chemistry, 2003, Pergamon; Y. Yamamoto, Science of Synthesis, Houben-Weyl Methods of Molecular Transformations; Six-Membered Hetarenes with Two Identical Heteroatoms, 2004, Thieme Verlag, Stuttgart, Vol. 16 Search PubMed.
- B. Robinson, The Fischer Indole Synthesis, 1982, Wiley, Chichester CrossRef; K. Masatane and T. J. Yutaka, J. Heterocycl. Chem., 1981, 18, 709–714 CrossRef; H. J. Knolker and K. R. Reddy, Chem. Rev., 2002, 102, 4303–4427 CrossRef.
- L. Ackermann, L. Kaspar and C. J. Gschrei, Chem. Commun., 2004, 2824–2825 RSC; A. R. Katritzky, S. Ledoux and S. Nair, J. Org. Chem., 2003, 68, 5728–5730 CrossRef CAS; J. Zhao and R. C. Larock, Org. Lett., 2005, 7, 701–704 CrossRef; R. B. Bedford and C. S. Cazin, Chem. Commun., 2002, 2310–2311 RSC; Z. Liu and R. C. Larock, Org. Lett., 2004, 6, 3739–3741 CrossRef; X. Cai and V. Snieckus, Org. Lett., 2004, 6, 2293–2395 CrossRef; B. K. Banik, S. Samajdar and I. Banik, J. Org. Chem., 2004, 69, 213–216 CrossRef.
- P. T. Anastas, J. C. Warner, Green Chemistry: Theory and Practice, 1998, Oxford University Press, Oxford Search PubMed.
- A. Loupy, Microwaves in Organic Synthesis, 2002, Wiley-VCH, Weinheim CrossRef CAS; C. O. Kappe, Angew. Chem., Int. Ed., 2004, 43, 6250–6284 CrossRef CAS; C. O. Kappe, A. Stadler, Microwaves in Organic and Medicinal Chemistry, 2005, Wiley-VCH, Weinheim; J. P. Tierney, P. Lidström, Microwave-Assisted Organic Synthesis, 2005, Blackwell, Oxford; C. O. Kappe, D. Dallinger and S. S. Murphree, Practical Microwave Synthesis for Organic Chemists-Stategies, Instruments, and Protocols, 2008, Wiley-VCH, Weinheim RSC; C. O. Kappe, Chem. Soc. Rev., 2008, 37, 1127–1139 RSC; W. Santagada, F. Frecentese, E. Perisutti, F. Fiorino, B. Severino and G. Caliendo, Mini Rev. Med. Chem, 2009, 9, 340–358 Search PubMed; C. O. Kappe and D. Dalinger, Mol. Diversity, 2009, 13, 71–193 CrossRef; N. Leadbeater, (ed.) Microwave Heating as a Tool for Sustainable Chemistry, 2010, CRC Press, Boca Raton, FL Search PubMed.
- T. J. Mason and J. P. Lorimer, Sonochemistry, 1988Ellis Horwood, Chichester; Synthetic Organic Sonochemistry, ed. J. L. Luche, 1998, Plenum Press, New York. K. S. Suslick, in Handbook of Heterogeneous Catalysis, ed. G. Ertl, H. Knözinger, J. Weitkamp, 1997, Wiley-VCH, Weinheim, Vol. 3, p. 1350 CrossRef CAS; B. Török, K. Balázsik, K. Felföldi and M. Bartók, Ultrason. Sonochem., 2001, 8, 191–200 CrossRef CAS; R. S. Varma, Green Chem. Lett. Rev., 2007, 1, 37–45 CrossRef.
- K. Mikami, Green Reaction Media in Organic Synthesis, 2005, Blackwell, Oxford CrossRef CAS; D. Dallinger and C. O. Kappe, Chem. Rev., 2007, 107, 2563–2591 CrossRef CAS; V. Polshettiwar and R. S. Varma, Aqueous Microwave Assisted Chemistry, 2010, 91–122 Search PubMed; V. Polshettiwar and R. S. Varma, Chem. Soc. Rev., 2008, 37, 1546–1557 RSC.
- G. A. Somorjai, Introduction to Surface Chemistry and Catalysis, 1994, Wiley, New York; J. M. Thomas and W. J. Thomas, Principles and Practice of Heterogeneous Catalysis, 1996, Wiley-VCH, New York, Weinheim; Encyclopedia of Catalysis, ed. I. Horvath, Wiley, New York, 2003; Handbook of Heterogeneous Catalysis, ed. G. Ertl, 2nd Ed. 2008, Wiley-VCH, Weinheim-New York Search PubMed.
- V. Polshettiwar and R. S. Varma, Pure Appl. Chem., 2008, 80, 777–790 CrossRef CAS; M. Abid, B. Török and X. Huang, Aust. J. Chem., 2009, 62, 208 CrossRef; V. P. Mehta, P. Appukkuttan and E. Van der Eycken, Curr. Org. Chem., 2011, 15, 265–283 CrossRef; A. Kruithof, E. Ruijter and R. V. A. Orru, Curr. Org. Chem., 2011, 15, 204 CrossRef; S. A. Patil, R. Patil and D. D. Miller, Curr. Med. Chem., 2011, 18, 615–637 CrossRef.
- S. Dasgupta and B. Török, Org. Prep. Proced. Int., 2008, 40, 1–65 CrossRef CAS; S. Dasgupta and B. Török, Curr. Org. Synth., 2008, 5, 321–342 CrossRef; M. A. P. Martins, C. P. Frizzo, D. N. Moreira, N. Dayse, L. Buriol and P. Machado Pablo, Chem. Rev., 2009, 109, 4140–4182 CrossRef.
- R. S. Varma, Tetrahedron, 2002, 58, 1235–1255 CrossRef CAS; V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629–639 CrossRef; V. Polshettiwar, M. N. Nadagouda and R. S. Varma, Aust. J. Chem., 2009, 62, 16–26 CrossRef.
- S. Bag, S. Dasgupta and B. Török, Curr. Org. Synth., 2011, 8, 237–261 CrossRef CAS.
- J. D. Moseley and C. O. Kappe, Green Chem., 2011, 13, 794–806 RSC.
- B. K. G. Theng, The Chemistry of Clay-Organic Rections, 1974, Halsted Press-a Wiley division, New York CrossRef CAS; H. A. Benesi and B. H. C. Winquest, Adv. Catal., 1978, 27, 97–182 CrossRef CAS; G. A. Olah, P. S. Iyer and G. K. S. Prakash, Synthesis, 1986, 513–531 CrossRef; B. Török and Á. Molnár, Compt. Rend. Acad. Sci. Paris Serie II c, 1998, 381–396 Search PubMed; M. Balogh and P. Laszlo, Organic Chemistry Using Clays, 1993, Springer-Verlag, Berlin, Heidelberg CrossRef; A. Vaccari, Appl. Clay Sci., 1999, 14, 161–198 CrossRef; I. V. Kozhevnikov, Catalysis by Polyoxometalates, 2002, Wiley, Chichester CrossRef; I. V. Kozhevnikov, Appl. Catal., A, 2003, 256, 3–18 CrossRef; M. Misono, Heteropoly Acids, in Encyclopedia of Catalysis,ed. I. T. Horvath, 2003, Wiley, New York, Vol. 3, pp. 433-447 CrossRef; B. C. Gates, Catalysis by Solid Acids, in Encyclopedia of Catalysis, ed. I. T. Horvath, 2003, Wiley, New York,Vol. 2, pp. 104-142 CrossRef; A. Kulkarni and B. Török, Curr. Org. Synth., 2011, 8, 187–207 CrossRef.
- P.-S. E. Dai, Catal. Today, 1995, 26, 3–11 CrossRef CAS; T. Baba, A. Kato, H. Takahashi, F. Toriyama, H. Handa, Y. Ono and H. Sugisawa, J. Catal., 1998, 176, 488–494 CrossRef; A. Béres, I. Pálinkó, I. Kiricsi, J. B. Nagy, Y. Kiyozumi and F. Mizukami, Appl. Catal., A, 1999, 182, 237–247 CrossRef; J. M. Lopez-Pestana, M. J. Avila-Rey and R. M. Martin-Aranda, Green Chem., 2002, 4, 628–630 RSC; F. Figueras, Top. Catal., 2004, 29, 189–196 CrossRef; F. Figueras, M. L. Kantam and B. M. Choudary, Curr. Org. Chem., 2006, 10, 1627–1637 CrossRef.
- A. G. Whittaker and D. M. P. Mingos, J. Chem. Soc., Dalton Trans., 2000, 1521–1526 RSC.
- M. H. C. L. Dressen, B. H. P. van de Kruijs, J. Meuldijk, J. A. J. M. Vekemans and L. A. Hulshof, Org. Process Res. Dev., 2007, 11, 865–869 CrossRef CAS.
- J. Ishiyama, Y. Senda and S. Imaizumi, Chem. Lett., 1983, 1243–1244 CrossRef CAS.
- A. J. Smith and D. L. Trimm, Annu. Rev. Mater. Res., 2005, 35, 127–147 CrossRef CAS.
- Á Molnár, G. V. Smith and M. Bartók, Adv. Catal., 1989, 36, 329–383 CrossRef; B. Török, Á. Molnár, K. Borszéky, E. Tóth-Kádár and I. Bakonyi, Stud. Surf. Sci. Catal., 1993, 78, 179–186 CrossRef.
- K. P. de Jong, Curr. Opin. Solid State Mater. Sci., 1999, 4, 55 CrossRef CAS; R. J. White, R. Luque, V. L. Budarin, J. H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 2009, 38, 481–494 RSC.
- A. Corma, Top. Catal., 1997, 4, 249 CrossRef CAS; R. J. Davis, J. Catal., 2003, 216, 396–405 CrossRef.
- Chiral Catalyst Immobilization and Recycling, eds D. E. de Vos, I. F. J. Vankelecom and P. Jacobs, 2000, Wiley-VCH, Weinheim CrossRef CAS; N. E. Leadbeater and M. Marco, Chem. Rev., 2002, 102, 3217–3273 CrossRef CAS.
- S. C. Mhadgut, K. Palaniappan, M. Thimmaiah, S. A. Hackney, B. Török and J. Liu, Chem. Commun., 2005, 3207–3209 RSC.
- B. Török, M. Abid, G. London, J. Esquibel, M. Török, S. C. Mhadgut, P. Yan and G. K. S. Prakash, Angew. Chem., Int. Ed., 2005, 44, 3086–3089 CrossRef CAS; V. Estévez, M. Villacampa and J. C. Menéndez, Chem. Soc. Rev., 2010, 39, 4402–4421 RSC; K. D. Sheikh, P. P. Banerjee, S. Jagadeesh, S. C. Grindrod, L. Zhang, M. Paige and M. L. Brown, J. Med. Chem., 2010, 53, 2376–2382 CrossRef.
- U. Bora, A. Saikia and R. C. Boruah, Org. Lett., 2003, 5, 435–438 CrossRef CAS.
- M. Abid, S. M. Landge and B. Török, Org. Prep. Proced. Int., 2006, 38, 495–500 CrossRef CAS.
- M. Abid, A. Spaeth and B. Török, Adv. Synth. Catal., 2006, 348, 2191–2196 CrossRef CAS.
- A. Kulkarni, P. Quang and B. Török, Synthesis, 2009, 4010–4014 CAS.
- V. Polshettiwar, B. Baruwati and R. S. Varma, Chem. Commun., 2009, 1837–1839 RSC.
- V. Polshettiwar and R. S. Varma, Tetrahedron, 2010, 66, 1091–1097 CrossRef CAS.
- D. Tejedor, D. González-Cruz, F. García-Tellado, J. Juan Marrero-Tellado and M López Rodríguez, J. Am. Chem. Soc., 2004, 126, 8390–8391 CrossRef CAS.
- T. Lipińska, Tetrahedron Lett., 2004, 45, 8831–8834 CrossRef.
- S. Sugasawa, M Terashima and Y. Kanaoka, Chem. Pharm. Bull., 1956, 4, 16–19 Search PubMed; R. Bently, T. S. Stevens and M. Thompson, J. Chem. Soc. (C), 1970, 791–795 RSC.
- T. Lipińska and S. J. Czarnocki, Org. Lett., 2006, 8, 367–370 CrossRef.
- Z.-P. Zhan, W.-Z. Yang and R.-F. Yang, Synlett, 2005, 2425–2428 CrossRef CAS.
- P. S. Baran and J. M. Richter, J. Am. Chem. Soc., 2005, 127, 15394–15396 CrossRef CAS.
- M. Laronze-Cochard, F. Cochard, E. Daras, A. Lansiaux, B. Brassart, E. Vanquelef, E. Prost, J. Nuzillard, B. Baldeyrou, J. Goosens, O. Lozach, L. Meijer, J. Riou, E. Henon and J. Sapi, Org. Biomol. Chem., 2010, 8, 4625–4636 CAS.
- H. A. De Wald, S. Lobbestael and B. P. H. Poschel, J. Med. Chem., 1981, 24, 982–987 CrossRef CAS.
- N. K. Terret, A. S. Bell, D. Brown and P. Ellis, Bioorg. Med. Chem. Lett., 1996, 6, 1819–1824 CrossRef.
- H. Matsui, Y. Sakanashi, T. M. Oyama, Y. Oyama, S. Yokota, S. Ishida, Y. Okano, T. B. Oyama and Y. Nishimura, Toxicology, 2008, 248, 142–150 CrossRef CAS; H. C. Steel, G. R. Tintinger and R. Anderson, Chem. Biol. Drug Des., 2008, 72, 225–228 Search PubMed; P. Puig-Parellada, G. García-Gasulla and P. Puig-Muset, Pharmacology, 1973, 10, 161–168 CrossRef.
- S. M. Landge, A. Schmidt, V. Outerbridge and B. Török, Synlett, 2007, 1600–1604 CAS.
- S. Balalaie and A. Arabanian, Green Chem., 2000, 2, 274–276 RSC.
- D. S. Raghuvanshi and K. N. Singh, Indian J. Chem. Sec B, 2010, 49B, 1394–1397 CAS.
- R. S. Varma and D. Kumar, Tetrahedron Lett., 1999, 40, 7665–7669 CrossRef CAS.
- H. C. Kolb and K. B. Sharpless, Drug Discovery Today, 2003, 8, 1128–1137 CrossRef CAS.
- B. H. Lipshutz and B. R. Taft, Angew. Chem., Int. Ed., 2006, 45, 8235–8238 CrossRef CAS.
- S. K. Yousuf, D. Mukherjee, B. Singh, S. Maity and S. C. Taneja, Green Chem., 2010, 12, 1568–1572 RSC.
- M. Chtchigrovsky, A. Primo, P. Gonzalez, K. Molvinger, M. Robitzer, F. Quignard and F. Taran, Angew. Chem., Int. Ed., 2009, 48, 5916–5920 CrossRef CAS.
- R. Kumar, A. Mittal and U. Ramachandran, Bioorg. Med. Chem. Lett., 2007, 17, 4613–4618 CrossRef CAS; R. H. Boecker and F. P. Guengerich, J. Med. Chem., 1986, 29, 1596–1603 CrossRef; P. S. Humphries, J. V. Almaden, S. J. Barnum, T. J. Carlson, Q.-Q. T. Do, J. D. Fraser, M. Hess, Y. H. Kim, K. M. Ogilvie and S. Sun, Bioorg. Med. Chem. Lett., 2006, 16, 6116–6119 CrossRef; J.-K. Son, L. –X. Zhao, A. Basnet, P. Thapa, R. Karki, Y. Na, Y. Jahng, T. C. Jeong, B. –S. Jeong, C. –S. Lee and E.-S. Lee, Eur. J. Med. Chem., 2008, 43, 675–682 CrossRef.
- V. R. Solomon, W. Haq, K. Srivastava, S. K. Puri and S. B. Katti, J. Med. Chem., 2007, 50, 394–398 CrossRef CAS; P. M. S. Chauhan and S. K. Srivastava, Curr. Med. Chem., 2001, 8, 1535–1542 Search PubMed; J. P. Michael, Nat. Prod. Rep., 2004, 21, 650–668 RSC; J. P. Michael, Nat. Prod. Rep., 2007, 24, 223–246 RSC; J. I. Kim, I.-S. Shin, H. Kim and J.-K. Lee, J. Am. Chem. Soc., 2005, 127, 1614–1615 CrossRef.
- K. N. Singh and S. K. Singh, Arkivoc, 2009, xiii, 153–160 Search PubMed.
- S. Kantevari, M. V. Chary, S. V. N. Vuppalapati and N. Lingaiah, J. Heterocycl. Chem., 2008, 45, 1099–1102 CrossRef CAS.
- S. K. Singh and K. N. Singh, J. Heterocycl. Chem., 2010, 47, 194–198 CAS.
- S. B. Sapkal, K. F. Shelke, B. B. Shingate and M. S. Shingare, Tetrahedron Lett., 2009, 50, 1754–1756 CrossRef CAS.
- O. De Paolis, J. Baffoe, S. M. Landge and B. Török, Synthesis, 2008, 3423–3428 CAS.
- A. Kulkarni, M. Abid, B. Török and X. Huang, Tetrahedron Lett., 2009, 50, 1791–1794 CrossRef CAS.
- A. Kulkarni and B. Török, Green Chem., 2010, 12, 875–878 RSC.
- I. Mohammadpoor-Baltork, S. Tangestaninejad, M. Moghadam, V. Mirkhani, S. Anvar and A. Mirjafari, Synlett, 2010, 3104–3112 CrossRef CAS.
- O. De Paolis, L. Teixeira and B. Török, Tetrahedron Lett., 2009, 50, 2939–2942 CrossRef CAS.
- D. Borkin, E. Morzhina, S. Datta, A. Rudnitskaya, A. Sood, M. Török and B. Török, Org. Biomol. Chem., 2011, 9, 1394–1401 CAS.
- L. R. Moore and D. A. Vicic, Chem.–Asian J., 2008, 3, 1046–1049 CrossRef CAS.
- U. Achterrathtuckermann, C. H. Weischer and I. Szelenyi, Pharmacology, 1988, 36, 265–271 CrossRef CAS; B. J. Druker, S. Tamura, E. Buchdunger, S. Ohno, G. M. Segal, S. Fanning, J. Zimmermann and N. B. Lydon, Nat. Med., 1996, 2, 561–566 CrossRef; C. O. Kappe, Eur. J. Med. Chem., 2000, 35, 1043–1052 CrossRef; Y. Lee and E. Shacter, J. Biol. Chem., 1999, 274, 19792–19798 CrossRef; W. McNeely and L. R. Wiseman, Drugs, 1998, 56, 91–114 CrossRef; M. H. Norman, G. C. Rigdon, F. Navas and B. R. Cooper, J. Med. Chem., 1994, 37, 2552–2563 CrossRef; V. Ralevic and V. G. Burnstock, Pharmacol. Rev., 1998, 50, 413–492 Search PubMed; A. Studer, S. Hadida, R. Ferrito, S. Y. Kim, P. Jeger, P. Wipf and D. P. Curran, Science, 1997, 275, 823–826 CrossRef; J. F. Wolfe, T. L. Rathman, M. C. Sleevi, J. A. Campbell and T. D. Greenwood, J. Med. Chem., 1990, 33, 161–166 CrossRef; M. Yamaguchi, K. Kamei, T. Koga, M. Akima, T. Kuroki and N. Ohi, J. Med. Chem., 1993, 36, 4052–4060 CrossRef.
- V. M. Outerbridge, S. M. Landge, H. Tamaki and B. Török, Synthesis, 2009, 1801–1806 CAS.
- B. V. Lingaiah, G. Ezikiel, T. Yakaiah, G. Venkat Reddy and P. Shanthan Rao, Synlett, 2006, 2507–2509 CAS.
- V. Kanagarajan, J. Thanusu and M. Gopalakrishnan, Journal of the Korean Chemical Society, 2009, 53, 731–741 CrossRef CAS.
- M. Gopalakrishnan, P. Sureshkumar, V. Kanagarajan, J Thanusu, R. Govindaraju and M. R. Ezhilarasi, Lett. Org. Chem., 2006, 3, 484–488 CrossRef CAS.
- X. Wang, Z. Quan, F. Wang, M. Wang, Z. Zhang and Z. Li, Synth. Commun., 2006, 36, 451–456 CrossRef CAS.
- D. W. McCallien and J. K. M. Sanders, J. Am. Chem. Soc., 1995, 117, 6611–6612 CrossRef CAS; A. Chouai and E. E. Simanek, J. Org. Chem., 2008, 73, 2357–2366 CrossRef.
- A. Díaz-Ortiz, A. de la Hoz, A. Moreno, A. Sánchez-Migallón and G. Valiente, Green Chem., 2002, 4, 339–343 RSC.
- F. Portela-Cubillo, J. S. Scott and J. C. Walton, J. Org. Chem., 2009, 74, 4934–4942 CrossRef CAS.
- J. Wang, Y. Shen, W. Hu, M. Hsieh, F. Lin, M. K. Hsu and M. H. Hsu, J. Med. Chem., 2006, 49, 1442–1449 CrossRef CAS; B. L. De Corte, J. Med. Chem., 2005, 48, 1689–1696 CrossRef.
- M. A. Chari and K. Syamasundar, Catal. Commun., 2005, 6, 67–70 CrossRef CAS.
- M. A. Chari, D. Shobha and K. Syamasundar, J. Heterocycl. Chem., 2007, 44, 929–932 CrossRef CAS.
- S. M. Landge and B. Török, Catal. Lett., 2008, 122, 338–343 CrossRef CAS.
- J. B. Bariwal, D. S. Ermolat'ev, T. N. Glasnov, K. Van Hecke, V. P. Mehta, L. Van Meervelt, C. O. Kappe and E. V. Van der Eycken, Org. Lett., 2010, 12, 2774–2777 CrossRef CAS.
- T. D. Conesa, J. M. Campelo, J. H. Clark, R. Luque, D. J. Macquarrie and A. A. Romero, Green Chem., 2007, 9, 1109–1113 RSC.
- T. L. Gilchrist, Heterocyclic Chemistry, 3rd Ed., 1997, Addison Wesley Longman Ltd., London Search PubMed.
- U. R. Pillai, E. Sahle-Demessie and R. S. Varma, Tetrahedron Lett., 2002, 43, 2909–2911 CrossRef CAS; U. R. Pillai, E. Sahle-Demessie and R. S. Varma, J. Mater. Chem., 2002, 12, 3199–3207 RSC.
- A. Baran and M. Balci, J. Org. Chem., 2009, 74, 88–95 CrossRef CAS.
- M. Dauria and G. Piancatelli, Chem. Lett., 1993, 1153–1156 CrossRef CAS.
- W. Adam, R. Curci, L. Daccolti, A. Dinoi, C. Fusco, F. Gasparrini, R. Kluge, R. Paredes, M. Schulz, A. K. Smerz, L. A. Veloza, S. Weinkotz and R. Winde, Chem.–Eur. J., 1997, 3, 105–109 CrossRef CAS; W. Adam and L. Hadjiarapoglou, Top. Curr. Chem., 1993, 164, 45–62 Search PubMed; W. Adam, L. Hadjiarapoglou and B. Nestler, Tetrahedron Lett., 1990, 31, 331–334 CrossRef; W. Adam, L. Hadjiarapoglou and A. Smerz, Chem. Ber., 1991, 124, 227–232 CrossRef; W. Adam and A. K. Smerz, Bull. Soc. Chim. Belg., 1996, 105, 581–599 Search PubMed.
- M. Jurado, M. Gracia, J. Campelo, R. Luque, J. Marinas and A. Romero, J. Mater. Chem., 2009, 19, 8603–8609 RSC.
- A. M. Balu, J. M. Hidalgo, J. M. Campelo, D. Luna, R. Luque, J. M. Marinas and A. A. Romero, J. Mol. Catal. A: Chem., 2008, 293, 17–24 CrossRef CAS.
- R. Luque, S. K. Badamali, J. H. Clark, M. Fleming and D. J. Macquarrie, Appl. Catal., A, 2008, 341, 154–159 CrossRef CAS.
- D. Y. Ok, N. Jiang, E. A. Prasetyanto, H. Jin and S. E. Park, Microporous Mesoporous Mater., 2011, 141, 2–7 CrossRef CAS.
- L. Palombi, F. Bonadies and A. Scettri, Tetrahedron, 1997, 53, 15867–15876 CrossRef CAS.
- A. Soriente, M. De Rosa, M. Lamberti, C. Tedesco, A. Scettri and C. Pellecchia, J. Mol. Catal. A: Chem., 2005, 235, 253–259 CrossRef CAS.
- H. Rao and S. Jothilingam, J. Org. Chem., 2003, 68, 5392–5394 CrossRef CAS.
- X. Qi, M. Watanabe, T. M. Aida and R. L. Smith, Green Chem., 2008, 10, 799–805 RSC.
- E. Martino, L. Bartalena, F. Bogazzi and L. E. Braverman, Endocr. Rev., 2001, 22, 240–254 CrossRef CAS; K. M. Zareba, Drugs Today, 2006, 42, 75–86 CrossRef; G. Zeni and R. C. Larock, Chem. Rev., 2004, 104, 2285–2309 CrossRef.
- R. S. Varma, D. Kumar and P. J. Liesen, J. Chem. Soc., Perkin Trans. 1, 1998, 4093–4096 RSC; R. S. Varma, J. Heterocycl. Chem., 1999, 36, 1565–1571 CrossRef CAS.
- Z. Wang, J. Z. Gu, H. W. Jing and Y. M. Liang, Synth. Commun., 2009, 39, 4079–4087 CrossRef CAS.
- G. W. Kabalka, L. Wang and R. M. Pagni, Tetrahedron Lett., 2001, 42, 6049–6051 CrossRef CAS.
- G. W. Kabalka, L. Wang and R. M. Pagni, Tetrahedron, 2001, 57, 8017–8028 CrossRef CAS.
- S. M. Al-Mousawi, M. A. El-Apasery and M. H. Elnagdi, Molecules, 2010, 15, 58–67 CrossRef CAS.
- A. Richel, P. Laurent, B. Wathelet, J. P. Wathelet and M. Paquot, Tetrahedron Lett., 2010, 51, 1356–1360 CrossRef CAS.
- D. K. Barange, B. R. Raju, V. Kavala, C. W. Kuo, Y. C. Tu and C. F. Yao, Tetrahedron, 2010, 66, 3754–3760 CrossRef CAS.
- S. M. Landge, M. Berryman and B. Török, Tetrahedron Lett., 2008, 49, 4505–4508 CrossRef CAS.
- M. M. Dharman, H. J. Choi, S. W. Park and D. W. Park, Top. Catal., 2010, 53, 462–469 CrossRef.
- K. C. Nicolaou, J. A. Pfefferkorn, S. Barluenga, H. J. Mitchell, A. J. Roecker and G. Q. Cao, J. Am. Chem. Soc., 2000, 122, 9968–9976 CrossRef CAS; K. C. Nicolaou, J. A. Pfefferkorn, H. J. Mitchell, A. J. Roecker, S. Barluenga, G. Q. Cao, R. L. Affleck and J. E. Lillig, J. Am. Chem. Soc., 2000, 122, 9954–9967 CrossRef; K. C. Nicolaou, J. A. Pfefferkorn, A. J. Roecker, G. Q. Cao, S. Barluenga and H. J. Mitchell, J. Am. Chem. Soc., 2000, 122, 9939–9953 CrossRef.
- A. Balbi, G. Roma, M. Mazzei, E. Sottofattori, S. Cadel and P. Schiantarelli, Farmaco, 1989, 44, 565–577 CAS; K. Iwasaki, T. Shiraga, K. Nagase, Z. Tozuka, K. Noda, S. Sakuma, T. Fujitsu, K. Shimatani, A. Sato and M. Fujioka, Drug Metab. Dispos., 1993, 21, 971–977 Search PubMed.
- Y. Q. Peng and G. H. Song, Catal. Commun., 2007, 8, 111–114 CrossRef CAS.
- H. R. Shaterian, A. Hosseinian and M. Ghashang, Phosphorus, Sulfur Silicon Relat. Elem., 2008, 183, 3136–3144 CrossRef CAS.
- X. Y. Hu, X. S. Fan, X. Y. Zhang, G. R. Qu and Y. Z. Li, Can. J. Chem., 2006, 84, 1054–1057 CrossRef CAS.
- I. Devi and P. J. Bhuyan, Tetrahedron Lett., 2004, 45, 8625–8627 CrossRef CAS.
- H. Hagiwara, A. Numamae, K. Isobe, T. Hoshi and T. Suzuki, Heterocycles, 2006, 68, 889–895 CrossRef CAS.
- S. J. Tu, Y. Gao, C. Guo, D. Q. Shi and Z. S. Lu, Synth. Commun., 2002, 32, 2137–2141 CrossRef CAS.
- L. Nagarapu, S. Kantevari, V. C. Mahankhali and S. Apuri, Catal. Commun., 2007, 8, 1173–1177 CrossRef CAS.
- M. P. Surpur, S. Kshirsagar and S. D. Samant, Tetrahedron Lett., 2009, 50, 719–722 CrossRef CAS.
- S. Frere, V. Thiery and T. Besson, Tetrahedron Lett., 2001, 42, 2791–2794 CrossRef CAS.
- B. P. Bandgar, L. S. Uppalla and D. S. Kurule, Green Chem., 1999, 1, 243–245 RSC.
- R. A. P. Castanheiro, M. M. M. Pinto, S. M. M. Cravo, D. Pinto, A. M. S. Silva and A. Kijjoa, Tetrahedron, 2009, 65, 3848–3857 CrossRef CAS.
- V. Polshettiwar and R. S. Varma, J. Org. Chem., 2007, 72, 7420–7422 CrossRef CAS.
- M. S. Al-Said, M. S. Bashandy, S. I. Al-Qasoumi and M. M. Ghorab, Eur. J. Med. Chem., 2011, 46, 137–141 CrossRef CAS; H. J. Cho, M. J. Jung, Y. Kwon and Y. Na, Bioorg. Med. Chem. Lett., 2009, 19, 6766–6769 CrossRef; R. Romagnoli, P. G. Baraldi, O. Cruz-Lopez, M. Tolomeo, A. Di Cristina, R. M. Pipitone, S. Grimaudo, J. Balzarini, A. Brancale and E. Hamel, Bioorg. Med. Chem. Lett., 2011, 21, 2746–2751 CrossRef.
- B. Kaboudin and H. Norouzi, Tetrahedron Lett., 2004, 45, 1283–1285 CrossRef CAS.
- Zeynizadeh and S. Yeghaneh, Phosphorus, Sulfur Silicon Relat. Elem., 2009, 184, 362–368 CrossRef CAS.
- M. Sridhar, R. M. Rao, N. H. K. Baba and R. M. Kumbhare, Tetrahedron Lett., 2007, 48, 3171–3172 CrossRef CAS.
- R. W. Sabnis, D. W. Rangnekar and N. D. Sonawane, J. Heterocycl. Chem., 1999, 36, 333–345 CrossRef CAS.
- W. Huang, J. Li, J. Tang, H. Liu, J. H. Shen and H. L. Jiang, Synth. Commun., 2005, 35, 1351–1357 CrossRef CAS.
- M. Melucci, G. Barbarella, M. Zambianchi, P. Di Pietro and A. Bongini, J. Org. Chem., 2004, 69, 4821–4828 CrossRef CAS.
- M. Melucci, G. Barbarella and G. Sotgiu, J. Org. Chem., 2002, 67, 8877–8884 CrossRef CAS.
- S. Alesi, F. Di Maria, M. Melucci, D. J. Macquarrie, R. Luque and G. Barbarella, Green Chem., 2008, 10, 517–523 RSC.
- S G. Küçükgüzel, E. E. Oruç, S. Rollas, F. Sahin and A. Ozbek, Eur. J. Med. Chem., 2002, 37, 197–206 CrossRef CAS; B. Chandrakantha, P. Shetty, V. Nambiyar, N. Isloor and A. M. Isloor, Eur. J. Med. Chem., 2010, 45, 1206–1210 CrossRef.
- V. Polshettiwar and R. S. Varma, Tetrahedron Lett., 2008, 49, 879–883 CrossRef CAS.
- Z. Li, A. G. Zhu, X. R. Mao, X. N. Sun and X. Gong, J. Braz. Chem. Soc., 2008, 19, 1622–1626 CrossRef CAS.
- A. R. Desai and K. R. Desai, J. Heterocycl. Chem., 2005, 42, 995–999 CrossRef CAS.
- Z. Li, J. Yu, R. Ding, Z. Wang and X. Wang, Synth. Commun., 2004, 34, 2981–2986 CrossRef CAS.
- A. Almasirad, M. Shafiee, A. Abdollahi, N. Noeparast, N. Shahrokhinejad, S. A. Vousooghi, R. G. Tabatabai and R. Khorasani, Med. Chem. Res., 2011, 20, 435–442 CrossRef CAS; E. Palaska, G. Sahin, P. Kelicen, N. T. Durlu and G. Altinok, Farmaco, 2002, 57, 101–107 CrossRef.
- B. Kaboudin and K. Navaee, Heterocycles, 2003, 60, 2287–2292 CrossRef CAS.
- B. Kaboudin and F. Saadati, J. Heterocycl. Chem., 2005, 42, 699–701 CrossRef CAS.
- B. Kaboudin and F. Saadati, Tetrahedron Lett., 2007, 48, 2829–2832 CrossRef CAS.
- S. Rostamizadeh, H. R. Ghaieni, R. Aryan and A. M. Amani, Tetrahedron, 2010, 66, 494–497 CrossRef CAS.
- J. P. Michael and G. Pattenden, Angew. Chem., Int. Ed. Engl., 1993, 32, 1–23 CrossRef CAS; K. S. Yeung and I. Paterson, Angew. Chem., Int. Ed., 2002, 41, 4632–4653 CrossRef.
- J. C. Lee and I. G. Song, Tetrahedron Lett., 2000, 41, 5891–5894 CrossRef CAS.
- D. Kumar, B. R. Vaddula, K. H. Chang and K. Shah, Bioorg. Med. Chem. Lett., 2011, 21, 2320–2323 CrossRef CAS; L. S. Varandas, C. A. M. Fraga, A. L. P. Miranda and E. J. Barreiro, Lett. Drug Des. Discovery, 2005, 2, 62–67 CrossRef; S. M. Xiao, Heterocycl. Commun., 2009, 15, 173–180 CrossRef.
- Z. Li, J. L. Yu, J. Y. Yang, S. Y. Shi and X. C. Wang, J. Chem. Res., 2005, 341–343 CrossRef.
- Y. M. Ha, J. Y. Park, Y. J. Park, D. Park, Y. J. Choi, J. M. Kim, E. K. Lee, Y. K. Han, J. A. Kim, J. Y. Lee, H. R. Moon and H. Y. Chung, Bioorg. Med. Chem. Lett., 2011, 21, 2445–2449 CrossRef CAS; M. Li, Z. Wang, Y. Lu, S. Ahn, R. Narayanan, J. D. Kearbey, D. N. Parke, W. Li, D. D. Miller and J. T. Dalton, Cancer Res., 2011, 71, 216–224 CrossRef; Y. Lu, C. M. Li, Z. Wang, C. R. Ross, J. Chen, J. T. Dalton, W. Li and D. D. Miller, J. Med. Chem., 2009, 52, 1701–1711 CrossRef.
- M. M. Hashemi, H. Asadollahi and R. Mostaghim, Russ. J. Org. Chem., 2005, 41, 623–624 CrossRef CAS.
- R. Fazaeli and H. Aliyan, Appl. Catal., A, 2009, 353, 74–79 CrossRef CAS.
- N. Benaamane, B. Nedjar-Kolli, Y. Bentarzi, L. Hammal, A. Geronikaki, P. Eleftheriou and A. Lagunin, Bioorg. Med. Chem., 2008, 16, 3059–3066 CrossRef CAS; I. S. Fomsgaard, A. G. Mortensen, J. Idinger, T. Coja and S. Blumel, J. Agric. Food Chem., 2006, 54, 1086–1092 CrossRef; Y. Hashimoto and K. Shudo, Phytochemistry, 1996, 43, 551–559 CrossRef; W. R. Huang, P. L. Zhang, J. F. Zuckett, L. Y. Wang, J. Woolfrey, Y. H. Song, Z. Z. J. Jia, L. A. Clizbe, T. Su, K. Tran, B. Huang, P. Wong, U. Sinha, G. Park, A. Reed, J. Malinowski, S. J. Hollenbach, R. M. Scarborough and B. Y. Zhu, Bioorg. Med. Chem. Lett., 2003, 13, 561–566 CrossRef; J. Poupaert, P. Carato and E. Colacillo, Curr. Med. Chem., 2005, 12, 877–885 CrossRef; P. J. Rybczynski, R. E. Zeck, J. Dudash, D. W. Combs, T. P. Burris, M. Yang, M. C. Osborne, X. L. Chen and K. T. Demarest, J. Med. Chem., 2004, 47, 196–209 CrossRef.
- J. Salvadori, E. Balducci, S. Zaza, E. Petricci and M. Taddei, J. Org. Chem., 2010, 75, 1841–1847 CrossRef CAS.
- L. D. S. Yadav, S. Singh and A. Singh, Tetrahedron Lett., 2002, 43, 8551–8553 CrossRef CAS; L. D. S. Yadav, B. S. Yadav and S. Dubey, Tetrahedron, 2004, 60, 131–136 CrossRef.
- A. Dandia, M. Sati and A. Loupy, Green Chem., 2002, 4, 599–602 RSC.
- S. H. A. Oskooie, M. M. Heravi, P. Khosrofar and F. Faridbod, Phosphorus, Sulfur Silicon Relat. Elem., 2002, 177, 2399–2402 CrossRef CAS.
- E. S. H. El Ashry, N. Rashed, L. F. Awad, E. Ramadan, S. M. Abdel-Maggeed and N. Rezki, Nucleosides, Nucleotides Nucleic Acids, 2007, 26, 423–435 Search PubMed; M. R. Aouad, N. Rezki and E. H. El Ahsry, J. Heterocycl. Chem., 2008, 45, 1321–1327 CrossRef CAS.
- L. D. S. Yadav and V. K. Rai, Tetrahedron, 2006, 62, 8029–8034 CrossRef CAS.
- L. D. S. Yadav and R. Kapoor, Lett. Org. Chem., 2007, 4, 218–221 CrossRef CAS.
- M. M. Heravi, H. R. Khademalfoghara, M. M. Sadeghi, S. Khaleghi and M. Ghassemzadeh, Phosphorus, Sulfur Silicon Relat. Elem., 2006, 181, 377–380 CrossRef CAS.
- L. D. S. Yadav, V. P. Srivastava, V. K. Rai and R. Patel, Tetrahedron, 2008, 64, 4246–4253 CrossRef CAS.
- I. Mohammadpoor-Baltork, M. Moghadam, S. Tangestaninejad, V. Mirkhani and S. F. Hojati, Polyhedron, 2008, 27, 750–758 CrossRef CAS.
- E. Ramesh and R. Raghunathan, Tetrahedron Lett., 2008, 49, 1812–1817 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
