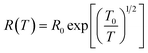Highly conductive poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) films treated with an amphiphilic fluoro compound as the transparent electrode of polymer solar cells
Yijie
Xia
,
Kuan
Sun
and
Jianyong
Ouyang
*
Department of Materials and Engineering, National University of Singapore, Singapore 117574. E-mail: mseoj@nus.edu.sg
First published on 9th November 2011
Abstract
Flexible transparent electrode materials are strongly needed for optoelectronic devices. We report a novel method to significantly enhance the conductivity of poly(3,4-ethylenedioxythiophene):poly(styrene sulfonate) (PEDOT:PSS) films through treatment with a fluoro compound, hexafluoroacetone (HFA). HFA hydrolyzes with water into a geminal diol, 1,1,1,3,3,3-hexafluoropropane-2,2-diol (HFP2OH) that has two –OH groups connected to the middle carbon atom. The conductivity increased from 0.3 to 1164 and 1325 S cm−1 after the treatment with HFA once and four times, respectively. The highly conductive HFA-treated PEDOT:PSS films can have a sheet resistances of 46 Ω □−1 and a transparency of around 83% at 550 nm. These values are comparable to those of indium tin oxide (ITO) on polyethylene terephthalate (PET). The conductivity enhancement is attributed to the HFP2OH-induced phase segregation of some hydrophilic PSSH chains from PEDOT:PSS and the conformational change of the conductive PEDOT chains, driven by the interactions between amphiphilic HFP2OH and PEDOT:PSS. The hydrophobic –CF3groups of HFP2OH preferentially interact with the hydrophobic PEDOT chains of PEDOT:PSS, while the hydrophilic –OH groups preferentially interact with hydrophilic PSS chains. The highly conductive PEDOT:PSS films were used to replace ITO as the transparent anode of polymer solar cells. Polymer solar cells based on poly(3-hexylthiophene) (P3HT) and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) exhibited a photovoltaic efficiency of 3.57% under simulated AM1.5G illumination, comparable to the control devices with ITO as the anode.
Broader contextOptoelectronic devices, such as liquid-crystal displays (LCDs), solar cells, and touch panel displays, are indispensable units in many advanced technologies. An optoelectronic device requires at least one electrode to be transparent to emit or harvest light. Indium tin oxide (ITO) is traditionally the popular material as the transparent electrode. However, the scarce indium on the earth and the brittleness of indium tin oxide (ITO) can limit the development of optoelectronic devices. Therefore, there is strong demand for new transparent conductive materials to replace ITO. In this paper, we report a facile way to significantly enhance the conductivity of poly(3,4-ethylene dioxythiophene):poly(styrene sulfonate) (PEDOT:PSS). The conductivity of PEDOT:PSS films is dramatically enhanced from 0.3 to 1164 S cm−1 after being treated once with an amphiphilic compound, hexafluoroacetone (HFA). The conductivity can increase to 1325 S cm−1 after PEDOT:PSS films are treated with HFA four times. HFA-treated PEDOT:PSS films can have a sheet resistances of 46 Ω □−1 and a transparency above 80% in the visible range. These values are comparable to that of ITO on polyethylene terephthalate (PET), rendering them as a substitute of ITO as the transparent electrode. High-performance polymer solar cells with HFA-treated PEDOT:PSS films as the transparent electrode are demonstrated. |
1. Introduction
Nowadays, optoelectronic devices, such as liquid crystal displays (LCDs), light-emitting diodes (LEDs), solar cells, touch panel displays, lasers, and detectors, have been attracting intense attention, due to their strong application in many aspects.1,2 Their market has been huge and has been rapidly expanding. For optoelectronic devices, at least one electrode is required to be transparent in order to emit or harvest light. Currently, the most popular material used as the transparent electrode is indium tin oxide (ITO). However, ITO is expensive. It substantially increases the cost of optoelectronic devices, particularly for the low-end optoelectronic devices like solar cells. The scarcity of indium also raises concerns of the ITO availability in the long term. In addition, the high mechanical brittleness of ITO makes it unsuitable for flexible electronic devices, which are regarded as the next-generation electronic devices.3,4 Therefore, new transparent conductive materials with high mechanical flexibility are urgently needed to replace ITO. Many materials, including conducting polymers,5–17carbon nanotubes,18–22graphenes,23,24 and metal nanowires,25 have been investigated as the transparent electrode of optoelectronic devices. Among them, poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) (chemical structure shown in Scheme 1) is very promising to be the next-generation transparent electrode material. PEDOT:PSS is the most successful conducting polymer in terms of commercial application. It can be dispersed in water and some organic solvents, and high-quality PEDOT:PSS films can be readily coated on substrates through conventional solution processing techniques, such as coating and printing.26–28 In addition, PEDOT:PSS films have high transparency in the visible range, high mechanical flexibility, and excellent thermal stability. Nevertheless, PEDOT:PSS suffers a problem of low conductivity. An as-prepared PEDOT:PSS film from aqueous PEDOT:PSS solution usually has a conductivity below 1 S cm−1, which is remarkably lower than ITO.27,29 Hence, it is important to significantly enhance the conductivity of PEDOT:PSS films for their application as the transparent electrode of optoelectronic devices.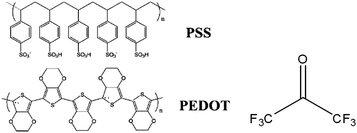 | ||
| Scheme 1 The chemical structures of PEDOT:PSS and HFA. | ||
Several methods have been reported to significantly enhance the conductivity of PEDOT:PSS, including the addition of an organic compound, such as ethylene glycol (EG), dimethyl sulfoxide (DMSO), an ionic liquid, an anionic surfactant, or dimethyl sulfate, into PEDOT:PSS aqueous solution15,30–39 and the treatment of PEDOT:PSS films with a polar organic compound, a certain salt, a zwitterion, a carboxylic or inorganic acid, or cosolvent.40–45 Most of the research work has been carried out on PEDOT:PSS aqueous solutions commercially supplied by H. C. Starck or Agfa, and the conductivity obtained depends on the grade of the PEDOT:PSS aqueous solutions. The conductivity of PEDOT:PSS films prepared from Clevios PH 1000 PEDOT:PSS aqueous solution can be enhanced to 680 ± 50 S cm−1 after treatment with EG or DMSO.46 Recently, Kim et al. observed conductivity up to 1418 S cm−1 by treating PEDOT:PSS films twice with EG.47 The first treatment is the addition of EG into the Clevios PH 1000 aqueous solution. The PEDOT:PSS films prepared from the Clevios PH 1000 aqueous solution with added EG are successively treated in an EG bath for 30 min at room temperature.
Here, we report a facile method to significantly enhance the conductivity of PEDOT:PSS films through treatment with a fluoro compound, hexafluoroacetone (HFA, chemical structure shown in Scheme 1). HFA hydrolyzes into 1,1,1,3,3,3-hexafluopropane-2,2-diol (HFP2OH) with water. HFP2OH is a geminal diol with two –OH groups connected to the middle carbon atom and is highly amphiphilic arising from the hydrophobic –CF3 and hydrophilic –OH groups. The conductivity of the PEDOT:PSS films is enhanced to 1164 S cm−1 after treatment once, which is much higher than that through the treatment with EG or DMSO once. We also demonstrated the application of HFA-treated PEDOT:PSS films as the transparent electrode of high-performance polymer solar cells (PSCs).
2. Experimental
2.1. Materials and treatment of PEDOT:PSS films
PEDOT:PSS aqueous solution (Clevios™ PH 1000) was purchased from H. C. Starck. The concentration of PEDOT:PSS was 1.3% by weight, and the weight ratio of PSS to PEDOT was 2.5 in solution. All other chemicals, including HFA·3H2O were obtained from Sigma-Aldrich. All the materials were used as received.PEDOT:PSS films were prepared by spin coating the Clevios PH1000 aqueous solution on 1.3 × 1.3 cm2 glass substrates, which were pre-cleaned successively with detergent, de-ionized (DI) water, acetone and IPA. The PEDOT:PSS films were dried at 120 °C on a hot plate for 15 min. The HFA treatment was performed by dropping 100 μL pure HFA·3H2O or an aqueous solution of HFA on a PEDOT:PSS film on a hot plate at 140 °C. The films dried after about 5 min. They were cooled down to room temperature, and then were rinsed with DI water and dried at 140 °C again. Some PEDOT:PSS films were treated multiple times. Thick PEDOT:PSS films were prepared by spin coating the PEDOT:PSS aqueous solution multiple times. The PEDOT:PSS films were dried after each spin coating, and an HFA treatment was carried out after each spin coating.
2.2. Characterization of PEDOT:PSS films
The conductivities of the polymer films were measured by the van der Pauw four-point probe technique with a Keithley 2400 source/meter. The electrical contacts were made by pressing indium on the four corners of each PEDOT:PSS film on a glass substrate. The temperature dependences of the resistivities of the untreated and HFA-treated PEDOT:PSS films were tested using a Janis Research VPF-475 dewar with liquid nitrogen as coolant and a Conductus LTC-11 temperature controller. The UV-Vis-NIR absorption spectra of the polymer films were taken with a Varian Cary 5000 UV-Vis-NIR spectrometer, and the AFM images of the polymer films were obtained using a Veeco NanoScope IV Multi-Mode AFM with the tapping mode. The X-ray photoelectron spectroscopy (XPS) spectra were collected with an Axis Ultra DLD X-ray photoelectron spectrometer equipped with an Al KαX-ray source (1486.6 eV). The thicknesses of the polymer films were determined with an Alpha 500 step profiler. Cyclic voltammetric (CV) measurements were carried out with a ECO CHEMIE AUTOLAB PGSTAT 302N + FRA2 system in 0.1 M NaCl solution with a Au disc coated with a PEDOT:PSS film as the working electrode. The PEDOT:PSS films for the CVs were prepared by dropping PEDOT:PSS aqueous solution on a Au disc with a diameter of 2 mm and subsequently drying at 120 °C. A Pt wire and Ag/AgCl (3 M NaCl) were used as the counter and reference electrode, respectively. The scan rate was 50 mV s−1. The X-ray diffractions (XRD) of the thin films were analyzed using a Bruker D8 Advance X-ray diffractometer equipped with Cu Ka1 radiation (40 kV, 40 mA).2.3. Fabrication and characterization of PSCs
The highly conductive PEDOT:PSS films on glass were used as the transparent anode of PSCs. Scheme 2a illustrates the device architecture of the ITO-free PSCs. Poly(3-hexylthiophene) (P3HT) and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM), whose chemical structures are shown in Scheme 2b, were used as the donor and acceptor in the active layer. The PSCs were fabricated through the following process. A 50 nm-thick less conductive PEDOT:PSS (Clevios P VP Al 4083) was spin coated on an HFA-treated PEDOT:PSS film as a buffer layer. The active layer was formed by spin coating a 1,2-dichlorobenzene solution consisting of 18 mg mL−1P3HT and 18 mg mL−1 PCBM at 500 rpm for 1 min in a glove box filled with nitrogen. It was dried at room temperature for 3 h and had a thickness of ca. 200 nm. The devices were completed by thermally depositing a 40 nm-thick Ca layer and subsequently a 200 nm-thick Al layer in a vacuum of 10−6 mbar. Each PSC had an active area of 0.11 cm2.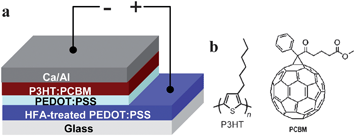 | ||
| Scheme 2 The device architecture (a) and the chemical structure (b) of P3HT and PCBM. | ||
The polymer solar cells were encapsulated with a UV-curable epoxy and glass sheets in the glove box. They were then taken out for the electrical testing. The photovoltaic performance of the devices was measured with a computer-programmed Keithley 2400 source/meter. The light source was a Newport's Oriel class A solar simulator, which simulated AM1.5G sunlight (100 mW cm−2) and was certified to the JIS C 8912 standard.
3. Results and discussion
3.1. HFA-induced conductivity enhancement of PEDOT:PSS films
Hydrated HFA, HFA·3H2O, was used as in this study. It is a liquid at room temperature. HFA readily hydrolyzes with water into HFP2OH, a geminal diol (Scheme 3). The formation of the geminal diol is due to the two electron-withdrawing –CF3groups. The equilibrium constant of the hydrolysis is 106 at room temperature. Thus, HFP2OH is the majority while HFA is the minority in HFA aqueous solution or HFA·3H2O. They are not strictly distinguished in this manuscript. Treatment of PEDOT:PSS with a geminal diol is expected to enhance the conductivity of PEDOT:PSS even more significantly than normal polyols with only one –OH group connected to a carbon atom, as normal diols like EG can significantly enhance the conductivity of PEDOT:PSS while alcohols with only one –OH group usually have a negligible effect on the conductivity of PEDOT:PSS.32 Moreover, HFP2OH is a highly amphiphilic compound with the hydrophobic –CF3 and hydrophilic –OH groups. The amphiphilic groups can preferentially interact with the hydrophobic PEDOT and hydrophilic PSS chains of PEDOT:PSS, and these interactions can give rise to conductivity enhancement as well.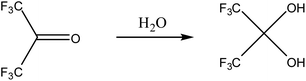 | ||
| Scheme 3 Hydrolysis of HFA. | ||
Treatment of PEDOT:PSS films with HFA can significantly enhance the conductivity. Fig. 1 presents the conductivities of PEDOT:PSS films after treatment with aqueous solutions of different HFA concentrations. The as-prepared PEDOT:PSS films had a conductivity of 0.3 S cm−1. The conductivity was significantly enhanced after the treatment, and the conductivity increased with the increasing HFA concentration. The highest conductivity of 1164 S cm−1 was observed for the treatment with pure HFA·3H2O. This conductivity is saliently higher than that of PEDOT:PSS treated with EG or DMSO once. The highest conductivity is only 735 S cm−1 for the PEDOT:PSS films treated with EG in our experiments, and it is 680 ± 50 S cm−1 by adding 5% DMSO into the PH1000 aqueous solution as reported by other labs.45,46 To the best of our knowledge, the conductivity of 1164 S cm−1 is the highest for PEDOT:PSS films through a treatment once.
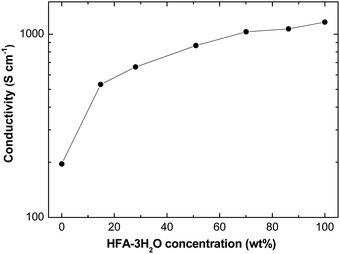 | ||
| Fig. 1 Conductivities of PEDOT:PSS films after treatment with HFA solutions of various concentrations at 140 °C. | ||
The conductivity of PEDOT:PSS films can be further enhanced through the treatment with HFA multiple times. It reaches 1325 S cm−1 after treatment with HFA·3H2O four times. This conductivity is comparable to that of the PEDOT:PSS films reported by Kim et al. by adding EG into the Clevious PH1000 PEDOT:PSS aqueous solution and subsequent treatment of the polymer films in an EG bath.47 We repeated the two-step treatment of PEDOT:PSS with EG as reported by Kim et al.47 and observed a conductivity of only 882 S cm−1. The lower conductivity than that of Kim et al. is probably related to the different Clevious PH1000 PEDOT:PSS batches used in our lab and their lab.
The conductivity enhancement also depends on the temperature during the treatment (Fig. 2). The conductivity increases with the elevating temperature from 80 to 140 °C, and then slightly drops when the temperature is further increased to 200 °C, but the conductivity is still more than 1100 S cm−1 even when the treating temperature is 200 °C. The HFA-treated PEDOT:PSS films are more thermally stable than those treated with other compounds, such as salts, zwitterions, acids and cosolvents.41–44 But the optimal treating temperature of 140 °C coincides with that of the treatment methods with other compounds.41–44 Hence, the effect of the temperature during the treatment on the conductivity of the PEDOT:PSS films should be related to the thermal properties of the PEDOT:PSS films.
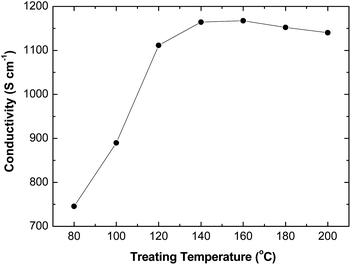 | ||
| Fig. 2 Conductivities of PEDOT:PSS films after treatment with HFA·3H2O at various temperatures. | ||
As-prepared PEDOT:PSS films had a thickness of 60 nm. The thickness decreased to 50 nm after treatment with HFA once, and HFA-treated PEDOT:PSS films had a sheet resistance of 172 Ω □−1. In order to lower the sheet resistance, thick PEDOT:PSS films were prepared by spin coating the Clevios PH1000 aqueous solution multiple times. An HFA treatment was carried out after spin coating each PEDOT:PSS layer. Four-layer PEDOT:PSS films had a thickness of 164 nm and a conductivity of 1319 S cm−1 after the HFA treatment. The corresponding sheet resistance was 46 Ω □−1. It is comparable or even lower than that of ITO coated on PET, which is about 50 Ω □−1.
3.2. Optical and electrical properties of PEDOT:PSS films before and after the HFA treatment
The PEDOT:PSS films are smooth and homogeneous after the HFA treatment. Their transmittance in the visible range is not affected by the HFA treatment. Fig. 3 presents the transmittance spectra of HFA-treated PEDOT:PSS films with different thicknesses in the visible range. The transmittance of the 50 nm-thick PEDOT:PSS film, which has a sheet resistance of 172 Ω □−1, is 94% at 550 nm. The transmittance is above 90% in the visible wavelength range from 400 to 700 nm. The transmittance of the 164 nm-thick PEDOT:PSS film, which has a sheet resistance of 46 Ω □−1, is more than 80% at 550 nm. The high conductivity and transparency indicate that the HFA-treated PEDOT:PSS films can be used as the transparent conductive electrode of optoelectronic devices.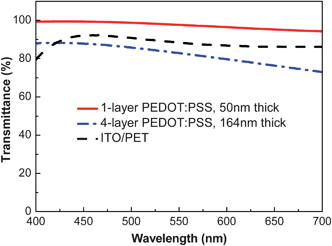 | ||
| Fig. 3 Transmittance spectra of PEDOT:PSS films. The transmittance spectrum of ITO/PET, which has a sheet resistance of 50 Ω □−1, is present for comparison. | ||
The conductivity of PEDOT:PSS films was studied from room temperature down to 110 K (Fig. 4a). When the temperature is below 200 K, the resistance of the HFA-treated PEDOT:PSS film decreases with the temperature increase like untreated PEDOT:PSS. It becomes almost constant at temperatures above 200 K. This suggests that the HFA-treated PEDOT:PSS film behaves almost like a metal or semimetal. It is very rare to observe metallic behavior for conductive PEDOT films, particularly when they are formed by solution processing.48 This is consistent with the high conductivity of the HFA-treated PEDOT:PSS films.
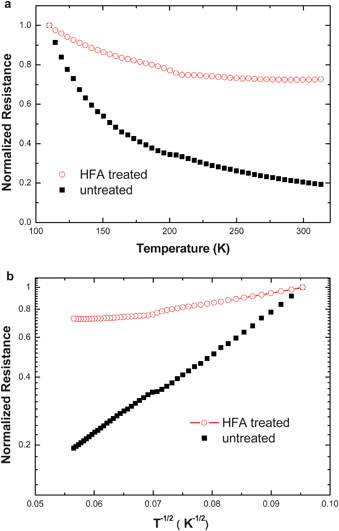 | ||
| Fig. 4 Temperature dependences of the normalized resistances of untreated and HFA-treated PEDOT:PSS films. a: Normalized resistances of untreated and HFA-treated versus temperature. b: Analysis of the resistance–temperature relationships of the untreated and HFA-treated PEDOT:PSS films with the VRH model. The resistances are normalized to that of the corresponding PEDOT:PSS films at 110 K. | ||
Fig. 4b shows the analysis of the temperature dependences of the resistances of untreated and HFA-treated PEDOT:PSS films with the one-dimensional variable range hopping (VRH) model,30,35,49
3.3. Mechanism for the conductivity enhancement by the HFA treatment
The mechanism for the conductivity enhancement though the HFA treatment was studied by various chemical and physical characterizations. The UV absorption spectra of untreated and HFA-treated PEDOT:PSS films are shown in Fig. 5. The two absorption bands in the UV range originate from the aromatic rings of PSS. Their intensity drops after the HFA treatment. This change indicates the decrease of the PSSH amount in PEDOT:PSS.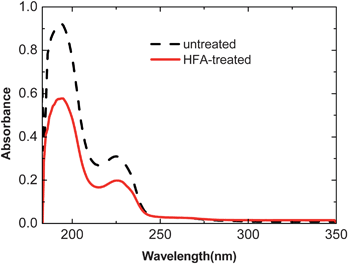 | ||
| Fig. 5 UV absorption spectra of PEDOT:PSS films before and after treatments with HFA. | ||
The reduction of PSSH from the PEDOT:PSS films after the HFA treatment is confirmed by XPS spectra of the PEDOT:PSS films (Fig. 6). The two XPS bands with binding energies between 166 and 172 eV are the S 2p band of the sulfur atoms in PSS, whereas the two XPS bands with binding energies between 162 and 166 eV are the S 2p band of the sulfur atoms in PEDOT.50,51 The S 2p XPS intensity ratio of PEDOT to PSS increases after the HFA treatment.
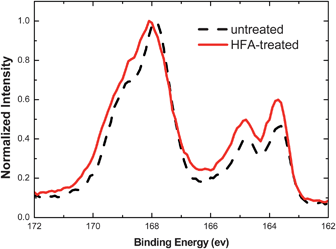 | ||
| Fig. 6 S 2p XPS spectra of untreated and HFA-treated PEDOT:PSS films. | ||
No fluorine XPS signal was detected on HFA-treated PEDOT:PSS films. Thus, no HFA remains in the PEDOT:PSS films after the treatment. HFA may completely vaporize during annealing of the PEDOT:PSS films, because the boiling points are 106 °C and −28 °C for HFA·3H2O and unhydrated HFA, respectively.
The surface morphology of the PEDOT:PSS films changes after the HFA treatment, as revealed by the AFM images (Fig. 7). Both the untreated and HFA-treated PEDOT:PSS films have a quite smooth surface. The rms roughnesses are 1.63 and 1.68 nm for the untreated and HFA-treated PEDOT:PSS films, respectively, but a remarkable difference in the AFM images can be observed. The untreated PEDOT:PSS film consists of mainly polymer particles with a size of about 50 nm. These polymer particles turn into entangled wires with a diameter of tens of nanometres after the HFA treatment. The AFM results are consistent with the energy barriers for the charge hopping obtained by the temperature dependences of the conductivities of the untreated and HFA-treated PEDOT:PSS films. The conductive nanowires can promote the charge hopping in comparison with the particles.
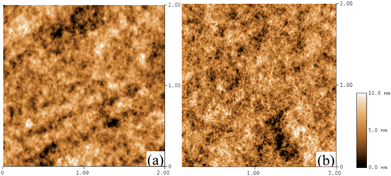 | ||
| Fig. 7 AFM height images of (a) untreated and (b) HFA-treated PEDOT:PSS films. The unit is μm. | ||
The AFM images suggest the conformational change of the polymer chains after the HFA treatment. It is further evidenced by the electrochemical activity of untreated and HFA-treated PEDOT:PSS films, which is quite sensitive to the chain conformation.33,52CVs of PEDOT:PSS films untreated, treated with HFA·3H2O and an aqueous solution of 15 wt% HFA·3H2O are presented in Fig. 8. The untreated PEDOT:PSS film exhibits electrochemical activity only at potentials higher than −0.2 V vs.Ag/AgCl, whereas additional electrochemical activity appears at potentials down to −0.8 V vs.Ag/AgCl for the HFA-treated PEDOT:PSS films. The electrochemical activities of the untreated and treated PEDOT:PSS films are consistent with the conductivities of the PEDOT:PSS films. The higher conductivity of the PEDOT:PSS films corresponds to more redox behavior in a more negative potential range. The PEDOT:PSS film treated with an aqueous solution of 15 wt% HFA·3H2O has a conductivity of 532 S cm−1, the peak potentials of the redox pair in the lowest potential range are −0.57 V and −0.54 V vs.Ag/AgCl, while this redox pair can not be observed for the untreated PEDOT:PSS film. The peak potentials of this redox pair shift to −0.68 V and −0.64 V vs.Ag/AgCl for the PEDOT:PSS film treated with HFA·3H2O. Presumably, the additional electrochemical activity for the HFA-treated PEDOT:PSS film can be ascribed to the decrease in the PSSH amount and the conformational change of the PEDOT chains. The PEDOT chains with a linear or extended-coil conformation can be electrochemically reduced and subsequently oxidized, while the reduction and oxidation are difficult for the PEDOT chains with a coil conformation surrounded by a relatively thick shell rich of PSSH.
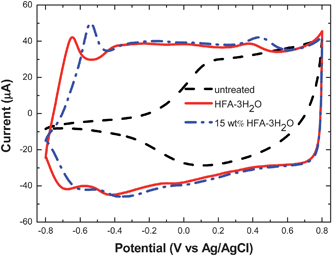 | ||
| Fig. 8 Cyclic voltammograms of PEDOT:PSS films untreated, treated with HFA·3H2O, and an aqueous solution of 15 wt% HFA·3H2O in 0.1 M NaCl solution. | ||
These experimental results indicate that HFA can induce the reduction of PSSH and the conformational change of the PEDOT chains in PEDOT:PSS films. The germinal diol, HFP2OH corresponding to HFA, is an amphiphilic compound with the hydrophobic –CF3 and hydrophilic –OH groups. The hydrophobic –CF3groups of HFP2OH preferentially interact with the hydrophobic PEDOT chains, whereas the hydrophilic –OH groups of HFP2OH preferentially interact with the hydrophilic PSS chains of PEDOT:PSS (Scheme 4). As a result of these interactions, the PEDOT chain is separated from the PSS chain by HFA. This gives rise to the reduction in the Coulombic attraction between PEDOT and PSS. Thus, phase separation occurs between the hydrophobic PEDOT and hydrophilic PSS chains. The Coulombic attraction even disappears when the PSS chains become PSSH chains by taking protons from other PSS chains. These PSSH chains can segregate from PEDOT:PSS and be removed during the post-treatment rinse with water.
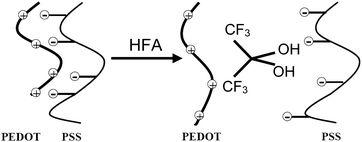 | ||
| Scheme 4 Schematic structures of PEDOT:PSS before and after HFA treatment. | ||
The germinal diol structure of HFP2OH can be the reason for the more significant conductivity enhancement than that by EG. The highly hydrophilic OH groups should be important for the conductivity enhancement. When hexachloroacetone was used to replace HFA for the treatment of the PEDOT:PSS films, no remarkable conductivity enhancement was observed. Hexachloroacetone hardly hydrolyzes into a geminal diol because of the weaker electronegativity of the chlorine atom than the fluorine atom.
It has been understood that PEDOT:PSS has a core/shell structure in water and in the as-prepared PEDOT:PSS film.53 The shell is rich of insulating PSS, while the core is rich of conductive PEDOT. The HFA-induced PSSH reduction can reduce the thickness of the less conductive shell and benefit the charge transport across the PEDOT chains. Thus, the energy barrier for the interchain and inter-domain charge hopping is lowered, giving rise to significant conductivity enhancement.
Moreover, HFA can induce the conformational change of the PEDOT chains in the PEDOT:PSS films. The hydrophobic PEDOT chains are stabilized by the hydrophilic PSS chains in water, so that the PEDOT chains follow the coil conformation of PSS chains due to the Coulombic attraction between them. The coil conformation is conserved in the as-prepared PEDOT:PSS films. The PEDOT chains turn to an extended-coil or linear conformation as a result of the Coulombic screening between PSS and PEDOT by HFA. The linear PEDOT chains can have stronger inter-chain interactions, which facilitates the inter-chain charge transport.
3.4. Application of highly conductive PEDOT:PSS in polymer PVs
The HFA treatment significantly enhances the conductivity while it does not affect the transparency in the visible range of the PEDOT:PSS films. As studied by ultraviolet photoelectron spectroscopy (UPS), the work function of PEDOT:PSS films almost does not change after the HFA treatment. The HFA-treated PEDOT:PSS films were thus used to replace ITO as the transparent electrode of PSCs.The device architecture is illustrated in Scheme 2. There is no ITO in these PSCs. The current density (J)–voltage (V) curves of these PSCs are presented in Fig. 9. The device with an HFA-treated PEDOT:PSS film as the anode exhibits high photovoltaic performance: short-circuit current density (Jsc) of 9.44 mA cm−2, open-circuit voltage (Voc) of 0.59 V, fill factor (FF) of 0.64, and power conversion efficiency (PCE) of 3.57%. The photovoltaic performance is comparable to that of the control device using an ITO anode. The photovoltaic performance of the control device is: Jsc of 10.00 mA cm−2, Voc of 0.59 V, FF of 0.70, and PCE of 4.13%.
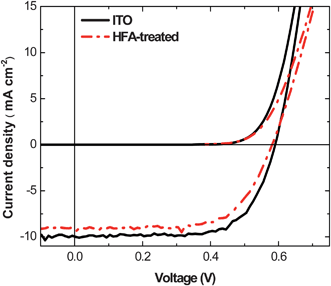 | ||
| Fig. 9 J–V characteristics of polymer PVs with ITO and HFA-treated PEDOT:PSS electrodes under 100 mW cm−2 AM1.5G illumination. | ||
4. Conclusions
In conclusion, the conductivity of as-prepared PEDOT:PSS films from the Clevios PH 1000 aqueous solution can be significantly enhanced through treatment with HFA. HFA hydrolyzes with water into a geminal diol, HFP2OH. Conductivity enhancement from 0.3 S to 1164 S cm−1 was observed after one treatment. It was further increased to 1325 S cm−1 after treatment four times. HFA-treated PEDOT:PSS films with multiple layers can have sheet resistances of 46 Ω □−1 and transparency over 80% in the visible range, comparable to ITO/PET. The conductivity enhancement is attributed to the HFP2OH-induced segregation of PSSH chains from PEDOT:PSS and the conformational change of the conductive PEDOT chains driven by the interactions between amphiphilic HFP2OH and PEDOT:PSS. The highly hydrophobic –CF3groups of HFP2OH preferentially interact with the hydrophobic PEDOT of PEDOT:PSS, while the highly hydrophilic –OH groups preferentially interact with hydrophilic PSS chains. The HFA-treated PEDOT:PSS films with high conductivity and high transparency can replace ITO as the transparent electrode of optoelectronic devices. PSCs with a HFA-treated PEDOT:PSS film as the transparent anode exhibit high photovoltaic performance, comparable to the control devices with ITO as the anode.Acknowledgements
This research work is financially supported by a research grant from the Ministry of Education, Singapore (R-284-000-073-112).Notes and references
- D. J. Lipomi and Z. Bao, Energy Environ. Sci., 2011, 4, 3314 RSC.
- D. Gendron and M. Leclerc, Energy Environ. Sci., 2011, 4, 1225 RSC.
- O. Inganäs, Nat. Photonics, 2011, 5, 201 CrossRef.
- A. Chipman, Nature, 2007, 449, 131 CrossRef CAS.
- S. I. Na, S. S. Kim, J. Jo and D. Y. Kim, Adv. Mater., 2008, 20, 4061 CrossRef CAS.
- W. H. Kim, Appl. Phys. Lett., 2002, 80, 3844 CrossRef CAS.
- F. Zhang, M. Johansson, M. R. Andersson, J. C. Hummelen and O. Inganäs, Adv. Mater., 2002, 14, 662 CrossRef CAS.
- C. S. Lee, J. Y. Kim, D. E. Lee, Y. K. Koo, J. Joo, S. Han, Y. W. Beag and S. K. Koh, Synth. Met., 2003, 135–136, 13 CrossRef CAS.
- A. A. Argun, A. Cirpan and J. R. Reynolds, Adv. Mater., 2003, 15, 1338 CrossRef CAS.
- J. Ouyang, C. W. Chu, F. C. Chen, Q. Xu and Y. Yang, Adv. Funct. Mater., 2005, 15, 203 CrossRef CAS.
- J. Ouyang and Y. Yang, Adv. Mater., 2006, 18, 2141 CrossRef CAS.
- J. E. Yoo, J. E. Yoo, K. S. Lee, A. Garcia, J. Tarver, E. D. Gomez, K. Baldwin, Y. Sun, H. Meng, T.-Q. Nguyen and Y.-L. Loo, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 5712 CrossRef CAS.
- J. H. Huang, D. Kekuda, C. W. Chu and K. C. Ho, J. Mater. Chem., 2009, 19, 3704 RSC.
- S. I. Na, G. Wang, S.-S. Kim, T.-W. Kim, S.-H. Oh, B.-K. Yu, T. Lee and D.-Y. Kim, J. Mater. Chem., 2009, 19, 9045 RSC.
- Y. S. Hsiao, W. T. Whang, C. P. Chen and Y. C. Chen, J. Mater. Chem., 2008, 18, 5948 RSC.
- Y. M. Chang, L. Wang and W. F. Su, Org. Electron., 2008, 9, 968 CrossRef CAS.
- M. W. Lee, M. Y. Lee, J. C. Choi, J. S. Park and C. K. Song, Org. Electron., 2010, 11, 854 CrossRef CAS.
- Z. C. Wu, Z. H. Chen, X. Du, J. M. Logan, J. Sippel, M. Nikolou, K. Kamaras, J. R. Reynolds, D. B. Tanner, A. F. Hebard and A. G. Rinzler, Science, 2004, 305, 1273 CrossRef CAS.
- M. Zhang, S. Fang, A. A. Zakhidov, S. B. Lee, A. E. Aliev, C. D. Williams, K. R. Atkinson and R. H. Baughman, Science, 2005, 309, 1215 CrossRef CAS.
- G. Gruner, J. Mater. Chem., 2006, 16, 3533 RSC.
- P. Joshi, L. Zhang, Q. Chen, D. Galipeau, H. Fong and Q. Qiao, ACS Appl. Mater. Interfaces, 2010, 2, 3572 CrossRef CAS.
- X. Mei and J. Ouyang, Carbon, 2011, 49, 5389 CrossRef CAS.
- K. S. Kim, K. S. Kim, Y. Zhao, H. Jang, S. Yoon Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi and B. H. Hong, Nature, 2009, 457, 706 CrossRef CAS.
- H. A. Becerril, J. Mao, Z. Liu, R. M. Stoltenberg, Z. Bao and Y. Chen, ACS Nano, 2008, 2, 463 CrossRef CAS.
- J. Y. Lee, S. T. Connor, Y. Cui and P. Peumans, Nano Lett., 2008, 8, 689 CrossRef CAS.
- G. Heywang and F. Jonas, Adv. Mater., 1992, 4, 116 CAS.
- L. Groenendaal, F. Jonas, D. Freitag, H. Peilartzik and J. R. Reynolds, Adv. Mater., 2000, 12, 481 CrossRef CAS.
- Q. Pei, G. Zuccarello, M. Ahlskogt and O. Inganäs, Polymer, 1994, 35, 1347 CrossRef CAS.
- Y. Cao, G. Yu, R. Menon and A. J. Heeger, Synth. Met., 1997, 87, 171 CrossRef CAS.
- J. Y. Kim, J. H. Jung, D. E. Lee and J. Joo, Synth. Met., 2002, 126, 311 CrossRef CAS.
- L. A. A. Pettersson, S. Ghosh and O. Inganäs, Org. Electron., 2002, 3, 143 CrossRef CAS.
- S. K. M. Jönsson, J. Birgersonb, X. Crispinb, G. Greczynskib, W. Osikowiczb, A. W. Denier van der Gonc, W. R. Salaneckb and M. Fahlman, Synth. Met., 2003, 139, 1 CrossRef CAS.
- J. Ouyang, Q. Xu, C.-W. Chu, Y. Yang, G. Li and J. Shinar, Polymer, 2004, 45, 8443 CrossRef CAS.
- X. Crispin, F. L. E. Jakobsson, A. Crispin, P. C. M. Grim, P. Andersson, A. Volodin, C. van Haesendonck, M. Van der Auweraer, W. R. Salaneck and M. Berggren, Chem. Mater., 2006, 18, 4354 CrossRef CAS.
- A. M. Nardes, M. Kemerinka, M. M. de Kokb, E. Vinkenc, K. Maturovaa and R. A. J. Janssen, Org. Electron., 2008, 9, 727 CrossRef CAS.
- A. M. Nardes, A. J. R. Janssen and M. A. Kemerink, Adv. Funct. Mater., 2008, 18, 865 CrossRef CAS.
- M. Döbbelin, R. Marcilla, M. Salsamendi, C. Pozo-Gonzalo, P. M. Carrasco, J. A. Pomposo and D. Mecerreyes, Chem. Mater., 2007, 19, 2147 CrossRef.
- B. H. Fan, X. G. Mei and J. Ouyang, Macromolecules, 2008, 41, 5971 CrossRef CAS.
- M. Reyes-Reyes, I. Cruz-Cruz and R. Lopez-Sandoval, J. Phys. Chem. C, 2010, 114, 20220 CrossRef CAS.
- Y. Xia and J. Ouyang, ACS Appl. Mater. Interfaces, 2010, 2, 474 CrossRef CAS.
- Y. Xia and J. Ouyang, Macromolecules, 2009, 42, 4141 CrossRef CAS.
- Y. Xia and J. Ouyang, Org. Electron., 2010, 11, 1129 CrossRef CAS.
- Y. Xia, H. M. Zhang and J. Ouyang, J. Mater. Chem., 2010, 20, 9740 RSC.
- Y. Xia and J. Ouyang, J. Mater. Chem., 2011, 21, 4927 RSC.
- K. Sun, Y. Xia and J. Ouyang, Sol. Energy Mater. Sol. Cells, 2011 DOI:10.1016/j.solmat.2011.09.039.
- Y. Zhou, H. Cheun, S. Choi, W. J. Potscavage, C. Fuentes-Hernandez and B. Kippelen, Appl. Phys. Lett., 2010, 97, 153304 CrossRef.
- Y. H. Kim, C. Sachse, M. L. Machala, C. May, L. Müller-Meskamp and K. Leo, Adv. Funct. Mater., 2011, 21, 1076 CrossRef CAS.
- K. Lee, S. Cho, S. H. Park, A. J. Heeger, C. W. Lee and S. H. Lee, Nature, 2006, 441, 65 CrossRef CAS.
- J. Joo, S. M. Long, J. P. Pouget, E. J. Oh, A. G. MacDiarmid and A. J. Epstein, Phys. Rev. B: Condens. Matter, 1998, 57, 9567 CrossRef CAS.
- U. Voigt, W. Jaeger, G. H. Findenegg and R. V. Klitzing, J. Phys. Chem. B, 2003, 107, 5273 CrossRef CAS.
- X. Crispin, S. Marciniak, W. Osikowicz, G. Zotti, A. W. Denier Van Der Gon, F. Louwet, M. Fahlman, L. Groenendaal, F. De Schryver and W. R. Salaneck, J. Polym. Sci., Part B: Polym. Phys., 2003, 41, 2561 CrossRef CAS.
- J. Ouyang and Y. Li, J. Appl. Polym. Sci., 1996, 59, 1827 CrossRef CAS.
- U. Lang, E. Müller, N. Naujoks and J. Dual, Adv. Funct. Mater., 2009, 19, 1215 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |