Highly active catalysts for olefin metathesis in water†
Krzysztof
Skowerski
*a,
Grzegorz
Szczepaniak
b,
Celina
Wierzbicka
a,
Łukasz
Gułajski
a,
Michał
Bieniek
a and
Karol
Grela
b
aApeiron Synthesis Sp. z o.o., Klecińska 125, 54-413 Wrocław, Poland. E-mail: krzysztof.skowerski@apeiron-synthesis.com; Fax: +48-71-7985-622; Tel: +48-71-7985-621
bUniversity of Warsaw, Faculty of Chemistry, Pasteura 1, 02-093 Warsaw, Poland. E-mail: klgrela@gmail.com; Tel: +48-22-822-28-92
First published on 20th June 2012
Abstract
Preparation of novel, highly water soluble Ru complexes, which contain quaternary ammonium chloride tags is presented. The “on-site” quaternisation method can be used to obtain polar metathesis catalysts in an easy and efficient manner. Application profiles of three representative catalysts are described.
Olefin metathesis found broad applications in many branches of organic chemistry, and is rapidly becoming a stock item in the toolbox of organic synthesis.1 However, conducting this transformation in water still remains a challenge.2 Perfecting metathesis in water would enable its wider use in many novel applications, such as protein modification.3
Two basic approaches can be used to conduct metathesis in water. The first one involves using standard, insoluble catalysts in combination with surfactants2,4 or ultrasound.5 The second approach, which is the primary focus of our group, is to modify the catalyst itself to make it water soluble. Examples of such catalysts are shown in the second and third row in Fig. 1. Typically a highly hydrophilic tag, such as a poly(ethylene glycol) chain (1)6 or a quaternary ammonium group (2–4),7 is added to the molecule.
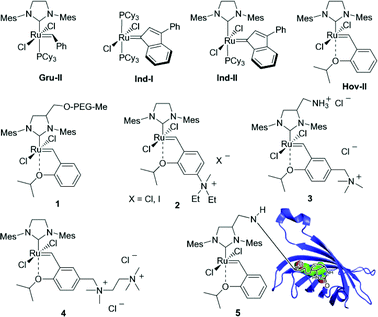 | ||
| Fig. 1 Popular water-insoluble catalysts (Gru-II, Ind-I, Ind-II, Hov-II) and complexes 1–5 designed for olefin metathesis in water (Mes = 2,4,6-trimethylphenyl, Cy = cyclohexyl). | ||
Furthermore, the recent pioneering work of Ward et al., who incorporated tagged Ru-catalysts into peptides (such as biotin-tagged complex 5 bonded to streptavidin) to produce artificial metalloenzymes active in RCM in aqueous solution, shows an interesting future direction.8 Although these results are impressive, still a lot of work must be invested to obtain a really practical water-soluble olefin metathesis catalyst.
Because catalyst 2, synthesized earlier in our group,7a suffered from low solubility in water (<1 mg mL−1), in the current project we decided to search for its more water soluble analogues. While catalyst 2 containing iodide counterions was synthesised in a relatively inexpensive manner, there is no simple and economic method for preparing such a complex with chloride counterions.9 Also other published methods of synthesis and purification of ammonium chloride tagged Ru-catalysts are relatively difficult to scale-up.7c
Being interested in the development of new, stable and efficient catalysts for olefin metathesis in neat water, we decided to prepare a catalyst bearing ammonium tags in both benzylidene and NHC parts of the complex. Having in mind possible applications in biological systems under neutral pH, we decided to synthesize catalysts containing in their structure only quaternary ammonium groups (–NR3+Cl−), and not the hydrochloride (–NH3+Cl−; compare with complex 3). To the best of our knowledge, the Ru-catalysts bearing a quaternary ammonium fragment in the NHC ligand are unknown.10
Unfortunately, we found previously that purification of a catalyst bearing a quaternary ammonium chloride fragment could be very difficult, probably due to interactions with a CuCl×PCy3 complex formed during the reaction. Other attempts, such as use of alternative ligand exchange protocols,11 were also ineffective in this case. It seems even more problematic to place a quaternary ammonium cation on the NHC ligand, due to strongly basic conditions required to generate the free NHC from the corresponding imidazolinium salt (Scheme 1). Therefore, we decided to develop a completely new, more universal and user-friendly method of synthesis and purification of ammonium-tagged olefin metathesis catalysts.
 | ||
| Scheme 1 Initial synthetic route to a bis-tagged Ru-complex. | ||
To do so, instead of using ligands with already present hydrophilic groups, we decided to form quaternary ammonium groups “on site” once the ruthenium complex is fully assembled.12 This strategy should allow us to work (till the final step) with uncharged non-polar compounds, which can be conveniently purified by standard techniques (distillation, column chromatography etc.). The synthesized Ru complexes (6, 8, 10), bearing trialkylamino groups after treatment with methyl chloride, shall yield ammonium tagged complexes (7, 9, 11, Scheme 2).
 | ||
| Scheme 2 New route to ammonium tagged Ru-complexes. | ||
First, we synthesized precursor 13 using the Mitsunobu reaction and used it in a ligand exchange reaction with indenylidene complex Ind-II to obtain compound 6 (Scheme 3). The yield of this reaction was lower than yields usually observed in syntheses of Hoveyda–Grubbs type catalysts.7 This might be associated with the increased bulkiness of the etheral fragment in the benzylidene ligand or with the presence of the basic amine, which could chelate the Ru-metal, leading to the formation of Ru-oligomers.
 | ||
| Scheme 3 Synthetic route to tagged catalysts 7, 9, 11 (DIAD = diisopropyl azodicarboxylate; TEOF = triethylorthoformate). | ||
The free-base 6 was then heated with methyl chloride in a pressurised vessel to obtain 7 in high yield. After excess CH3Cl was evaporated, the final purification of salt 7 was very simple and involved only filtration through a short pad of neutral aluminium oxide. Subsequently, we prepared catalysts 9 and 11 containing a quaternary ammonium chloride in the NHC fragment. Salt 18 was synthesized in four steps in 20% overall yield. Upon action of potassium tert-amylate, a free NHC was generated, which was reacted in situ with Ind-I to yield 19. This complex was then reacted with benzylidene ligand precursors 20 and 13, to give amine-containing catalysts 8 and 10. Quaternization of these neutral complexes afforded catalysts 9 and 11 in high yields. These entities are, to the best of our knowledge, the first reported metathesis catalysts with a quaternary ammonium group placed in the NHC ligand. The alkylation reaction was selective and affected only the less crowded piperazinic nitrogen atom (Scheme 3).†
Catalysts 7 and 9 turned out to be sparingly soluble in water (2 mg mL−1 and 3 mg mL−1, respectively), highlighting the fact that one quaternary ammonium group is not sufficient to provide good solubility. The presence of the second ammonium group in 11 resulted, however, in a dramatic improvement in solubility (35 mg mL−1).
The catalytic activity of 7, 9, and 11 was checked by conducting a number of reactions in neat non-degassed D2O under air. The results are summarized in Table 1. Complex 7 was generally highly active but surprisingly provided lowest yield in RCM of 23. Complex 9 was less active in isomerization of 21 but proved to be very effective in CM of 22 and RCM of 23. Complex 11 exhibits the highest solubility, but this property not always matched the highest catalytic activity in water. All catalysts gave moderate yields in the cycloisomerisation of 25. According to our knowledge, this transformation is the first successful enyne metathesis in neat water. Complexes 7 and 11 showed unprecedented activity in the isomerization of (Z)-21 (Fig. 2).
| Entry | Substrate | Product | Catalyst (mol %) | Time/h | Yield (%) |
|---|---|---|---|---|---|
| a Yields calculated from NMR. b E/Z = 16.7/1. c E/Z = 12.5/1. | |||||
| 1 |

|

|
7 (0.5) | 0.13 | 94 |
| 2 | 9 (0.5) | 1.1 | 71 | ||
| 3 | 11 (0.5) | 0.16 | 94 | ||
| 4 |

|

|
7 (5) | 24 | 74b |
| 5 | 9 (5) | 24 | 77b | ||
| 6 | 11 (5) | 24 | 38c | ||
| 7 |

|

|
7 (2.5) | 3.5 | 49 |
| 8 | 9 (2.5) | 2.5 | 96 | ||
| 9 | 11 (2.5) | 2.5 | 88 | ||
| 10 |

|

|
7 (5) | 5 | 62 |
| 11 | 9 (5) | 5 | 46 | ||
| 12 | 11 (5) | 5 | 41 | ||
 | ||
| Fig. 2 Isomerization of (Z)-21 with catalysts 7, 9, and 11. | ||
This is probably due to sterical modification of the benzylidene ligand or electronic activation through weakening of the Ru–O bond by strongly electron withdrawing quaternary ammonium groups.
In addition to metathesis in neat water, we decided to test our polar catalysts with a model water-insoluble substrate with the intention to extract 11 and any ruthenium containing impurities in water. To do so, we conducted the RCM reaction of diethyl diallylmalonate 27 promoted by 11 in non-degassed DCM in air. After 30 min the reaction was completed (Fig. 3a). To a yellow-green coloured reaction mixture D2O was added (Fig. 3b) and the formed two phase mixture was vigorously shaken. After the phases separated, we were pleased to find that after this single extraction event the DCM phase containing product 28 became colourless, and the Ru complex migrated to D2O (Fig. 3c). Similar approach to ruthenium residues removal was reported by Grubbs et al.13 Next (Z)-21 was added to the green D2O fraction and the reaction mixture was analyzed by NMR. (E)-21 was obtained with 94% yield after 1 h.
 | ||
| Fig. 3 Example of catalyst 11 extraction and reuse. | ||
A more detailed study concerning product purification from ruthenium residues and catalyst reuse is in progress.
In conclusion, we reported a simple synthetic protocol for the preparation of quaternary ammonium chloride tagged catalysts. The last step, quaternization of the preformed metal complex, allows for very simple isolation of the resulted highly polar catalyst. This method allows us to obtain Ru-complexes containing quaternary ammonium groups in the NHC ligand. Catalysts synthesized using this method are stable in water, even in the presence of air, and promote CM, RCM, and enyne reactions of various water-soluble substrates. The use of water as a solvent for metathesis has significance for biological applications, which are recently increasing in number. Moreover, these catalysts can also be used in metathesis of water-insoluble substrates in “classical” organic solvents, allowing for easy purification of product and catalyst reuse.14a Immobilization of these catalysts on solid supports14b or in ionic liquids15 will be studied in due time.
Apeiron Synthesis acknowledges the National Centre for Research and Development for financial support within “IniTech” programme. MB acknowledges the Foundation for Polish Science for financial support within “INNOWATOR” programme.
KG and GS acknowledge the Foundation for Polish Science for ‘TEAM’ Programme co-financed by the European Regional Development Fund, Operational Program Innovative Economy 2007–2013.
Notes and references
- (a) For selected reviews, see: R. H. Grubbs, Handbook of Metathesis, Wiley-VCH, Weinheim, 2003, vol. 1–3 Search PubMed; (b) M. Michalak, Ł. Gułajski and K. Grela, Alkene Metathesis, in Science of Synthesis: Houben–Weyl Methods of Molecular Transformations, Alkenes, ed. A. de Meijere, Georg Thieme Verlag KG, 2010, vol. 47a, pp. 327–438 Search PubMed; (c) Y. Vidavsky, A. Anaby and G. N. Lemcoff, Dalton Trans., 2012, 41, 32–43 RSC; (d) G. C. Vougioukalakis and R. H. Grubbs, Chem. Rev., 2010, 110, 1746–1787 CrossRef CAS.
- For selected reviews, see: (a) D. Burtscher and K. Grela, Angew. Chem., Int. Ed., 2009, 48, 442–454 CrossRef CAS; (b) S. Zaman, O. J. Curnow and A. D. Abell, Aust. J. Chem., 2009, 62, 91–100 CrossRef CAS; (c) C. Torborg, C. Samojłowicz and K. Grela, Metathesis Reactions, in Science of Synthesis: Methods of Molecular Transformations Vol. 2011/5: Water in Organic Synthesis, ed. S. Kobayashi, Georg Thieme Verlag KG, Section 3.6, 2012, pp. 225–256 Search PubMed.
- For selected reviews, see: (a) Y. A. Lin, J. M. Chalker and B. G. Davis, ChemBioChem, 2009, 10, 959–969 CrossRef CAS; (b) J. M. Chalker, G. J. L. Bernardes, Y. A. Lin and B. G. Davis, Chem.–Asian J., 2009, 4, 630–640 CrossRef CAS; (c) Y. A. Lin and B. G. Davis, Beilstein J. Org. Chem., 2010, 6, 1219–1228 CrossRef CAS.
- For a review, see: B. H. Lipshutz and S. Ghorai, Aldrichimica Acta, 2008, 41, 59–72 CAS.
- Ł. Gułajski, P. Śledź, A. Lupa and K. Grela, Green Chem., 2008, 10, 271–282 RSC.
- (a) J. P. Gallivan, J. P. Jordan and R. H. Grubbs, Tetrahedron Lett., 2005, 46, 2577–2580 CrossRef CAS; (b) S. H. Hong and R. H. Grubbs, J. Am. Chem. Soc., 2006, 128, 3508–3509 CrossRef CAS.
- (a) A. Michrowska, Ł. Gułajski, Z. Kaczmarska, K. Mennecke, A. Kirschning and K. Grela, Green Chem., 2006, 8, 685–688 RSC; (b) Ł. Gułajski, A. Michrowska, J. Narożnik, Z. Kaczmarska, L. Rupnicki and K. Grela, ChemSusChem, 2008, 1, 103–109 CrossRef; (c) J. P. Jordan and R. H. Grubbs, Angew. Chem., Int. Ed., 2007, 46, 5152–5155 CrossRef CAS.
- (a) C. Lo, M. R. Ringenberg, D. Gnandt, Y. Wilson and T. R. Ward, Chem. Commun., 2011, 47, 12065–12067 RSC; (b) C. Mayer, D. G. Gillingham, T. R. Ward and D. Hilvert, Chem. Commun., 2011, 47, 12068–12070 RSC.
- Ł. Gułajski and K. Grela, Green Metathesis Chemistry, NATO Science for Peace and Security Series A: Chemistry and Biology, ed. V. Dragutan, A. Demonceau, I. Dragutan, E. Sh. Finkelshtein A. and Springer Science Netherlands, 2010, pp. 49–56 Search PubMed.
- A review on NHC-bearing Ru-metathesis catalysts: C. Samojłowicz, M. Bieniek and K. Grela, Chem. Rev., 2009, 109, 3708–3742 CrossRef.
- S. Monsaert and F. Verpoort, Patent Application WO/2011/091980.
- There are only few chemical transformations conducted on preformed Ru-alkylidene complexes known, for example: (a) Boc deprotection – ref. 7c; (b) esterification – M. Mayr, D. Wang, R. Kroll, N. Schuler, S. Prühs, A. Fürstner and M. R. Buchmeiser, Adv. Synth. Catal., 2005, 347, 484–492 CrossRef CAS; (c) TMS deprotection – S. Prühs, C. W. Lehmann and A. Fürstner, Organometallics, 2004, 23, 280 CrossRef and Patent Application WO/2007/017047 A1.
- S. H. Hong and R. H. Grubbs, Org. Lett., 2007, 9, 1955–1957 CrossRef CAS.
- (a) M. R. Buchmeiser, Chem. Rev., 2009, 109, 303–321 CrossRef CAS; (b) H. Clavier, K. Grela, A. Kirschning, M. Mauduit and S. P. Nolan, Angew. Chem., Int. Ed., 2007, 46, 6786–6801 CrossRef CAS.
- P. Sledź, M. Mauduit and K. Grela, Chem. Soc. Rev., 2008, 37, 2433–2442 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Full experimental data and copies of spectra. See DOI: 10.1039/c2cy20320k |
| This journal is © The Royal Society of Chemistry 2012 |
