Synthesis of coumarins via Pechmann reaction catalyzed by 3-methyl-1-sulfonic acid imidazolium hydrogen sulfate as an efficient, halogen-free and reusable acidic ionic liquid
Nader Ghaffari
Khaligh
*
Department of Chemistry, College of Sciences, Guilan University, Rasht 41335-19141, Iran. E-mail: ngkhaligh@gmail.com; ngkhaligh@guilan.ac.ir; Fax: +98 66934046; Tel: +98 2166431738
First published on 3rd May 2012
Abstract
3-Methyl-1-sulfonic acid imidazolium hydrogen sulfate has been used as an acidic task-specific ionic liquid for Pechmann condensation reaction. The coumarin products could simply be separated and the ionic liquid could be recycled and reused several times without noticeable decrease in the catalytic activity.
Coumarin and its derivatives have attracted great interest because of their importance in the synthetic organic and medicinal chemistry. They are widely used as additives in food, perfumes, agrochemicals, cosmetics, pharmaceuticals1 and in the preparation of insecticides, optical brightening agents, dispersed fluorescent and tunable laser dyes.2 Coumarin and its derivatives have various bioactivities such as antimicrobial,3 antithrombotic,4 anticoagulant, antipsoriasis activity,5 anticancer,6 anti-HIV,7 antioxidant activity, antiproliferative activity,8 inhibitory activity on viral proteases,9 estrogen-like effects10 and central nervous system modulating activities.11 Coumarins also act as intermediates in the synthesis of furocoumarins, chromenes, coumarones and 2-acylresorcinols.12
Coumarins have been synthesized by several routes including Pechmann,13 Perkin,14 Knoevenagel,15 Reformatsky,16 Wittig reactions17, and by flash vacuum pyrolysis.18 Among these, the Pechmann reaction is the most widely used method, as the reaction involves the use of simple starting materials, that is, phenols and β-ketoesters, in the presence of acidic condensing agents. The use of various reagents such as H2SO4, P2O5, FeCl3, ZnCl2, POCl3, AlCl3, PPA, HCl, phosphoric acid, trifluoroacetic acid, montmorillonite and other clays is well documented in the literature.19 Most of these methods suffer from severe drawbacks including the use of a large amount of catalysts, sometimes long reaction times, low yields and very often temperatures to the extent of 150 °C.
Nowadays acidic task-specific ionic liquids (TSILs), which possess the advantageous characteristics of solid acids and mineral acids, attract much interest as environmentally benign catalysts or excellent alternatives to organic solvents. TSILs are designed to replace traditional mineral liquid acids, such as sulfuric acid and hydrochloric acid, as catalysts in organic synthetic procedures. The introduction of Brönsted-acidic functional groups into the cation or anion of ionic liquids, especially the SO3H and SO4H functional groups, obviously enhanced their acidity and water solubility.20–22 Moreover, their polar nature makes them useful for use under solvent-free conditions. An ever-increasing interest has been focused on TSILs for catalytic reactions where the catalyst is in one phase and the product in another, which makes product-isolation easy and catalyst-reuse convenient.
To date, reports on some ‘‘greener’’ halogen-free ionic liquids with phosphate or sulfate anions and the effects of the anion and toxicology have appeared in the literature.23 Typical ionic liquids consist of halogen-containing anions (for example [Cl]−, [PF6]−, [BF4]−, [CF3SO3]−, or [(CF3SO2)2N]−) which to some extent limits their ‘‘greenness’’.23,24 Therefore, it is necessary to synthesize novel efficient and halogen-free TSILs.
Based on the Zolfigol's report on the preparation of 3-methyl-1-sulfonic acid imidazolium chloride ([Msim]Cl),25 in our previous work a novel SO3H-functionalized halogen-free acidic ionic liquid that bears a sulfonic acid group in an imidazolium cation and hydrogen sulfate as a halogen-free anion was synthesized and its application in the promotion of the protection of hydroxyl groups was also investigated.26 In continuation of our work in studying acid-catalyzed reactions in ionic liquids, we report here the synthesis of coumarin derivatives by the Pechmann condensation in the halogen-free acidic ionic liquid 3-methyl-1-sulfonic acid imidazolium hydrogen sulfate [Msim]HSO4. To the best of our knowledge of the open literature, Pechmann-type reactions catalyzed by [Msim]Cl and [Msim]HSO4 ionic liquids have not been reported.
For beginning this study and comparing the catalytic performance of [Msim]Cl and [Msim]HSO4 ionic liquids (TSILs), resorcinol and ethyl acetoacetate (EEA) were employed as the model reactants. The Pechmann condensation of model reactants with equivalent ratio was carried out in the presence of various amounts of TSILs (2 and 5 mol%) at different temperatures ranging from 25 to 60 °C under solvent-free conditions (Table 1). As shown, after stirring for 1 h at 100 °C in the absence of ionic liquids, the reaction did not proceed as monitored by TLC (Table 1, entry 1). On the other hand, in the presence of a certain amount of ionic liquids and a specific temperature, the reaction was completed with 100% conversion. When the temperature of the reaction was raised from 25 to 40 °C, the reaction times and yields were improved considerably (Table 1, entries 2, 3, 5, 6, 8, 9, 11, and 12). However, with further increase in temperature, no significant improvement was observed in the yield and reaction time. So the temperature was not the only important factor for Pechmann reaction. According to the results in Table 1, it can be concluded that both ionic liquids were effective for Pechmann reaction at higher temperature, but [Msim]Cl is a halogen-containing ionic liquid, hence, [Msim]HSO4 should be the best catalyst for the Pechmann condensation among the two TSILs. Under the optimized reaction conditions, the reaction gave 94% yield of 7-hydroxy-4-methylcoumarin after 25 min in the presence of 2 mol% [Msim]HSO4 (Table 1, entry 9). The higher amount of [Msim]HSO4 and increasing the reaction temperature did not improve the results to a greater extent.
| Entry | TSIL | Amount of TSILb (mol%) | Temp (°C) | Time (min) | Yieldc (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 5 mmol resorcinol, 5 mmol ethyl acetoacetate. b Molar ratio of TSILs to phenol. c Isolated yield. | |||||
| 1 | — | — | 100 | 180 | — |
| 2 | [Msim]Cl | 2 | 25 | 180 | 42 |
| 3 | [Msim]Cl | 2 | 40 | 60 | 82 |
| 4 | [Msim]Cl | 2 | 60 | 60 | 85 |
| 5 | [Msim]Cl | 5 | 25 | 180 | 44 |
| 6 | [Msim]Cl | 5 | 40 | 60 | 82 |
| 7 | [Msim]Cl | 5 | 60 | 60 | 86 |
| 8 | [Msim]HSO4 | 2 | 25 | 180 | 48 |
| 9 | [Msim]HSO4 | 2 | 40 | 25 | 94 |
| 10 | [Msim]HSO4 | 2 | 60 | 25 | 96 |
| 11 | [Msim]HSO4 | 5 | 25 | 180 | 52 |
| 12 | [Msim]HSO4 | 5 | 40 | 25 | 96 |
| 13 | [Msim]HSO4 | 5 | 60 | 25 | 96 |
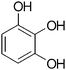
The Pechmann condensation reaction of other phenols and ethyl acetoacetate in the presence of [Msim]HSO4 was accomplished under the optimized reaction conditions described above (Scheme 1) and the results are presented in Table 2. It can easily be seen that the Pechmann condensation proceeded smoothly under solvent-free conditions and gave good to high yields ranging from 75% to 98%. Many phenols, such as resorcinol, orcinol, pyrogallol, phloroglucinol, 4-chlorophenol and 3-methoxyphenol, could be converted to corresponding coumarins in high yields (Table 2, entries 1, 4, 5, 8, 10 and 13). The reactivities of 3-methylphenol and 1-naphthol seem to be inferior as compared with that of the former (Table 2, entries 15 and 16), only 82% and 78% yields were obtained, respectively.
![The synthesis of substituted coumarins in the presence of [Msim]HSO4 at 40 °C under solvent-free conditions.](/image/article/2012/CY/c2cy20196h/c2cy20196h-s1.gif) | ||
| Scheme 1 The synthesis of substituted coumarins in the presence of [Msim]HSO4 at 40 °C under solvent-free conditions. | ||
| Entry | Substrate | β-Keto ester | Time (min) | Yieldb (%) | Ref. |
|---|---|---|---|---|---|
| a Reaction conditions: substrate, 5.0 mmol; EAA or MEAA, 5.0 mmol. b Isolated yield. c Obtained both a linear benzocoumarin (75%, mp: 267 °C) and an angular benzocoumarin (14%, mp: 276–277 °C).38 | |||||
| 1 |

|
EAA | 25 | 94 | 28 |
| MEAA | 30 | 92 | |||
| 2 |

|
EAA | 40 | 88 | 29 |
| 3 |

|
EAA | 40 | 92 | 30 |
| 4 |

|
EAA | 38 | 98 | 28 |
| MEAA | 42 | 96 | |||
| 5 |

|
EAA | 22 | 96 | 31 |
| MEAA | 26 | 92 | 32 | ||
| 6 |

|
EAA | 30 | 92 | 29 |
| 7 |

|
EAA | 32 | 88 | 29 |
| 8 |
|
EAA | 28 | 98 | 33 |
| MEAA | 34 | 96 | 32 | ||
| 9 |

|
EAA | 42 | 88 | 29 |
| 10 |

|
EAA | 40 | 90 | 34 |
| 11 |

|
EAA | 46 | 85 | 31 |
| 12 |

|
EAA | 34 | 92 | 33 |
| 13 |

|
EAA | 40 | 94 | 35 |
| 14 |

|
EAA | 42 | 93 | 36 |
| 15 |

|
EAA | 38 | 82 | 37 |
| 16 |

|
EAA | 44 | 78 | 28 |
| 17 |

|
EAA | 42 | 88 | 2 |
| 18 |

|
EAA | 45 | 75c | 38 |
The reaction of resorcinol with EAA (Table 2, entry 1) occurred within 25 minutes. This could be due to the presence of only two hydroxyl groups, which were meta to each other. However, in the case of orcinol, the reaction (Table 2, entry 4) was somewhat slower, which could be due to the steric hindrance of the methyl group ortho to the position of hydroxylalkylation. Reaction of phloroglucinol with EAA (Table 2, entry 5) took place faster within 22 minutes. This was mainly due to the presence of three hydroxyl groups that cooperated in activating the aromatic ring. Similarly, reaction of pyrogallol with EAA (Table 2, entry 8) proceeded for 28 minutes, which was slower than the reactions of resorcinol and phloroglucinol presumably due to steric hindrance of hydroxyl groups.
To compare the activities of various β-keto esters with various phenols, two different types of β-keto esters were used, namely, ethyl acetoacetate [EAA] and α-methyl ethyl acetoacetate [MEAA] (Table 2, entries 1, 4, 5 and 8). Reactivity order for β-keto esters was found to be EAA > MEAA (Table 2, entries 1, 4, 5 and 8). In all instances except two, coumarins were obtained. As exceptions, 2,7-dihydroxynaphthalene and 2-naphthol gave a mixture of linear and angular coumarins and a chromone.
The possible mechanism of the synthesis of 7-hydroxy-4-methylcoumarin in the presence of [Msim]HSO4 as a promoter under solvent-free conditions is shown in Scheme 2. On the basis of this mechanism, [Msim]HSO4 catalyzes the reaction of hydroxyalkylation by the activation of the EAA, making the carbonyl group susceptible to nucleophilic attack by resorcinol. The transesterification and dehydration occur concomitantly condensing together the two reactants at two sites to form coumarin products and regenerating [Msim]HSO4 in the reaction.
 | ||
| Scheme 2 Proposed mechanism of Pechmann reaction of resorcinol with EAA at 40 °C under solvent-free conditions. | ||
As an air and moisture stable ionic liquid, [Msim]HSO4 was superior than chloroaluminate ionic liquids in the aspect of recycle and reuse.27 The recycling performance of [Msim]HSO4 was then investigated in the reaction of resorcinol and EEA. The results listed in Fig. 1 showed that the [Msim]HSO4 could be reused five times with only a slight decrease in the catalytic activity.
![Reuse of the catalyst in Pechmann reaction in the presence of [Msim]HSO4 at 40 °C under solvent-free conditions.](/image/article/2012/CY/c2cy20196h/c2cy20196h-f1.gif) | ||
| Fig. 1 Reuse of the catalyst in Pechmann reaction in the presence of [Msim]HSO4 at 40 °C under solvent-free conditions. | ||
In summary, to the best of our knowledge, the Pechmann coupling in the presence of [Msim]HSO4 as an activator under solvent-free conditions is reported by us for the first time. The methodology has several advantages such as: green aspects by avoiding toxic catalysts and the traditional solvents, high reaction rates and excellent yields, no side reactions, ease of preparation and handling of the catalyst, effective recovery and reusability of the catalyst, use of a halogen-free catalyst with lower loading and a simple experimental procedure. Further work to explore this novel catalyst in other organic transformations is in progress.
General procedure for the preparation of coumarins
In a 25 mL batch reactor equipped with a distillation condenser the mixture of phenols (5 mmol), β-keto esters (5 mmol) and the [Msim]HSO4 prepared according to the literature26 (25 mg, 0.096 mmol) was stirred at 40 °C under solvent-free conditions for the time mentioned in Table 2. After completion of the reaction (monitored by TLC), the resulting solidified mixture was diluted with ethyl acetate (10 mL) and the catalyst was separated through decantation. The organic phase obtained was washed with water (2 × 5 mL) and the solvent was evaporated under reduced pressure, which yielded the crude product, which was purified by recrystallization in ethanol or an ethanol–water system to give the pure product as colorless prisms, pale yellow or white powder. The recovered ionic liquid, after drying, could be reused directly in the next run without further purification. All synthesized coumarin derivatives were characterized using analytical techniques like IR, 1H NMR, 13C NMR and mass spectroscopy. Also the melting points were measured for all synthesized coumarin derivatives and were compared with the corresponding reported melting points.28–38The spectral data of selected compounds
5-Hydroxy-3,4,7-trimethylcoumarin (Table 2, entry 4)
White crystals, mp: 249–251 °C (ethanol) (lit. mp: 249 °C)27; IR (KBr): ν = 3216, 3028, 2986, 1675, 1620, 1283, 1120, 1062 cm−1; 1H NMR (300 MHz, DMSO-d6) δ = 2.04 (s, 3H, CH3), 2.27 (s, 3H, CH3), 2.53 (s, 3H, CH3), 6.57 (s, 2H, ArH), 10.32 (brs, 1H, OH). MS (EI, 70 eV): m/z = 204 [M+].8-Hydroxy-4-methyl-2H-naphtho[2,3-b]pyran-2-one (Table 2, entry 18)
Yellow powder, mp: 275–277 °C (ethanol–water) (lit. mp: 267 °C)37; IR (KBr) ν = 3360, 3031, 1705, 1564, 1238, 1062 cm−1; 1H NMR (400 MHz, DMSO-d6) δ = 2.46 (s, 3H, CH3), 6.30 (s, 1H, ArH), 7.08 (dd, J = 8.8 and 1.8 Hz, 1H, ArH), 7.14 (d, J = 1.8 Hz, 1H, ArH), 7.54 (s, 1H, ArH), 7.88 (d, J = 8.8 Hz, 1H, ArH), 8.21 (s, 1H, ArH), 10.15 (brs, 1H, OH). MS (EI, 70 eV): m/z = 226 [M+].Acknowledgements
The author is thankful to Guilan University Research Council for partial support of this work.References
- R. O'Kennedy and R. D. Thornes, Coumarins: biology application and mode of action, Wiley, Chichester, 1997 Search PubMed.
- M. Zabradnik, The production and application of fluorescent brightening agents, Wiley, New York, 1992 Search PubMed.
- I. N. Costova, N. M. Nikolov and L. N. Chipilska, J. Ethnopharmacol., 1993, 39, 205–208 CrossRef.
- A. K. Mitra, A. De, N. Karchaudhuri, S. K. Misra and A. K. Mukhopadhyay, J. Indian Chem. Soc., 1998, 75, 666–671 CAS.
- G. Bravic, J. Gaultier and C. Hauw, C. R. Acad. Sci., Paris Ser. IIc: Chim., 1968, 267, 1790–1793 CAS.
- C. J. Wang, Y. J. Hsieh, C. Y. Chu, Y. L. Lin and T. H. Tseng, Cancer Lett., 2002, 183, 163–168 CrossRef CAS.
- C. J. Palmer and J. L. Josephs, J. Chem. Soc., Perkin Trans. 1, 1995, 3135–3152 RSC.
- M. Taniguchi, Y. Q. Xiao, X. H. Liu, A. Yabu, Y. Hada, L. Q. Guo, Y. Yamazoe and K. Baba, Chem. Pharm. Bull., 1999, 47, 713–715 CrossRef CAS.
- D. E. Nettleton, Drugs Future, 1996, 34, 1257–1264 Search PubMed.
- Y. Jacquot, C. Rojaz, B. Refouvelet, J. F. Robert, G. Leclercq and A. Xicluna, Mini-Rev. Med. Chem., 2003, 3, 387–400 CrossRef CAS.
- M. Noeldner, H. Hauer and S. S. Chatterjee, Drugs Future, 1996, 21, 779–781 CAS.
- S. M. Sethna and N. P. Kong, Chem. Rev., 1945, 36, 1–62 CrossRef CAS.
- H. Von Pechmann and C. Duisberg, Ber. Dtsch. Chem. Ges., 1884, 17, 929–936 CrossRef.
- J. R. Johnson, Org. React., 1942, 1, 210–265 Search PubMed.
- G. Brufola, F. Fringuelli, O. Piermatti and F. Pizzo, Heterocycles, 1996, 43, 1257–1266 CrossRef CAS.
- R. L. Shirner, Org. React., 1942, 1, 1–37 Search PubMed.
- I. Yavari, R. Hekmat-Shoar and A. Zonouzi, Tetrahedron Lett., 1998, 39, 2391–2392 CrossRef CAS.
- G. A. Cartwright and W. McNab, J. Chem. Res. (S), 1997, 296–297 RSC.
- T. S. Li, Z. H. Zhang, F. Yang and C. G. Fu, J. Chem. Res. (S), 1998, 38–39 RSC.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes, J. James and H. Davis, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS.
- A. Arfan and J. P. Bazureau, Org. Process Res. Dev., 2005, 9, 743–748 CrossRef CAS.
- P. Wasserscheid, M. Sesing and W. Korth, Green Chem., 2002, 4, 134–138 RSC.
- (a) P. Wasserscheid, R. van Hal and A. Bösmann, Green Chem., 2002, 4, 400–404 RSC; (b) J. Fraga-Dubreuil, K. Bourahla, M. Rahmouni, J. P. Bazureau and J. Hamelin, Catal. Commun., 2002, 3, 185–190 CrossRef CAS; (c) M. T. Garcia, N. Gathergood and P. J. Scammells, Green Chem., 2005, 7, 9–14 RSC; (d) Y. Gu, J. Zhang, Z. Duan and Y. Deng, Adv. Synth. Catal., 2005, 347, 512–516 CrossRef CAS; (e) P. Kalita and R. Kumar, Microporous Mesoporous Mater., 2012, 149, 1–9 CrossRef CAS.
- N. Gathergood, M. T. Garcia and P. J. Scammells, Green Chem., 2004, 6, 166–175 RSC.
- M. A. Zolfigol, A. Khazaei, A. R. Moosavi-Zare, A. Zare and V. Khakyzadeh, Appl. Catal., A, 2011, 400, 70–81 CrossRef CAS.
- N. G. Khaligh, J. Mol. Catal. A: Chem., 2011, 349, 63–70 CrossRef CAS.
- (a) H. Olivier-Bourbigou and L. Magna, J. Mol. Catal. A: Chem., 2002, 182–183, 419–437 CrossRef CAS; (b) R. A. Sheldon, Chem. Commun., 2001, 2399–2407 RSC.
- D. O. Bennardi, D. M. Ruiz, G. P. Romanelli, G. T. Baronettic, H. J. Thomas and J. C. Autino, Lett. Org. Chem., 2008, 5, 607–615 CrossRef CAS.
- D. Prajapati and M. Gohain, Catal. Lett., 2007, 119, 59–63 CrossRef CAS.
- M. M. Heravi, M. Khorasani, F. Derikvand, H. A. Oskooie and F. F. Bamoharram, Catal. Commun., 2007, 8, 1886–1890 CrossRef CAS.
- S. S. Bahekar and D. B. Shinde, Tetrahedron Lett., 2004, 45, 7999–8001 CrossRef CAS.
- B. M. Reddy, B. Thirupathi and M. K. Patil, Open Catal. J., 2009, 2, 33–39 CrossRef CAS.
- J. C. Rodrüíguez-Domínguez and G. Kirsch, Synthesis, 2006, 1895–1897 Search PubMed.
- F. Chavan, B. Madje, J. Bharad, M. Ubale, M. Ware, M. Shingare and N. Shinde, Bull. Catal. Soc. India, 2008, 7, 41–45 Search PubMed.
- S. Peipei and H. Zhixin, Synth. Commun., 2005, 33, 1875–1880 Search PubMed.
- V. Singh, J. Singh, K. P. Kaur and G. L. Kad, J. Chem. Res. (S), 1997, 58–59 RSC.
- S. Kumar, A. Saini and J. S. Sandhu, ARKIVOC, 2007, XV, 18–23 Search PubMed.
- (a) J. H. Pardanani and S. J. Sethna, J. Indian Chem. Soc., 1978, 55, 806–809 CAS; (b) Ng. Ph. Buu-Hoi and D. Lavit, J. Chem. Soc., 1956, 1743–1748 RSC.
| This journal is © The Royal Society of Chemistry 2012 |
