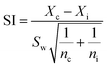Toxicity of propargylic alcohols on green alga—Pseudokirchneriella subcapitata
Chung Yuan
Chen
*,
Kwan-Liang
Kuo
and
Je-Wei
Fan
Institute of Environmental Engineering, National Chiao Tung University, 75 Po-Ai Street, Hsinchu, Taiwan 300. E-mail: chen1954@mail.nctu.edu.tw; Fax: +886-3-5714839; Tel: +886-3-5731915
First published on 21st November 2011
Abstract
The present study evaluates the toxicity of 34 propargylic alcohols, including primary, primary homo-, secondary, and tertiary alcohols, based on their effects on phytoplankton. A closed-system algal toxicity test was applied because the closed-system technique presents more realistic concentration–response relationships for the above compounds than the conventional batch tests. The green alga, Pseudokirchneriella subcapitata, was the test organism and final yield and growth rate were chosen as the test endpoints. Among all the propargylic alcohols tested, 1-pentyn-3-ol is the most toxic compound with its EC50 equal to 0.50 mg L−1, which can be classified as a “R50” compound (very toxic to aquatic organisms, EC50/LC50 < 1 mg L−1), following the current practice for classification of chemicals in the European Union (EU). There are several other compounds including 2-decyn-1-ol, 3-decyn-1-ol, 1-hexyn-3-ol, 3-butyn-2-ol, and 3-hexyne-2,5-diol, which deserve more attention for their possible adverse impact on the aquatic environment, because these alcohols can be classified as “R51” compounds (toxic to aquatic organisms, EC50/LC50 between 1 and 10 mg L−1). Compared to the base-line toxicity relationship (narcosis QSAR) derived previously, tertiary propargylic alcohols can be identified as nonpolar narcotic chemicals, while secondary alcohols and primary alcohols with low molecular weight generally exhibit obvious excess toxicity in relation to the base-line toxicity. Finally, quantitative structure–activity relationships were established for deriving a preliminary estimation of the toxicity of other propargylic alcohols.
Environmental impactPropargylic alcohols have a wide range of industrial applications and are produced in mass volumes. However, there are insufficient data for assessing the risks of propargylic alcohols to aquatic organisms. Furthermore, the existing algal toxicity database for organic toxicants was mainly derived by the conventional batch technique, which has been found to be inadequate and failing to display certain important toxicological characteristics. More efforts are needed to revise the existing database using the closed-system test technique which presents more realistic and meaningful concentration–response relationships of organic compounds than the conventional batch tests. The present study evaluates the toxicity of various propargylic alcohols, using a closed-system algal testing technique. The toxicity data and the quantitative structure–activity relationships (QSARs) derived from this study will be useful for risk assessment and protection of the aquatic environments. |
1. Introduction
Propargylic alcohols are semi-volatile aliphatic compounds. These substances have a wide range of industrial applications including reactant/chemical intermediate, corrosion inhibitor, solvent stabilizer, soil fumigant and polymer modifier, hence, they are produced in mass volumes. It was estimated that over 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pounds of propargylic alcohols were released into ground water annually in Texas, USA.1 The US EPA High Production Volume Challenge Program1 has thus concluded that there are insufficient aquatic data for assessing the risks of propargylic alcohols; in particular, daphnia and algae data are needed to strengthen the information of aquatic toxicity.
000 pounds of propargylic alcohols were released into ground water annually in Texas, USA.1 The US EPA High Production Volume Challenge Program1 has thus concluded that there are insufficient aquatic data for assessing the risks of propargylic alcohols; in particular, daphnia and algae data are needed to strengthen the information of aquatic toxicity.
The effects of propargylic alcohols on fish and ciliate have been reported by previous researchers.2–8 Lipnick2 found that the toxicity of primary and secondary propargylic alcohols was considerably more (7–4600 times) than that estimated by the baseline toxicity relationship. Such a phenomenon was mainly due to the formation of Michael-type acceptor electrophiles through biological transformation.3 The related mechanism involves metabolism of the parent alcohol to the corresponding α,β-unsaturated aldehyde or ketone by alcohol dehydrogenase.4 On the other hand, tertiary propargylic alcohols were found to be narcotic in nature.4 Based on test results from Tetrahymena pyriformis, Schultz et al.5,6 have successfully established quantitative structure–activity relationships (QSARs) by using the logarithm of the 1-octanol–water partition coefficient (KOW) to estimate the toxicity of primary and primary homo-propargylic alcohols. However, no valid QSAR was identified with respect to secondary propargylic alcohols.7 Similarly, based on data from fathead minnow tests, Mekenyan et al.8 have demonstrated that hydrophobicity (log Kow) is a good descriptor for the toxicity of tertiary propargylic alcohols.
In recent years, quantitative structure–activity relationships (QSARs) have become important tools in estimating aquatic toxicity of various chemicals for regulatory purposes.9 To successfully predict the adverse effects of chemical compounds based on QSARs, the applied ecotoxicological database should be reliable and of high quality. Currently, most standard algal test protocols for the evaluation of phytotoxicity10,11 are basically open-system tests and have been questioned for their adequacies for testing volatile organic toxicants (European Centre for Ecotoxicology and Toxicology of Chemicals12), in consideration of their open test environment and the vigorous mixing provided during testing. In addition, a previous analysis13 indicated that the existing database derived by these protocols failed to display certain fundamental toxicological characteristics such as species correlation, baseline toxicity relationship, relative toxicity relationship among nonpolar, polar, and reactive organic compounds with respect to chemical's hydrophobicity, etc. On the other hand, algal toxicity data based on the closed-system technique reveal satisfactory test sensitivity and good agreement in the aforementioned toxicological characteristics.13–18 Compared to the conventional batch tests, the closed-system technique presents more meaningful concentration–response relationships and more adequate assessments for organic chemicals.13–18 It is thus advisable to revise the existing algal toxicity database using the closed-system test technique.
Currently, information on the effects of propargylic alcohols on phytoplankton is not yet available from the existing aquatic toxicity database. The objective of the present study is to estimate the toxicity of propargylic alcohols on Pseudokirchneriella subcapitata using the aforementioned closed-system test technique and to establish quantitative structure–activity relationships (QSARs) to enhance the predictability of the toxic effects of various propargylic alcohols.
2. Materials and methods
Toxicity testing
Algal inoculum (Pseudokirchneriella subcapitata, formerly known as Selenastrum capricornutum, UTEX 1648) was withdrawn from a chemostat operated under steady state and transferred into 300 mL, biochemical-oxygen-demand (BOD) test bottles together with dilution water (with growth medium) and toxicants. The BOD bottles were filled completely with no headspace left. A water seal was provided to ensure a closed test environment. The bottles were then placed on an orbital shaker (Ferstek, Model S103; Medclub, Hsinchu, Taiwan) and operated at 100 rpm for 48 h (test duration). Temperature and light intensity were kept at 24 ± 1 °C and 65 μEm-2s-1 (±10%), respectively. US EPA11 bottle medium, with no EDTA content, was used for toxicity testing. Two response endpoints were used to evaluate the toxicity of the toxicants: the final yield (FY: final cell density—initial cell density) and algal growth rate (GR) based on cell density counts. The initial inoculated cell density was 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL, which was determined using an electronic particle counter (Culter Electronics, Luton, UK). The initial pH for toxicity testing was set at 7.5. Thirty-four propargylic alcohols, including primary, primary homo-, secondary, and tertiary alcohols, were tested in the present study. All chemicals used were of reagent grade and were tested at least twice, i.e., range finding test and definitive test. For the definitive test, one control and 6 (or 7) different treatments were performed in triplicate. Stock solution was freshly prepared, and its concentration was analyzed with a total organic carbon (TOC) analyzer before commencing the experiment. Toxicants with low solubility were dissolved in dimethyl sulfoxide (DMSO). Solvent controls were conducted using the highest DMSO concentration applied in treatments.
000 cells per mL, which was determined using an electronic particle counter (Culter Electronics, Luton, UK). The initial pH for toxicity testing was set at 7.5. Thirty-four propargylic alcohols, including primary, primary homo-, secondary, and tertiary alcohols, were tested in the present study. All chemicals used were of reagent grade and were tested at least twice, i.e., range finding test and definitive test. For the definitive test, one control and 6 (or 7) different treatments were performed in triplicate. Stock solution was freshly prepared, and its concentration was analyzed with a total organic carbon (TOC) analyzer before commencing the experiment. Toxicants with low solubility were dissolved in dimethyl sulfoxide (DMSO). Solvent controls were conducted using the highest DMSO concentration applied in treatments.
Data analyses
Probit analysis was applied to determine the concentration–response relationship and the median effective concentration (EC50). One-tail Dunnett's procedure was applied for the estimation of NOEC and LOEC values at 5% level of significance. The studentized range (SI) can be calculated using the following expression:where Xc and Xi are mean observations from controls and treatments, respectively, Sw is the square root of the within-group variance, and nc and ni are the numbers of replicates for the control and treatment. A specific treatment is considered to be significantly different from the controls if the corresponding SI value is greater than the critical value (T) specified by the Dunnett's T table.
The lowest unoccupied molecular orbital energies (Elumo) were calculated with the Gaussian 98 program package (Cambridge Soft Corporation, Cambridge, MA, USA). Specific chemicals were first analyzed at the density functional theory level (B3LYP/6-31G) and the results were submitted to subsequent ab initio calculations.19 The 1-octanol–water partition coefficients (KOW) were obtained using the US EPA Estimation Program Interface Suite (EPI Suite; http://www.epa.gov/opptintr/exposure/pubs/episuite.htm). Correlation analyses were performed using MINITAB (Ver 14.2; MINITAB, State College, PA, USA) to establish QSARs. Leave-one-out cross-validation was carried out to test the significance of each QSAR. The statistical quality was judged by the square of the correlation coefficient (r2), the Fisher criterion (F), the root mean square error (S), and the predictivity of the model (Q2).
3. Results and discussion
3.1 Toxicity of propargylic alcohols
Table 1 lists the EC50, NOEC, and EC10 values of various propargylic alcohols, with respect to the endpoints of final yield and algal growth rate. Literature data and the physical/chemical properties of propargylic alcohols are also displayed for discussions. Median effective concentrations (EC50) range from 0.50 to 1465 mg L−1 with respect to the final yield endpoint, and 1.0 to 4568 mg L−1 for the growth rate endpoint. In general, EC50 values based on algal growth rate are at least twice greater than that derived by final yield. For primary propargylic alcohols, there is a general trend that toxicity increases with increased carbon-chain length and hydrophobicity (log Kow), except for 2-propyn-1-ol. For tertiary propargylic alcohols, toxicity is also generally increased with greater log Kow values. On the other hand, secondary propargylic alcohols appeared to be more toxic than primary and tertiary alcohols.| Chemicals | Molecular weight | log Kow | E lumo/eV | Algae/mg L−1 | Tetrahymena b (IGC50)/mg L−1 | Fathead minnow (LC50)/mg L−1 | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Final yield | Growth rate | ||||||||||||
| EC50 | NOEC | EC10 | ACR1 | ACR2 | EC50 | EC10 | ACR3 | ||||||
| a EC50, IGC50, LC50 units: mg L−1; *: identical NOEC values obtained by both final yield and growth rate endpoints. ACR1: EC50/NOEC by final yield; ACR2: EC50/EC10 by final yield; ACR3: EC50/EC10 by growth rate. b Scultz et al.7 c Veith et al.4 d Mekenyan et al.8 | |||||||||||||
| Primary propargylic alcohols | |||||||||||||
| 2-Propyn-1-ol | 56.1 | −0.4 | 1.7 | 28.9 | 2.56* | 8.74 | 11.28 | 3.3 | 55.8 | 17.41 | 3.21 | 658.7 | 1.53c |
| 2-Butyn-1-ol | 70.1 | 0.4 | 1.6 | 47.2 | <5.16 | 18.62 | >9.14 | 2.53 | 67.5 | 33.56 | 2.01 | 519.0 | 10.1c |
| 2-Butyn-1,4-diol | 86.1 | −0.7 | 1.4 | 464.2 | <55.67 | 104.4 | >8.34 | 4.45 | 1214.2 | 233.43 | 5.20 | 6530.0 | 53.6c |
| 2-Pentyn-1-ol | 84.1 | 0.9 | 1.6 | 42.5 | 2.41* | 9.2 | 17.62 | 4.62 | 109.5 | 23.42 | 4.68 | 314.6 | |
| 2-Hexyn-1-ol | 98.1 | 1.4 | 1.6 | 32.2 | 2.43* | 5.47 | 13.25 | 5.88 | 86.5 | 16.32 | 5.30 | 237.6 | |
| 2-Heptyn-1-ol | 112.2 | 2.0 | 1.6 | 38.4 | <4.99 | 2.1 | >7.69 | 18.27 | 285.2 | 8.76 | 32.56 | 173.5 | |
| 2-Octyn-1-ol | 126.2 | 2.5 | 1.6 | 24.4 | <2.49 | 1.52 | >9.82 | 16.08 | 78.6 | 9.14 | 8.60 | 81.0 | |
| 2-Nonyn-1-o | 140.2 | 3.0 | 1.6 | 7.8 | <0.53 | 1.68 | >14.66 | 4.63 | 21.3 | 3.86 | 5.52 | 31.4 | |
| 2-Decyn-1-ol | 154.3 | 3.5 | 1.6 | 1.4 | <0.25* | 0.3 | 5.4 | 4.5 | 3.6 | 0.38 | 9.47 | 15.8 | 1.07c |
| Primary homo-propargylic alcohols | |||||||||||||
| 3-Butyn-1-ol | 70.1 | −0.2 | 1.8 | 262.3 | 9.22* | 16.71 | 28.45 | 15.7 | 2262.9 | 69.0 | 32.80 | 4849.0 | 36.1c |
| 3-Pentyn-1-ol | 84.1 | 0.3 | 1.7 | 158.5 | <12.8* | 11.39 | >12.33 | 13.91 | 492.1 | 51.11 | 9.63 | 1273.2 | |
| 3-Hexyn-1-ol | 98.1 | 0.9 | 1.7 | 37.8 | <6.17 | 10.01 | >6.12 | 3.77 | 89.7 | 15.94 | 5.63 | 1027.7 | |
| 3-Heptyn-1-ol | 112.2 | 1.4 | 1.7 | 83.6 | 3.82* | 5.16 | 21.89 | 16.21 | 525.2 | 23.27 | 22.57 | 234.3 | |
| 3-Octyn-1-ol | 126.2 | 1.9 | 1.7 | 40.8 | 1.24* | 4.19 | 32.87 | 10.4 | 140.5 | 14.12 | 9.95 | 120.5 | |
| 3-Nonyn-1-ol | 140.2 | 2.5 | 1.7 | 10.1 | <1.36 | 3.7 | >7.43 | 2.73 | 16.8 | 7.17 | 2.34 | 64.1 | |
| 3-Decyn-1-ol | 154.3 | 3.0 | 1.7 | 4.8 | <0.51 | 1.31 | >9.49 | 3.69 | 10.1 | 2.53 | 3.99 | 11.4 | |
| Secondary propargylic alcohols | |||||||||||||
| 1-Hexyn-3-ol | 98.2 | 1.2 | 1.7 | 1.7 | <0.48 | 0.280 | >3.563 | 6.099 | 5.9 | 0.529 | 11.15 | 21.5 | |
| 1-Pentyn-3-ol | 84.1 | 0.7 | 1.7 | 0.5 | 0.060 | 0.112 | 8.591 | 4.597 | 1.0 | 0.165 | 6.06 | 1273.2 | |
| 3-Butyn-2-ol | 70.1 | 0.1 | 1.8 | 3.6 | 0.200 | 0.118 | 17.96 | 30.43 | 40.4 | 0.543 | 74.40 | 176.1 | 11.63d |
| 3-Hexyne-2,5-diol | 114.2 | −0.1 | 1.4 | 1.4 | 0.050 | 0.082 | 27.80 | 16.89 | 9.5 | 0.350 | 27.14 | 329.2 | |
| 4-Heptyn-2-ol | 112.2 | 1.2 | 2.0 | 64.1 | <12.25 | 8.554 | >5.231 | 7.491 | 218.7 | 27.67 | 7.90 | 467.6 | |
| 4-Heptyn-3-ol | 112.2 | 1.7 | 1.7 | 40.9 | 9.560 | 11.31 | 4.278 | 3.615 | 96.0 | 16.12 | 5.96 | 120.2 | |
| 4-Hexyn-3-ol | 98.2 | 1.2 | 1.7 | 8.0 | 1.480 | 1.653 | 5.431 | 4.863 | 23.3 | 2.833 | 8.22 | 152.0 | |
| 3-Hexyn-2-ol | 98.2 | 1.2 | 1.7 | 5.8 | <1.24 | 0.899 | 4.652 | 6.417 | 21.9 | 1.845 | 11.87 | 30.3 | |
| 2-Methyl-5-octyn-4-ol | 140.2 | 2.7 | 1.6 | 17.7 | 3.920* | 3.117 | 4.507 | 5.667 | 40.0 | 9.243 | 4.33 | 55.8 | |
| 5-Methyl-1-hexyn-3-ol | 112.2 | 1.6 | 1.7 | 3.2 | 0.080* | 0.203 | 40.10 | 15.82 | 14.0 | 0.936 | 14.96 | 26.9 | |
| Tertiary propargylic alcohols | |||||||||||||
| 2-Methyl-3-butyn-2-ol | 84.1 | 0.3 | 1.8 | 1464.8 | 239.0 | 287.8 | 6.13 | 5.09 | 4567.8 | 724.4 | 6.31 | 1717.5 | 3290c |
| 3-Methyl-1-pentyn-3-ol | 98.2 | 1.1 | 1.7 | 355.9 | 61.56 | 78.68 | 5.78 | 4.52 | 945.9 | 192.0 | 4.93 | 2050.6 | 1220c |
| 1-Ethynyl-1-cyclohexanol | 124.2 | 1.7 | — | 72.6 | <12.06 | 7.194 | >6.016 | 10.09 | 305.4 | 29.59 | 10.32 | 256c | |
| 2,5-Dimethyl-3-hexyne-2,5-diol | 142.2 | 0.7 | 1.6 | 530.2 | <61.88* | 53.43 | >8.567 | 9.92 | 2098.5 | 193.5 | 10.84 | 5406.3 | |
| 3,5-Dimethyl-1-hexyne-3-ol | 126.2 | 2.0 | 1.7 | 50.7 | 9.640* | 5.966 | 5.26 | 8.80 | 198.4 | 24.07 | 8.24 | 447.8 | |
| 2-Phenyl-3-butyn-2-ol | 146.2 | 1.7 | 0.2 | 111.6 | <12.21 | 24.28 | >9.139 | 4.60 | 233.0 | 48.49 | 4.81 | 221.3 | 113c |
| 1,1-Diphenyl-2-propyn-1-ol | 208.3 | 2.7 | 0.2 | 7.2 | <0.99 | 0.994 | >7.287 | 7.26 | 27.3 | 2.969 | 9.20 | 11.1c | |
| 3,4-Dimethyl-1-pentyn-3-ol | 112.2 | 1.3 | — | 84.7 | <9.37 | 9.429 | >9.037 | 8.98 | 352.3 | 32.29 | 10.91 | 205c | |
| Mean | 11.62 | 8.583 | 11.79 | ||||||||||
Among all the propargylic alcohols tested, 1-pentyn-3-ol is the most toxic compound with its EC50 equal to 0.50 mg L−1, which can be classified as a “R50” compound (very toxic to aquatic organisms, EC50/LC50 < 1 mg L−1), following the current practice for classification of chemicals in the European Union (EU).20 There are several other compounds, such as 2-decyn-1-ol, 3-decyn-1-ol, 1-hexyn-3-ol, 3-butyn-2-ol, and 3-hexyne-2,5-diol, that deserve more attention for their possible adverse impact to the aquatic environment because these alcohols can be classified as “R51” compounds (toxic to aquatic organisms, EC50/LC50 between 1 and 10 mg L−1).
NOECs are within the range of 0.05–239 mg L−1. For about one-third (12 of 34, as indicated in Table 1) of the test compounds, identical NOEC values were obtained by both final yield and growth rate endpoints. For the rest of propargylic alcohols, NOEC values based on final yield are smaller than those derived by the growth rate endpoint. Currently, the growth rate endpoint is preferred by most ecotoxicologists to the biomass type endpoint (e.g., final yield) because algal growth rate is considered to be more stable, comparable, and ecologically relevant.21 However, the actual NOEC should be the toxicant concentration that caused no statistically significant difference as compared to the controls, with respect to both test endpoints (i.e., growth rate and final yield). Therefore, only NOECs based on final yield are listed in Table 1. The ratios between EC50 and NOEC (ACR1) ranged from 4.5 to 40, with the mean value equal to 11.6. ACR1 ratios for primary and tertiary propargylic alcohols appear to be slightly smaller than those for primary homo- and secondary alcohols. However, no significant difference was found among these propargylic alcohols.
In Table 1, the EC10 value derived from the final yield endpoint varies from 0.082 (3-hexyne-2,5-diol) to 287.8 mg L−1 (2-methyl-3-butyn-2-ol). In most cases, EC10s are apparently greater than NOEC values, which are in agreement with the present authors' previous work dealing with the low-toxic-effect concentrations derived by the closed-system technique.18 The mean value for ACR2 ratio (EC50/EC10) is 8.58. Similarly, based on algal growth rate, EC10s and acute-to-chronic ratios (ACR3) were calculated and are displayed in Table 1. The ACR3 value, with its mean equal to 11.79, is subject to large variations (from 2.0 to 74.0). Overall, the above NOECs, EC10s and ACR ratios will be useful for estimating the ultimate safety levels for various propargylic alcohols, for the protection of the aquatic environment.
Fig. 1(a) and (b) compare the species sensitivities for alga (final yield endpoint), ciliate,4 and fish.8 The units for LC50, IGC50, and EC50 are mmol L−1. From Fig. 1(a), one may find that the alga (Pseudokirchneriella subcapitata) is clearly more sensitive than ciliate (Tetrahymena pyriformis). For most primary propargylic alcohols, the differences are approximately one order in magnitude. With respect to secondary alcohols (reactive in nature), the difference can be greater than 3 orders in magnitude. On the other hand, fathead minnow revealed similar sensitivity as algae (Fig. 1(b)). However, primary propargylic alcohols appeared to be more toxic to fathead minnow than algae. In contrast, tertiary alcohols exerted stronger toxic effects on algae than those on fish.
 | ||
| Fig. 1 Comparison of species sensitivities. (a) Algae vs. ciliate, and (b) algae vs. fish. | ||
3.2 Modes of action
Fig. 2(a) and (b) display the relationships between toxicity (as log EC50−1) and log Kow, based on the final yield and growth rate endpoints. The straight lines represent the baseline toxicity relationships for narcotic mode of action (eqn (1) and (2)), which were derived in the authors' previous works:13,16| log (1/EC50)FY = 0.90log kow − 1.40, n = 48, r2 = 0.87 | (1) |
| log (1/EC50)GR = 0.974log kow − 1.95, n = 26, r2 = 0.943 | (2) |
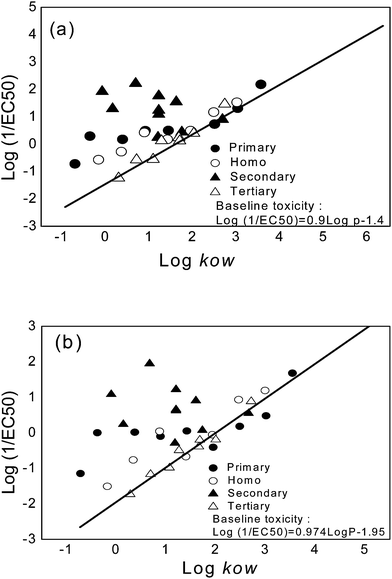 | ||
| Fig. 2 Excess toxicity of various propargylic alcohols in relation to the baseline toxicity. (a) The final yield and (b) the growth rate endpoints. | ||
The units for EC50 values are mmol L−1. For both endpoints, primary propargylic alcohols with small molecular weight (<100) and low hydrophobicity (log Kow < 1.5) exhibit apparently greater toxicity than that estimated by the baseline toxicity relationships. Primary propargylic alcohols with high molecular weight and hydrophobicity, on the other hand, appeared to be narcotic in nature. Such an observation agrees with the previous findings based on fathead minnow and ciliate.4–8 Furthermore, for secondary propargylic alcohols, the observed toxicity was 2 to 3 orders of magnitude higher than that predicted by the narcosis toxic action. Lipnick2 proposed that the excess toxicity observed from primary alcohols (with low molecular weight) and secondary alcohols was due to a proelectrophile toxicity mechanism. The observations from algae (the present study) provide additional support to Lipnick's theory. Finally, tertiary alcohols are obviously nonpolar narcotic compounds because the related data points fit very well with the baseline toxicity relationship. It is also clear that the baseline toxicity relationships (eqn (1) and (2)) derived previously are valid representations for the effects of narcotic compounds on phytoplankton.
3.3 Quantitative structure–activity relationships
Hydrophobicity (1-octanol–water partition coefficient, Kow) and the lowest unoccupied molecular orbital energy (Elumo) were used as descriptors for deriving QSARs for various propargylic alcohols. All EC50 values are in terms of mmol L−1. For primary propargylic alcohols (including primary homo-propargylic alcohols), descriptor log Kow was found to provide satisfactory description for chemical's toxicity (eqn (3) and (4)), except for 2-propyn-1-ol:| log (1/EC50)FY = 0.57log Kow − 0.37, n = 15, r2 = 0.9, Q2 = 0.87, S = 0.25, F = 118.9 | (3) |
| log (1/EC50)GR = 0.6log Kow − 0.9, n = 15, r2 = 0.76, Q2 = 0.69, S = 0.50, F = 42.1 | (4) |
The compound 2-propyn-1-ol, however, is an obvious outlier because a significant excess toxicity (>2.0) was observed. The same phenomenon was also reported by previous researchers4–6 and was owing to an enhanced Michael-type addition reaction as a result of biodegradation of 2-propyn-1-ol to the more reactive 2-propyn-l-al.22
log Kow and the lowest unoccupied molecular orbital energies (Elumo) were used to model the toxicity of secondary propargylic alcohols. The results of QSAR analyses show that neither of these descriptors alone is capable of describing the toxicity of secondary alcohols. Furthermore, several different QSAR formulations were applied for regression analyses, in order to improve data fitting. Eqn (5) and (6) are the final forms for describing the effects of secondary propargylic alcohols. The product term in the equations suggests that the influences of kow and Elumo on toxicity are not independent:
| log (1/EC50)FY = 2.20log kow − 1.10Elumo − 1.56log kow × Elumo + 3.40, n = 7, r2 = 0.93, S = 0.22, F = 12.6 | (5) |
| log (1/EC50)GR = 0.0014log kow − 2.10Elumo − 0.024log kow × Elumo + 4.00, n = 7, r2 = 0.85, S = 0.25, F = 5.74 | (6) |
With respect to the above expressions, three outliers (1-hexyn-3-ol, 1-pentyn-3-ol, and 5-methyl-1-hexyn-3-ol) have to be removed from regression in order to establish QSARs. A common point for these three compounds is that they all have the alkyne group attached to the 1-position on the carbon chain. It shows that, for secondary propargylic alcohols, 1-position substitution resulted in less steric hindrance on reactivity as compared to substitution on other positions. Therefore, these compounds are highly reactive and toxic. Similar phenomena can also be found from previous reports based on ciliate.7,23
For tertiary propargylic alcohols, as expected, the observed toxicity of all tertiary alcohols can be adequately described by log Kow because these compounds act via a narcotic mode of action. Eqn (7) and (8) are the QSARs with respect to the endpoints of final yield and growth rate:
| log (1/EC50)FY = 1.025log kow − 1.46, n = 8, r2 = 0.95, Q2 = 0.91, S = 0.20, F = 108.0 | (7) |
| log (1/EC50)GR = 1.01log kow − 1.96, n = 8, r2 = 0.97, Q2 = 0.94, S = 0.16, F = 164.7 | (8) |
Overall, the above QSARs provide satisfactory descriptions of the toxicity of primary and tertiary propargylic alcohols. The correlation coefficient (r2) varies from 0.76 to 0.97 and the cross-validation coefficient Q2 is between 0.69 and 0.94, suggesting that the correlation relationships are quite significant. For secondary alcohols, however, the QSARs (eqn (5) and (6)) provide satisfactory statistical fitting, but very poor predictivities (Q2 is equal to 0.483 and 0.0, respectively). This could be the reason that, in previous study, no valid QSAR was obtained with respect to secondary propargylic alcohols.7 Clearly, more effort is needed to further improve the QSARs for secondary propargylic alcohols.
4. Conclusions
The present study presents the toxicity data of 34 propargylic alcohols, including primary, primary homo-, secondary, and tertiary alcohols, on P. subcapitata in terms of EC50 and NOEC values. Among all the propargylic alcohols tested, 1-pentyn-3-ol is the most toxic compound with its EC50 equal to 0.50 mg L−1, which can be classified as a “R50” compound (very toxic to aquatic organisms, EC50/LC50 < 1 mg L−1), following the current practice for classification of chemicals in the European Union (EU). There are several other compounds, including 2-decyn-1-ol, 3-decyn-1-ol, 1-hexyn-3-ol, 3-butyn-2-ol, and 3-hexyne-2,5-diol, which deserve more attention for their possible adverse impact on the aquatic environment because these alcohols can be classified as “R51” compounds (toxic to aquatic organisms, EC50/LC50 between 1 and 10 mg L−1). Compared to the base-line toxicity relationship (narcosis QSAR) derived previously, tertiary propargylic alcohols can be identified as nonpolar narcotic chemicals, while secondary alcohols and primary alcohols with low molecular weight generally exhibit obvious excess toxicity in relation to the base-line toxicity. Finally, quantitative structure–activity relationships were established to correlate the observed toxicity with log Kow and Elumo values. The above toxicity data and QSARs will be useful for risk assessment and protection of the aquatic environments, because such information is not yet available in the existing toxicological databases.Acknowledgements
This research was supported by grants from the National Science Council, Taiwan, Republic of China (NSC 96-2221-E-009-057-MY3) and NCTU Program for Promoting Academic Excellence of Universities (NCTU 95W701-4).References
- U.S. EPA, HPV Challenge Program.http://www.epa.gov/chemrtk/volchall.htm, revision dated 9/18/2001, downloaded 10/18/2001 Search PubMed.
- R. L. Lipnick, QSAR in Toxicology and Xenobiochemistry, ed. M. Tichy, Elsevier, Amsterdam, 1985, pp. 39–52 Search PubMed.
- S. P. Bradbury and G. M. Christensen, Environ. Toxicol. Chem., 1991, 10, 1155–1160 Search PubMed.
- G. D. Veith, R. L. Lipnick and C. L. Russom, Xenobiotica, 1989, 19, 555–565 CrossRef CAS.
- T. W. Schultz and M. Tichy, Bull. Environ. Contam. Toxicol., 1993, 51, 681–688 Search PubMed.
- T. W. Schultz, T. S. Kissel and M. Tichy, Bull. Environ. Contam. Toxicol., 1994, 179–185 Search PubMed.
- T. W. Schultz, J. Seward-Nagel, K. A. Foster and V. A. Tucker, Environ. Toxicol., 2004, 19, 1–10 Search PubMed.
- O. G. Mekenyan, G. D. Veith, S. P. Bradbury and C. L. Russom, Quant. Struct.–Act. Relat., 1993, 12, 132–136 CrossRef CAS.
- J. D. Walker, THEOCHEM, 2003, 622, 167–184 Search PubMed.
- Organisation for Economic Co-operation and Development, Aquatic Toxicity Testing of Difficult Substances and Mixtures, Guidance Document 23, Environmental Health and Safety Publications, Paris, France, 2000 Search PubMed.
- U.S. Environmental Protection Agency, Ecological Effect Test Guidelines. OPPTS 850.5400. Algal Toxicity, Tiers I and II, Washington, DC, 1996 Search PubMed.
- European Centre for Ecotoxicology and Toxicology of Chemicals, Aquatic Toxicity Testing of Sparingly Soluble, Volatile and Unstable Substances. Monograph 26, Brussels, Belgium, 1996 Search PubMed.
- K. P. Tsai and C. Y. Chen, Environ. Toxicol. Chem., 2007, 26, 1931–1939 Search PubMed.
- J. H. Lin, W. C. Kao, K. P. Tsai and C. Y. Chen, Water Res., 2005, 39, 1869–1877 CrossRef CAS.
- C. Y. Chen and J. H. Lin, Chemosphere, 2006, 62, 503–509 CrossRef CAS.
- S. H. Hsieh, C. H. Hsu, D. Y. Tasi and C. Y. Chen, Environ. Toxicol. Chem., 2006, 25, 2920–2926 Search PubMed.
- C. Y. Chen, C. W. Ko and P. I. Lee, Environ. Toxicol. Chem., 2007, 26, 1158–1164 Search PubMed.
- C. Y. Chen, Y. J. Wang and C. F. Yang, Ecotoxicol. Environ. Saf., 2009, 72, 1514–1522 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, V. G. Zakrzewski, J. A. Montgomery, R. E. Stratmann, J. C. Burant, S. Dapprich, J. M. Millam, A. D. Daniels, K. N. Kudin, M. C. Strain, O. Farkas, J. Tomasi, V. Barone, M. Cossi, R. Cammi, B. Mennucci, C. Pomelli, C. Adamo, S. Clifford, J. Ochterski, G. A. Petersson, P. Y. Ayala, Q. Cui, K. Morokuma, D. K. Malick, A. D. Rabuck, K. Raghavachari, J. B. Foresman, J. Ciolowski, J. V. Ortiz, B. B. Stefanov, G. Liu, A. Liashenko, P. Piskorz, I. Komaromi, R. Gomperts, R. L. Martin, D. J. Fox, T. Keith, M. A. Al-Laham, C. Y. Peng, A. Nanayakkara, C. Gonzalez, M. Challacombe, P. M. W. Gill, B. G. Johnson, W. Chen, M. W. Wong, J. L. Andres, M. Head-Gordon, E. S. Replogle and J. A. Pople, Gaussian 98, Rev A.7, Gaussian, Pittsburgh, PA, USA, 1999 Search PubMed.
- European Commission, Council Directive 92/32/EEC of 5 June 1992 Amending for the 7th time Council Directive 67/548/EEC, Official Journal of European Communities L152, Brussels, Belgium, 1992 Search PubMed.
- A. Weyers and G. Vollmer, Chemosphere, 2000, 41, 1007–1010 Search PubMed.
- E. G. DeMaster, T. Dahlseid and B. Redfern, Chem. Res. Toxicol., 1994, 7, 414–419 Search PubMed.
- T. W. Schultz, J. W. Yarbrough and T. B. Pilkington, Environ. Toxicol. Pharmacol., 2007, 23, 10–17 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |