Gold(III)–oxo complexes as catalysts in intramolecular hydroamination†
James A. T.
O'Neill
,
Georgina M.
Rosair
and
Ai-Lan
Lee
*
Institute of Chemical Sciences, School of Engineering and Physical Sciences, Heriot-Watt University, Edinburgh EH14 4AS, UK. E-mail: A.Lee@hw.ac.uk; Tel: +44 (0)131-4518030
First published on 21st June 2012
Abstract
A variety of Au(III)–oxo complexes 1 were evaluated as catalysts for the first time and shown to be active catalysts for the alkyne hydroamination reaction.
In less than a decade, homogenous gold catalysis has undergone a transformation from rarity to an incredibly active and mainstream field of research.1 Its popularity is partly the result of the excellent selectivity and efficiency of gold catalysts as π-Lewis acids for activating C–C π bonds such as in alkynes, allenes, dienes and alkenes, and also the ability to tune gold catalysts (via ligands and counterions) in order to vary the reactivity and selectivity of the reactions.2,3 The latter has been explored thoroughly with Au(I) complexes but less so with Au(III) complexes. A recent commentary has highlighted the need for development of Au(III) based enantioselective catalysis,4 and in order for progress to be made in this area, more investigations on organometallic Au(III) catalysts, with tuneable and modifiable ligands, need to be carried out. While many organometallic groups have dedicated their efforts to synthesising new N-ligated Au(III) complexes, investigations into their applications have been primarily aimed at biological (especially cytotoxic) activity rather than catalysis.5,6 In this communication, we present our investigations into the use of 6,6′-dimethyl-2,2′-bipyridine and 2,9-dimethyl-1,10-phenanthroline based Au(III) complexes as catalysts. In particular, a variety of gold(III)–oxo complexes 1 (Fig. 1, left) are evaluated as catalysts for the first time and shown to be active catalysts for the alkyne hydroamination reaction.
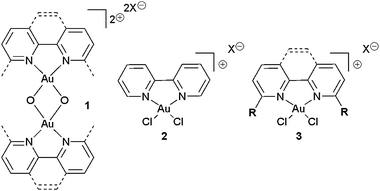 | ||
| Fig. 1 Gold(III)–oxo complex 1, bipy-complex 2 and desired R-substituted complex 3. | ||
We began our investigations in this area because we observed that while unsubstituted bipy Au(III) complexes such as 2 (Fig. 1, centre) are known and have been evaluated as catalysts,7 the 6,6′-disubstituted bipy (or 2,9-disubstituted phenanthroline) equivalent (3) (Fig. 1, right) is not known. However, in terms of catalysis, there are compelling reasons to investigate whether R substitutions on 3 at the positions indicated are tolerated. Firstly, if chiral versions of these Au(III) complexes are to be used for catalysis, the substituents usually need to be introduced at the 6,6′ positions for good chiral induction.8 Secondly, macrocyclic versions of complex 3 have potential utility for the synthesis of interlocked architectures (such as rotaxanes and catenanes) via an active template approach, but once again, the R substituents (in this case forming a macrocycle) must be at the 6,6-positions as indicated.9 With these issues in mind, we initially set out to (a) discover whether complexes 3, with R substituents at the positions indicated, can be synthesised, and (b) evaluate these complexes as catalysts in a model reaction.
Initially, we set out to synthesise 3 (bipy, R = Me) where X− is a weakly-coordinating counterion. Using a variety of methods which proved to successfully form 2 in the unsubstituted bipyridine and phenanthroline versions7,10,11 unfortunately produced compound 411 instead (e.g.Scheme 1). This clearly shows that the steric hindrance from R-substitution at 6,6′ positions on the bipy ligand changes the outcome of the reaction, and that complexes 3 are not as easy to access as 2.
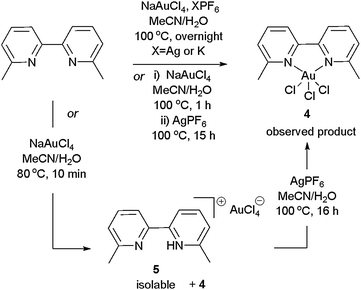 | ||
| Scheme 1 Attempted synthesis of 2 results in the formation of 4. | ||
Next, various attempts were made to form 3 from 4, by reacting with various AgX salts (AgOAc, AgTFA, AgBF4, AgSbF6, AgPF6, AgOTf, AgNO3, AgClO4) and HX (HBF4, HPF6, HClO4), but all failed to produce 3. For example, treatment of 4 with AgClO4 produced protonated dimethyl bipy 5 instead in 75% yield (Scheme 2, confirmed by X-ray crystallography, see ESI†).12 Once again, these results demonstrate that the R substituents at 6,6′ hinder the synthesis of a [(N–N)Au(III)Cl2]+X− complex (3).
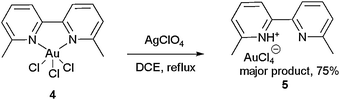 | ||
| Scheme 2 Attempted synthesis of 3 from 4. | ||
To our surprise, treatment of 4 with a saturated aq. solution of NaClO4 produced a new gold(III)–oxo complex 7 (Scheme 3 and confirmed by X-ray crystallography, Fig. 2).13 Gold(III)–oxo complexes have been synthesised and studied by Cinellu and co-workers, particularly for their anti-cancer properties but also as precursors to gold–alkene complexes.14 However, these gold(III)–oxo complexes, to the best of our knowledge, have never been investigated as homogenous catalysts or pre-catalysts in gold-catalysed reactions.15 Since gold(III)–oxo complex 7 fulfils our original criteria of a gold(III) bipy complex bearing substituents at the 6,6′ positions, and being cationic with a weakly coordinating counterion, we decided that it would be judicious to investigate whether this complex, along with other related gold(III)–oxo complexes, exhibits any catalytic activity.
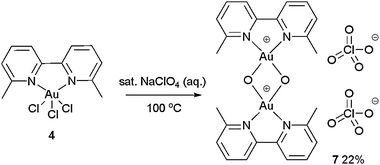 | ||
| Scheme 3 Synthesis of Au(III)–oxo complex 7. | ||
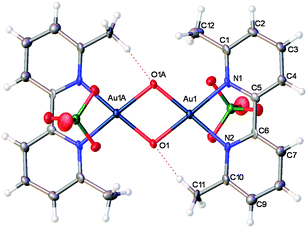 | ||
| Fig. 2 X-ray structure of Au(III)–oxo complex 7. | ||
Thus, a series of gold(III)–oxo complexes and other gold(III) bipy complexes, 8,14c9,1610,17 and 11,17 were synthesised (Fig. 3) and evaluated along with 7, 4 and ligandless complexes AuCl3 and NaAuCl4 as catalysts in the intramolecular alkyne hydroamination reaction (Table 1).18 Pleasingly, oxo complex 8 proved to be a good pre-catalyst for the hydroamination reaction at 70 °C (2 h, entry 1). The reaction also proceeds well at room temperature to give the 5-exo-dig product in 70% yield (entry 2). Changing the counterion from PF6− to ClO4− (7) resulted in a slight drop in yield (60%, entry 3). Replacing the 6,6′-dimethyl-2,2′-bipyridine ligand with the 2,9-dimethyl-1,10-phenanthroline ligand (9) still produces an active pre-catalyst, although the yield is slightly lower (62%, entry 4). Next, we wanted to ascertain whether the 6,6′-Me substituents had any effect on the catalysis reaction. Thus, complex 10, with just unsubstituted bipy ligands, was evaluated. Complex 10 performed worse than the more sterically hindered complex 8 (55%, entry 5), implying that some degree of steric protection around the gold is beneficial. Since bipy-complexes 2, unlike 3, can be easily accessed, we decided to evaluate complex 11 in the hydroamination reaction. Complex 11 performed worse than the equivalent oxo compound, producing 50% yield but with several unidentified side products and traces of starting material still present (entry 6). Next, the neutral bipy–gold complex 4 was also evaluated for comparison. Both complex 4 (entry 7) and the uncomplexed AuCl3 (entry 8) provided identical results: 50% yield, 25% starting material remaining. These last results show the importance of using a cationic complex to achieve ligand-tunable catalytic Au(III) systems with bipy complexes. Next, the simple salt NaAuCl4 was evaluated for comparison18c and also provided poorer results (incomplete conversion) than with Au(III)–oxo complexes at room temperature (entry 9). Finally, controls using AuCl3 + 6,6′-dimethyl-bipy (entry 10) and AuCl + 6,6′dimethyl-bipy (entry 11) both gave poor yields of 14.
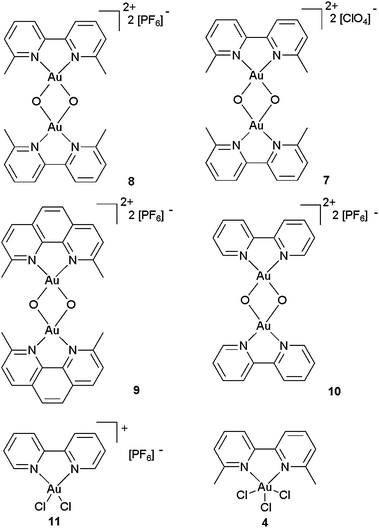 | ||
| Fig. 3 Au(III) complexes evaluated as catalysts in the hydroamination reaction. | ||
| Entry | Catalyst | Yielda (%) | Unreacted starting materialc |
|---|---|---|---|
| a Yield determined by 1H-NMR analysis with mesitylene as an internal standard. b 70 °C, 2 h. c The 6-endo-dig product is not observed. | |||
| 1b | 8 | 70 | — |
| 2 | 8 | 70 | — |
| 3 | 7 | 60 | — |
| 4 | 9 | 62 | — |
| 5 | 10 | 55 | — |
| 6 | 11 | 50 | Trace + side products |
| 7 | 4 | 50 | 25% |
| 8 | AuCl3 | 50 | 25% |
| 9 | NaAuCl4 | 54 | 10% |
| 10 | AuCl3 + 6,6′-diMe-bipy | 27 | — |
| Unidentified side products | |||
| 11 | AuCl + 6,6′-diMe-bipy | 21 | — |
| Unidentified side products | |||
The Au(III) reaction investigated here can be carried out in air, is a mild room temperature reaction, and the gold(III)–oxo complexes have better activity than the simple AuCl3 or NaAuCl4 salts, thus showing the potential of such Au(III)–oxo complexes in catalysis.
Conclusions
In summary, Au(III)–oxo complexes (7–10) have been evaluated as catalysts for the first time and shown to be catalytically active in the alkyne hydroamination reaction. Complex 8, with 6,6′-dimethyl-2,2′-bipyridine ligand, is found to be a better catalyst than the equivalent phenanthroline complex 9, or the unsubstituted bipy complex 10. The cationic oxo complexes are also better catalysts than the neutral complex 4, AuCl3 or NaAuCl4. Crucially, the formation of Au(III)–oxo complexes such as 7 is tolerant of substituents at the 6,6′ positions of the bipy ligand, and 2,9 positions of the phenanthroline ligand, unlike the formation of Au(III) dichloride complexes 2/3. Thus, looking forward, these Au(III)–oxo complexes, unlike 2, have the potential to be adapted for and applied in asymmetric gold(III) catalysis.Acknowledgements
We would like to thank EPSRC (EP/G006695/1), the EPSRC Mass Spectrometry services at Swansea for analytical support and Johnson Matthey for a generous loan of gold salts.Notes and references
- Selected reviews: (a) D. J. Gorin and F. D. Toste, Nature, 2007, 446, 395 CrossRef CAS; (b) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS; (c) E. Jimenez-Nunez and A. M. Echavarren, Chem. Commun., 2007, 333 RSC; (d) Z. G. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS; (e) N. Marion and S. P. Nolan, Chem. Soc. Rev., 2008, 37, 1776 RSC; (f) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef; (g) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS; (h) H. C. Shen, Tetrahedron, 2008, 64, 3885 CrossRef CAS; (i) H. C. Shen, Tetrahedron, 2008, 64, 7847 CrossRef CAS.
- For a review on ligand effects in homogenous gold catalysis, see: D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS.
- For examples of such gold-catalysed reactions developed in our group, see: (a) J. T. Bauer, M. S. Hadfield and A. L. Lee, Chem. Commun., 2008, 6405 RSC; (b) M. S. Hadfield, J. T. Bauer, P. E. Glen and A. L. Lee, Org. Biomol. Chem., 2010, 8, 4090 RSC; (c) M. S. Hadfield and A. L. Lee, Org. Lett., 2010, 12, 484 CrossRef CAS; (d) A. Heuer-Jungemann, R. G. McLaren, M. S. Hadfield and A.-L. Lee, Tetrahedron, 2011, 67, 1609 CrossRef CAS; (e) K. J. Kilpin, U. S. D. Paul, A. L. Lee and J. D. Crowley, Chem. Commun., 2011, 47, 328 RSC; (f) M. S. Hadfield and A.-L. Lee, Chem. Commun., 2011, 47, 1333 RSC; (g) P. C. Young, M. S. Hadfield, L. Arrowsmith, K. M. Macleod, R. J. Mudd, J. A. Jordan-Hore and A.-L. Lee, Org. Lett., 2012, 14, 898 CrossRef CAS; (h) M. S. Hadfield, L. J. Häller, A.-L. Lee, S. A. Macgregor, J. A. T. O'Neill and A. M. Watson, Org. Biomol. Chem., 2012, 10, 4433 RSC.
- F. D. Toste, Synlett, 2012, 46 CrossRef CAS.
- For a review on gold(III) complexes with N- and O-ligands, see: M. A. Cinellu, in Gold Chemistry Applications and Future Directions in the Life Sciences, ed. F. Mohr, Wiley-VCH, Weinhem, 2009, pp. 47–92 Search PubMed.
- For reviews on the therapeutic application of gold complexes, see: (a) C.-M. Che and R. W.-Y. Sun, Chem. Commun., 2011, 47, 9554 RSC; (b) S. J. Berners-Price and A. Filipovska, Metallomics, 2011, 3, 863 RSC.
- Selected examples, catalysis: (a) Y. Zhang, H. Peng, M. Zhang, Y. Cheng and C. Zhu, Chem. Commun., 2011, 47, 2354 RSC ; synthesis: ; (b) A. P. Shaw, M. Tilset, R. H. Heyn and S. Jakobsen, J. Coord. Chem., 2011, 64, 38 CrossRef CAS; (c) M. A. Ivanov, M. V. Puzyk and K. P. Balashev, Russ. J. Gen. Chem., 2003, 73, 2003 Search PubMed; (d) C. N. Harris and T. N. Lockyer, J. Chem. Soc., 1959, 3083 CAS . Unlike the 6,6′ positions, substitution at the 4,4′ positions of the bipy ligand is tolerated, see: A. Cassini, M. C. Diawara, R. Scopelliti, S. M. Zakeeruddin, M. Gratzel and P. J. Dyson, Dalton Trans., 2010, 39, 2239 RSC.
- For reviews on chiral bipyridine ligands for asymmetric induction, see: (a) A. V. Malkov and P. Kocovsky, Curr. Org. Chem., 2003, 7, 1737 CrossRef CAS; (b) N. C. Fletcher, J. Chem. Soc., Perkin Trans. 1, 2002, 1831 RSC; (c) G. Chelucci and R. P. Thummel, Chem. Rev., 2002, 102, 3129 CrossRef CAS.
- Review: J. D. Crowley, S. M. Goldup, A.-L. Lee, D. A. Leigh and R. T. McBurney, Chem. Soc. Rev., 2009, 38, 1530 RSC.
- F. Abbate, P. Orioli, B. Bruni, G. Marcon and L. Messori, Inorg. Chim. Acta, Rev., 2000, 311, 1 CrossRef CAS.
- F. Cocco, M. A. Cinellu, G. Minghetti, A. Zucca, S. Stoccoro, L. Maiore and M. Manassero, Organometallics, 2010, 29, 1064 CrossRef CAS.
- Similar observations were reported when substituents were placed at the 2,9-positions of 1,10-phenanthroline ligands: (a) Z. D. Hudson, C. D. Sanghvi, M. A. Rhine, J. J. Ng, S. D. Bunge, K. I. Hardcastle, M. R. Saadein, C. E. MacBeth and J. F. Eichler, Dalton Trans., 2009, 7473 RSC. For X-ray structure of a related [Au(phen)Cl2]Cl, see: (b) F. Abbate, P. Orioli, B. Bruni, G. Marcon and L. Messori, Inorg. Chim. Acta, Rev., 2000, 311, 1 CrossRef CAS.
- See ESI† for the proposed mechanism.
- For selected examples, see: (a) C. Gabbiani, A. Casini, L. Messori, A. Guerri, M. A. Cinellu, G. Mingghetti, M. Corsini, C. Rosani, P. Zanello and M. Arca, Inorg. Chem., 2008, 47, 2368 CrossRef CAS; (b) M. A. Cinellu, G. Minghetti, F. Cocco, S. Stoccoro, A. Zucca, M. Manassero and M. Arca, Dalton Trans., 2006, 5703 RSC; (c) M. A. Cinellu, G. Minghetti, M. V. Pinna, S. Stoccoro, A. Zucca, M. Manassero and M. Sansoni, J. Chem. Soc., Dalton Trans., 1998, 1735 RSC; (d) M. A. Cinellu, G. Minghetti, F. Cocco, S. Stoccoro, A. Zucca and M. Manassero, Angew. Chem., Int. Ed., 2005, 44, 6892 CrossRef CAS; (e) M. A. Cinellu, G. Minghetti, S. Stoccoro, A. Zucca and M. Manassero, Chem. Commun., 2004, 1618 RSC , see also ref. 5.
- Gold(III)–oxo complexes have never been applied to catalysis, but gold(I)–oxo complex {[(Ph3PAu)3O]BF4} has. For example, see: (a) B. D. Sherry and F. D. Toste, J. Am. Chem. Soc., 2004, 126, 15978 CrossRef CAS; (b) B. D. Sherry, L. Maus, B. N. Laforteza and F. D. Toste, J. Am. Chem. Soc., 2006, 128, 8132 CrossRef CAS; (c) R. L. LaLonde, W. E. Brenzovich, Jr., D. Benitez, E. Tkatchouk, K. Kelley, W. A. Goddard III and F. D. Toste, Chem. Sci., 2010, 1, 226 RSC; (d) J. M. W. Chan, G. W. Amarante and F. D. Toste, Tetrahedron, 2011, 67, 4306 CrossRef CAS; (e) O. F. Jeker and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3474 CrossRef CAS.
- M. A. Cinellu, L. Maiore, M. Manassero, A. Casini, M. Arca, H.-H. Fiebig, G. Kelter, E. Michelucci, G. Pieraccini, C. Gabbiani and L. Messori, ACS Med. Chem. Lett., 2010, 1, 336 CrossRef CAS.
- M. A. Cinellu, G. Minghetti, M. V. Pinna, S. Stoccoro, A. Zucca and M. Manassero, J. Chem. Soc., Dalton Trans., 2000, 1261 RSC.
- For examples of gold(III)-catalysed intramolecular hydroamination (using AuCl3 and NaAuCl4): (a) Y. Fukuda and K. Utimoto, Synthesis, 1991, 975 CrossRef CAS; (b) T. E. Müller, Tetrahedron Lett., 1998, 39, 5961 CrossRef; (c) Y. Fukuda, K. Utimoto and H. Nozaki, Heterocycles, 1987, 25, 297 CrossRef CAS; (d) K. Iritani, S. Matsubara and K. Utimoto, Tetrahedron Lett., 1988, 29, 1799 CrossRef CAS . Reviews: ; (e) R. A. Widenhoefer and X. Han, Eur. J. Org. Chem., 2006, 4555 CrossRef CAS; (f) A. S. K. Hashmi and M. Bührle, Aldrichimica Acta, 2010, 43, 27 CAS. See also: (g) J. M. Carney, P. J. Donoghue, W. M. Wuest, O. Wiest and P. Helquist, Org. Lett., 2008, 10, 3903 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and spectral data, and crystallographic data. CCDC reference numbers 878431–878433. For crystallographic data in CIF or other electronic format see DOI: 10.1039/c2cy20255g |
| This journal is © The Royal Society of Chemistry 2012 |

