 Open Access Article
Open Access ArticleEvaluation of a temporal trend heavy metals contamination in Posidonia oceanica (L.) Delile, (1813) along the western coastline of Sicily (Italy)
Chiara
Copat
*a,
Riccardo
Maggiore
b,
Giovanni
Arena
a,
Stanislao
Lanzafame
b,
Roberto
Fallico
a,
Salvatore
Sciacca
a and
Margherita
Ferrante
a
aDepartment of Hygiene and Public Health “G.F. Ingrassia”, University of Catania, Via Santa Sofia 87, 95123, Catania, Italy. E-mail: chiaracopat@hotmail.com; marfer@unict.it; Fax: +39-0953782177; Tel: +39-0953782095
bDepartment of Chemical Science, University of Catania, Viale Andrea Doria 6, 95125, Catania, Italy
First published on 21st November 2011
Abstract
The use of biological species in the monitoring of marine environmental quality allows the evaluation of biologically available levels of contaminants in the ecosystem and the effects of contaminants on living organisms. The seagrass Posidonia oceanica is a useful bioindicator because through the lepidochronology technique it is possible to obtain a historical contamination trend of a given area. This study aims to assess the temporal trend contamination by heavy metal investigations on dead sheaths of 100 samples of P. oceanica collected in the Protected Marine Area of “Plemmirio” (Sicily) and in the Siracusa bay. Important results were obtained because data show a significant negative temporal trend for the metals analysed especially for As, Co, Cr, Hg, Pb, Se, U and V that in the past had higher concentrations, with a stronger contamination in the Plemmirio area, the site much more exposed to the pollution of the nearby petrochemical complex. This study confirms the relevance of the use of P. oceanica as a biological indicator of metal contamination in coastal ecosystems. Thus the usefulness of P. oceanica as a tracer of spatial metal contamination and as a good tool for water quality evaluation is reinforced.
Environmental impactThis manuscript “Evaluation of a temporal trend heavy metals contamination in Posidonia oceanica (L.) Delile, (1813) along the western coastline of Sicily (Italy)” by analysing heavy metal concentrations in Posidonia oceanica from 1999 to 2009 contributes to improved knowledge of the quality of seawater, and the degree of metals contamination over a long period. This is the first report of coastal contamination of Sicily seas. Indeed, there are few published studies on this topic in the area of Sicily and in particular near one of the largest petrochemical complexes of Italy. |
1. Introduction
Heavy metals contamination on the global scale is intensely studied because of their persistence in the environment, their toxicity and the risk of biomagnification through food chains.1–12The accumulation of toxic substances in hazardous concentrations in biota has led scientists in recent years to deepen the environmental monitoring programs using bioindicator organisms.5,6,12–20
The species considered is the endemic seagrass Posidonia oceanica that holds a central position in the ecology of the Mediterranean Sea. The importance of this species lies in its extension, in its high productivity and stability, and also because its meadows are functional as spawning area, hunting territory or permanent habitat for numerous plant and animal species.3,21–25
This marine phanerogame has all the qualities required to be used as a biological indicator of chemical pollution as a benthic species widely spread throughout the Mediterranean basin, with a great power of concentration of chemical pollutants. For twenty years P. oceanica has been studied by some authors with the aim of assessing its usefulness as a trace metal biomonitor.14,22,26–35
Since the early 50's, the southeast area of Sicily (Priolo) has become one of the largest and most complex petrochemical sites in Europe, containing several industrial plants, mainly consisting of oil refineries, petrochemical plants, power stations, military base and many other industrial installations. This has led to a growing concern about the possible health effects caused by environmental exposure of residents in neighboring towns until 1990, when the industrial area of Augusta-Priolo, in the Siracusa area, was declared an “area at high risk of environment crisis ” in accordance with Italian law 349 of 8/7/86.
Sampling sites are located a few kilometres to the south from this industrial complex, and are examples of coastal environments with a relatively high influx of unregulated industrial and domestic effluents. In the petrochemical area there is a well documented bioavailability of contaminants such as heavy metals, PCBs and PAHs:36–38 a study conducted in this area on surveillance of congenital malformations and abortions revealed a frequency much higher than the national average in 2001, probably correlated with the petrochemical pollution source.38,39 Another study revealed high concentrations of Hg, PCB and HCB in milk and hair of pregnant women residents in Augusta with respect to the control area of Catania.38
The aim of our study was to monitor the status of the marine environment in two stations of the southern Ionic coast of Sicily, the Protected Marine Area (P.M.A.) of “Plemmirio”, since 2003, and Siracusa bay. Matching the lepidochronological technique for the identification of yearly cycles of leaf production of P. oceanica rhizomes with a mass spectrometry technique for heavy metals analysis, we measured the temporal trend accumulation of heavy metals from 1999 to 2009.
2. Materials and methods
2.1 Sampling and sample preparation
In the summer of 2009 samples of P. oceanica were collected by scuba diving at a depth of 10 m in the Plemmirio P.M.A. station and in the Siracusa bay station (Fig. 1).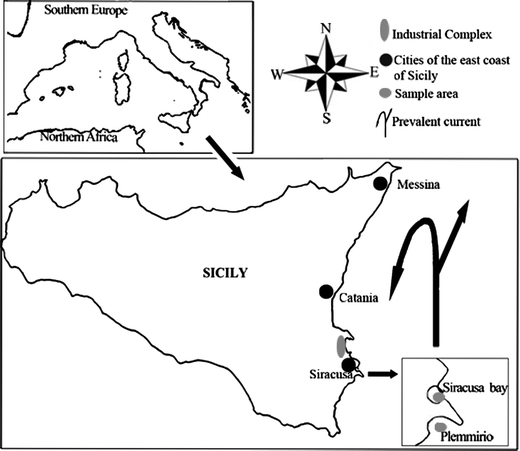 | ||
| Fig. 1 Study area. Data on prevalent surface Ionian current are from Brandt et al. (1999).40 | ||
Each sample area was divided into 10 grids of 1 m2 of about 1 m away from each other. At each grid point were collected 10 plants. In each plant, dead sheaths were separated by year with the lepidochronology technique, till 1999 and no more, due to the reduction of biomass to be analyzed.
Sheaths from each grid of the same year (sample group) were mixed and washed first in fresh water to have the mechanical removal of epiphyte and sediment, then in ultrapure water. Each sample group was dried and frozen at −20 °C before analysis.
2.2. Trace metals analysis
For the lab tests 1 g of each analytical sample group was weighed with an analytical scale (Mettler Toledo xs105) and mineralized in a microwave system Ethos Touch Control (MILESTONE S.r.l.—Italy) with a digestion solution prepared with 6 ml of 65% nitric acid (HNO3) (Carlo Erba) and 2 ml of 30% peroxide hydrogen (H2O2) (Carlo Erba). After mineralization, ultrapure water (Merck) was added to the samples up to 30 ml.The test for heavy metals analysis was performed with an ICP-MS Elan DRCe (PERKIN ELMER-USA). Standards for the instrument calibration were prepared on the basis of multi-element certified reference solution ICP Standard (Merck).
The system was calibrated using a calibration line made up of 5 points of increasing concentration for each element, with a linear regression (R2) for the calibration line between 0.9931 and 0.9991.
Analytical blanks and recovery samples were run in the same way as the real samples and concentrations were determined using standard solutions prepared in the same acid matrix to validate the calibration.
Analytical quality control was performed with certified reference material BCR-670-Aquatic Plant, and with NIST-1946-Lake Superior Fish (Table 1).
| Metals | BCR-670 | |
|---|---|---|
| Certified | Found | |
| As | 1.98 ± 0.19 | 1.9 ± 0.07 |
| Cd | 0.075 ± 0.002 | 0.065 ± 0.001 |
| Cr | 2.05 ± 0.10 | 2.66 ± 0.32 |
| Cu | 1.82 ± 0.30 | 1.66 ± 0.05 |
| Pb | 2.06 ± 0.12 | 1.90 ± 0.06 |
| Zn | 24 ± 2.1 | 23.05 ± 2.05 |
| Metals | Not certified | Found |
|---|---|---|
| Ni | 2.12–2.58 | 2.63 ± 0.39 |
| Se | 0.149–0.273 | 0.238 ± 0.05 |
| Metal | NIST-1946 | |
|---|---|---|
| Certified | Found | |
| Hg | 0.433 ± 0.009 | 0.442 ± 0.02 |
To compare the total metal content in different years and at different stations, the metal pollution index (MPI) defined by Usero et al. (2005)41 was used. It is obtained with the following equation: MPI = (Cf1 × Cf2 × Cfn)1/n, where Cfn is the concentration of the metal n in the sample.
2.3. Statistical analysis
The difference between metals concentrations of the stations was evaluated with a Student's t-test for the paired sample, applying p = 0.001 as the level of significance, using the software package SPSS 14.0.3. Results
3.1. Comparison of the stations
The concentrations of most of the metals vary considerably depending on the location of the sampling stations: As, Cr, Cd, Cu, Ni (P < 0.001) and Hg (P < 0.05) concentrations in the Plemmirio station are significantly higher than in the Siracusa bay station; the highest Zn concentration is recorded in the Siracusa bay station (P < 0.001); Co, Pb, Se, U and V concentrations do not show significant differences (Fig. 2). Although there are differences in concentrations, for some metals there is a clear decrease of pollution load in both sites, as it can be seen from the value of the metal pollution index, MPI (Tables 2 and 3). | ||
| Fig. 2 Temporal trend contamination of heavy metals in P. oceanica dead sheaths of the Siracusa bay and Plemmirio (μg g −1 dry wt). | ||
| Year | Cd | Pb | Zn | Co | V | U | As | Cu | Ni | Cr | Hg | Se | MPI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | 0.69 ± 0.21 | 3.43 ± 1.34 | 12.62 ± 1.23 | 1.42 ± 0.44 | 16.44 ± 3.77 | 4.54 ± 1.65 | 6.65 ± 2.21 | 5.10 ± 1.42 | 6.31 ± 1.80 | 2.94 ± 1.30 | 0.08 ± 0.01 | 0.68 ± 0.19 | 2.63 |
| 2001 | 0.68 ± 0.12 | 3.62 ± 0.75 | 13.39 ± 1.44 | 1.43 ± 0.53 | 18.60 ± 6.56 | 4.14 ± 1.08 | 5.99 ± 1.12 | 5.70 ± 1.04 | 8.18 ± 1.96 | 3.50 ± 1.48 | 0.06 ± 0.01 | 0.71 ± 0.21 | 2.69 |
| 2003 | 0.67 ± 0.11 | 3.02 ± 0.78 | 14.99 ± 1.38 | 1.26 ± 0.23 | 16.15 ± 3.19 | 3.62 ± 0.94 | 5.33 ± 0.86 | 5.74 ± 0.10 | 9.49 ± 1.54 | 3.21 ± 0.92 | 0.04 ± 0.01 | 0.73 ± 0.17 | 2.52 |
| 2005 | 0.63 ± 0.07 | 2.00 ± 0.22 | 15.73 ± 1.69 | 0.94 ± 0.14 | 11.67 ± 2.11 | 2.25 ± 0.80 | 3.59 ± 0.36 | 4.77 ± 0.75 | 8.26 ± 1.90 | 2.42 ± 0.43 | 0.05 ± 0.01 | 0.35 ± 0.08 | 1.93 |
| 2007 | 0.63 ± 0.11 | 2.17 ± 0.61 | 17.68 ± 2.36 | 1.05 ± 0.41 | 11.30 ± 3.45 | 1.75 ± 0.53 | 2.93 ± 0.80 | 5.35 ± 1.73 | 9.29 ± 0.94 | 2.68 ± 1.10 | 0.04 ± 0.01 | 0.39 ± 0.11 | 1.95 |
| 2009 | 0.56 ± 0.09 | 1.14 ± 0.46 | 14.02 ± 4.21 | 0.57 ± 0.12 | 6.23 ± 3.21 | 0.97 ± 0.32 | 1.43 ± 0.55 | 4.46 ± 1.02 | 7.57 ± 2.47 | 1.88 ± 0.63 | 0.01 ± 0.02 | 0.31 ± 0.10 | 1.22 |
| Year | Cd | Pb | Zn | Co | V | U | As | Cu | Ni | Cr | Hg | Se | MPI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1999 | 1.13 ± 0.19 | 8.05 ± 2.79 | 4.11 ± 2.22 | 1.89 ± 0.80 | 36.20 ± 11.39 | 7.21 ± 1.70 | 16.38 ± 8.68 | 17.68 ± 8.20 | 15.23 ± 6.90 | 9.70 ± 5.98 | 0.23 ± 0.13 | 1.08 ± 0.10 | 4.90 |
| 2001 | 0.96 ± 0.32 | 4.18 ± 1.94 | 5.34 ± 0.64 | 1.37 ± 0.29 | 19.11 ± 4.67 | 3.18 ± 0.37 | 11.34 ± 3.21 | 19.61 ± 4.35 | 15.39 ± 4.86 | 7.42 ± 3.02 | 0.09 ± 0.04 | 0.70 ± 0.17 | 3.43 |
| 2003 | 0.79 ± 0.09 | 2.72 ± 0.65 | 5.07 ± 0.92 | 1.26 ± 0.26 | 14.05 ± 3.23 | 2.68 ± 0.61 | 7.89 ± 1.54 | 13.89 ± 3.55 | 14.69 ± 4.99 | 5.13 ± 1.52 | 0.09 ± 0.10 | 0.53 ± 0.12 | 2.75 |
| 2005 | 0.83 ± 0.14 | 1.86 ± 0.48 | 5.00 ± 0.71 | 0.97 ± 0.14 | 9.12 ± 2.00 | 1.86 ± 0.31 | 5.69 ± 2.00 | 9.89 ± 1.90 | 11.46 ± 2.36 | 2.68 ± 0.88 | 0.07 ± 0.06 | 0.42 ± 0.08 | 2.06 |
| 2007 | 0.83 ± 0.16 | 1.37 ± 0.35 | 4.34 ± 0.81 | 0.72 ± 0.11 | 7.69 ± 1.89 | 1.44 ± 0.37 | 3.65 ± 0.88 | 9.08 ± 1.92 | 14.66 ± 4.95 | 2.72 ± 0.28 | 0.05 ± 0.03 | 0.35 ± 0.08 | 1.77 |
| 2009 | 0.89 ± 0.10 | 0.91 ± 0.40 | 5.40 ± 1.80 | 0.38 ± 0.08 | 3.16 ± 1.77 | 0.59 ± 0.06 | 3.26 ± 2.59 | 16.18 ± 9.33 | 14.16 ± 2.69 | 5.82 ± 2.43 | 0.06 ± 0.03 | 0.44 ± 0.07 | 1.62 |
4. Discussions and conclusion
As shown in Fig. 2, for many metals such as As, Co, Hg, Pb, Se, V and U there is a clear downward trend over time in both stations analyzed. Cd, Ni and Zn show no significant temporal fluctuations, Cr and Cu do not show temporal oscillations in the Siracusa bay, while in Plemmirio there is a downward trend from 1999 to 2009.The dead sheaths, although less sensitive to environmental changes than the living parts of the plant, are an index of environmental contamination over long periods.33 The persistence of metals from leaves to dead sheaths is not clear, and consequently it is unknown with certainty which are the metals that, found in the scale, reflect perfectly the concentrations in the living plant.
Some authors in fact noted that Cr, Ni, Cd, Pb and Zn accumulate preferably in leaves, as a result of a longer exposure to the metal load of the marine environment.14,34,42 Gosselin et al. (2006) instead observed concentrations of Cr and Pb major in dead sheaths, and Ni, Cd and Zn highest in the leaves. Kljakovic-Gaspic et al. (2004)43 however showed no significant difference in Cd and Pb between the two parties, as Gosselin et al. (2006) reported for Cu.
A comparison with bibliographic data on bioaccumulation of heavy metals in P. oceanica revealed that concentrations we found are consistently lower than those found by other authors.
In particular the values of Cu found in the Siracusa bay (Table 2) are lower than those found in the literature, but those of Plemmirio (Table 3) are in the range of concentrations found by other authors in contaminated and non-contaminated sites, except for values found by Conti et al. (2007) in Ustica (Sicily, Italy) and Malea (1994)44 in Antikyra Gulf (Greece), significantly higher than ours. The presence of Cu concentrations >16 ppm in some areas is linked to the use of this element as a fungicide in vineyards,33 which are widespread in the province of Siracusa, or as antifouling agent in boat paints, as our study area has a heavy vessel traffic.
The data on the bioaccumulation of Cd, whose uptake occurs through a passive process that depends on the leaf surface exposed to a subsequent translocation in the roots and rhizomes,22,45 are among average values found in the literature.33,46
With regard to Ni, only Gosselin et al. (2006) evaluated the concentrations in dead sheaths, finding values between 9 and 37 ppm in contaminated sites, and 111 ppm in sites contaminated by mining operation, values significantly higher than those detected by us in all sample groups, except one dated 1999 from Plemmirio, which corresponds to the year with greater accumulation of all metals in this area (Tables 2 and 3).
Zn is the only element found in higher concentrations in the dead sheaths of P. oceanica sampled in the Siracusa bay. The increased presence of Zn in this area could result from galvanizing operations of vessels, because within the bay there is a small marina and a small area for mooring vessels. The concentration range of Zn rated by other authors in dead sheaths (14–61 ppm)33,46 appears to be greater than ours (Tables 2 and 3). This indicates a lower contamination of Zn in our study area.
The temporal trend of Pb found in the sites is in agreement with the decreasing contamination of this element in the atmosphere and the sea surface, and is likely to be the result of progressive reduction in the use of lead-based additives in car fuels.46 The concentration of lead found at both sites is among the lowest values reported in the literature on analysis made both in living leaves26,28,33,44,47,48 and in dead sheaths,33,46 except for the values reported by Conti et al. (2010)49 in Linosa Island (Sicily, Italy), values that fall within our concentration range.
The concentrations of Cr in studies in different areas of the Mediterranean Sea in leaves of P. oceanica range from a minimum of 0.11 to a maximum of 5.73 ppm (dry wt).27,33,34,42,47,50,51 The average concentrations of this element in studies of dead sheaths by Gosselin et al. (2006) at contaminated and non-contaminated sites in the north of Corsica are significantly higher than ours (Tables 2 and 3); instead Pergent in Pointe-Martini Chèvre (France) found an average concentration of 2.8 ppm (dry wt), comparable to our results.
The Hg concentration in the living sheaths remains stable throughout the year following the death of the dead sheaths, and it shows that no appreciable mercury flux (absorption or desorption) occurs in this tissue after the blade shedding.52 So the analysis of Hg in dead sheaths of P. Oceanica allows reconstruction of the trend contamination of coastal sites.52 Results obtained by other authors in sites considered contaminated have average concentrations ranging between 0.09 and 0.51 ppm25,30,53,54 comparable to those found in Plemmirio from 1999 to 2003 (Table 3). Although the Mediterranean Sea is rich in natural emissions of this element,37,55 our data attest to a past contamination of industrial origin, but at the same time seem to reflect a decrease.
About the accumulation of As in dead sheaths Gosselin et al. (2006) found average values ranging between 14 and 20 ppm, in the years between 1999 and 2003. The values of Siracusa bay are lower (Table 2) than those found in the bibliography. Plemmirio values (Table 3) are within the range reported by other authors. The presence of this metal in the area is due both to natural events, such as volcanic activity, and to human activities, such as the use of herbicides and antifouling agents.
The results obtained for U, V, Co and Se show a similar decreasing trend over time with concentrations of metals overlapping in both sites, as well as for Pb.
The first consideration is certainly the greatest occurrence of metal load in Plemmirio, probably due to greater movement of water with respect to the Siracusa bay, the latter being a half-closed area with low hydrodynamic movement of water, despite being closer to the industrial area. The increased exposure of Plemmirio to pollutants is also underlined by the values of MPI when compared year to year with that of the Siracusa bay (Tables 2 and 3).
Certainly, important results were obtained because data show a negative temporal trend for the metals analysed especially for As, Co, Cr, Hg, Pb, Se, U and V that in the past had higher concentrations.
References
- M. Barwick and W. Maher, Mar. Environ. Res., 2003, 56, 471–502 CrossRef CAS.
- J. C. McGeer, K. V. Brix, J. M. Skeaff, D. K. DeForest, S. I. Brigham, W. J. Adams and A. Green, Environ. Toxicol. Chem., 2003, 22, 1017–1037 CrossRef CAS.
- S. Ancora, N. Bianchi, A. Butini, M. C. Buia, M. C. Gambi and C. Leonzio, Environ. Toxicol. Chem., 2004, 23, 1093–1099 CrossRef CAS.
- T. Agusa, T. Kunito, G. Yasunaga, H. Iwata, A. Subramanian, A. Ismail and S. Tanabe, Mar. Pollut. Bull., 2005, 51, 896–911 CrossRef CAS.
- M. A. Abdallah and A. M. Abdallah, Environ. Monit. Assess., 2008, 146, 139–145 CrossRef CAS.
- J. Burger, Sci. Total Environ., 2008, 389, 37–45 CrossRef CAS.
- M. Maanan, Environ. Pollut., 2008, 153, 176–183 CrossRef CAS.
- O. Altun, M. T. Sacan and A. K. Erdem, Environ. Monit. Assess., 2009, 151, 345–362 CrossRef CAS.
- L. Cadena-Cardenas, L. Mendez-Rodriguez, T. Zenteno-Savin, J. Garcia-Hernandez and B. Acosta-Vargas, Arch. Environ. Contam. Toxicol., 2009, 57, 96–102 CrossRef CAS.
- N. Dogan-Saglamtimur and H. Kumbur, Biol. Trace Elem. Res., 2010, 136, 55–70 CrossRef CAS.
- J. W. Nichols, M. Bonnell, S. Dimitrov, B. Escher, X. Han and N. I. Kramer, Integr. Environ. Assess. Manage., 2009, 5, 577–597 CrossRef CAS.
- C. Tigano, B. Tomasello, V. Pulvirenti, V. Ferrito, C. Copat, G. Carpinteri, E. Mollica, S. Sciacca and M. Renis, Ecotoxicol. Environ. Saf., 2009, 72, 1278–1286 CrossRef CAS.
- L. T. Budnik and X. Baur, Dtsch. Arztebl. Int., 2009, 106, 91–97 Search PubMed.
- L. Campanella, M. E. Conti, F. Cubadda and C. Sucapane, Environ. Pollut., 2001, 111, 117–126 CrossRef CAS.
- M. D. Fernandez, E. Cagigal, M. M. Vega, A. Urzelai, M. Babin, J. Pro and J. V. Tarazona, Ecotoxicol. Environ. Saf., 2005, 62, 174–184 CrossRef CAS.
- M. L. Martin-Diaz, S. R. Tuberty, C. L. McKenney, Jr, J. Blasco, C. Sarasquete and T. A. Delvalls, Environ. Monit. Assess., 2006, 116, 169–184 CrossRef CAS.
- C. J. Passos, D. Mergler, M. Lemire, M. Fillion and J. R. Guimaraes, Sci. Total Environ., 2007, 373, 68–76 CrossRef CAS.
- Q. Zhou, J. Zhang, J. Fu, J. Shi and G. Jiang, Anal. Chim. Acta, 2008, 606, 135–150 CrossRef CAS.
- M. Ebrahimi and M. Taherianfard, Environ. Monit. Assess., 2010, 168, 575–585 CrossRef CAS.
- H. Wang and Y. Jia, J. Environ. Sci. (Beijing, China), 2009, 21, 1409–1414 CAS.
- C. F. Boudouresque and A. Meinesz, Découverte de l'herbier de Posidonie. Cahier Parc Nat. Port-Cros, 4, 1–79 Search PubMed.
- T. J. Ward, Mar. Biol., 1987, 95, 315–321 CrossRef CAS.
- L. Ballesta, G. Pergent, V. Pasqualini and C. Pergent-Martini, C. R. Acad. Sci., Ser. III, 2000, 323, 407–414 CAS.
- J. M. Ruiz and J. Romero, Mar. Pollut. Bull., 2003, 46, 1523–1533 CrossRef CAS.
- C. Pergent-Martini, V. Pasqualini, L. Ferrat and G. Pergent, Environ. Manage., 2006, 38, 889–895 CrossRef.
- S. Costantini, R. Giordano, L. Ciaralli and E. Beccaloni, Mar. Pollut. Bull., 1991, 22, 362–363 CrossRef CAS.
- V. A. Catsiki and P. Panayotidis, Chemosphere, 1993, 26, 963–978 CrossRef CAS.
- M. Warnau, G. Ledent, A. Temara, J. M. Bouquegneau, M. Jangoux and P. Dubois, Sci. Total Environ., 1995, 171, 95–99 CrossRef CAS.
- M. A. Schlacher-Hoenlinger and T. A. Schlacher, Mar. Biol., 1998, 131, 401–410 CrossRef CAS.
- A. Capiomont, L. Piazzi and G. Pergent, J. Mar. Biol. Assoc. UK, 2000, 80, 1119–1123 CrossRef CAS.
- C. F. Boudouresque and M. Verlaque, Mar. Pollut. Bull., 2002, 44, 32–38 CrossRef CAS.
- L. Ferrat, C. Pergent-Martini and M. Romeo, Aquat. Toxicol., 2003, 65, 187–204 CrossRef CAS.
- M. Gosselin, J. M. Bouquegneau, F. Lefebvre, G. Lepoint, G. Pergent, C. Pergent-Martini and S. Gobert, BMC Ecol., 2006, 6, 12 CrossRef.
- M. E. Conti, B. Bocca, M. Iacobucci, M. G. Finoia, M. Mecozzi, A. Pino and A. Alimonti, Arch. Environ. Contam. Toxicol., 2007, 58, 79–95 CrossRef.
- C. Lafabrie, G. Pergent and C. Pergent-Martini, Sci. Total Environ., 2009, 407, 2440–2446 CrossRef CAS.
- A. Ausili, M. Gabellini, G. Cammarata, D. Fattorini, M. Benedetti, B. Pisanelli, S. Gorbi and F. Regoli, Mar. Environ. Res., 2008, 66, 215–217 CrossRef CAS.
- R. Di Leonardo, G. Tranchida, A. Bellanca, R. Neri, M. Angelone and S. Mazzola, Chemosphere, 2006, 65, 2366–2376 CrossRef CAS.
- A. Madeddu and S. Sciacca, Ann. Ig., 2008, 20, 59–64 CAS.
- F. Bianchi, S. Bianca, N. Linzalone and A. Madeddu, Epidemiologia e Prevenzione, 2004, 28, 87–93 Search PubMed.
- P. Brandt, A. Rubino, D. Quadfasel and W. Alpers, J. Phys. Oceanogr., 1999, 29, 1071–1080 CrossRef.
- J. Usero, J. Morillo and I. Gracia, Chemosphere, 2005, 59, 1175–1181 CrossRef CAS.
- C. Lafabrie, C. Pergent-Martini and G. Pergent, Mar. Pollut. Bull., 2008, 57, 155–159 CrossRef CAS.
- Z. Kljakovic-Gaspic, B. Antolic, T. Zvonaric and A. Baric, Fresenius Environ. Bull., 2004, 13, 1210–1215 CAS.
- P. Malea, Environ. Pollut., 1994, 85, 77–85 CrossRef CAS.
- B. H. Brinkhuis, W. F. Penello and A. C. Churchill, Mar. Biol., 1980, 58, 187–196 CrossRef CAS.
- M. Roméo, M. Gnassia-Barelli, T. Juhel and A. Meinesz, Mar. Ecol.: Prog. Ser., 1995, 120, 211–218 CrossRef.
- C. Pergent-Martini, V. Rico-Raimondino and G. Pergent, Mar. Biol., 1994, 120, 9–15 Search PubMed.
- L. Tranchina, S. Micciche, A. Bartolotta, M. Brai and R. N. Mantegna, Environ. Sci. Technol., 2005, 39, 3006–3012 CrossRef CAS.
- M. E. Conti, B. Bocca, M. Iacobucci, M. G. Finoia, M. Mecozzi, A. Pino and A. Alimonti, Arch. Environ. Contam. Toxicol., 2010, 58, 79–95 CrossRef CAS.
- M. Warnau, G. Ledent, A. Temara, J. M. Bouquegneau, M. Jangoux and P. Dubois, Sci. Total Environ., 1995, 171, 95–99 CrossRef CAS.
- V. A. Catsiki and F. Bei, Fresenius Environ. Bull., 1992, 1, 60–65 Search PubMed.
- C. Lafabrie, G. Pergent, C. Pergent-Martini and A. Capiomont, Environ. Pollut., 2007, 148, 688–692 CrossRef CAS.
- B. E. Maserti, R. Ferrara and P. Paterno, Mar. Pollut. Bull., 1988, 19, 381–382 CrossRef CAS.
- L. Ferrat, M. Gnassia-Barelli, C. Pergent-Martini and M. Romeo, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2003, 134, 147–155 CrossRef.
- R. Ferrara, B. Mazzolai, E. Lanzillotta, E. Nucaro and N. Pirrone, Sci. Total Environ., 2000, 259, 115–121 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
