An aqueous-phase catalytic process for the selective hydrogenation of acetylene with monodisperse water soluble palladium nanoparticles as catalyst†
Huoli
Zhang
a,
Yuanyi
Yang
ab,
Wei
Dai
*b,
Dong
Yang
b,
Shuliang
Lu
*b and
Yuanyuan
Ji
b
aDepartment of Chemical Engineering, Beijing University of Chemical Technology, Beijing, China
bSinopec Beijing Research Institute of Chemical Industry, Beijing, China. E-mail: lusl.bjhy@sinopec.com; Fax: +86 6420 8694; Tel: +86 5920 2749
First published on 30th April 2012
Abstract
We report herein an aqueous-phase catalytic process with palladium(0) nanoparticles (PdNPs) stabilized by semi-natural water soluble cellulose as catalyst. PdNPs stabilized by sodium carboxymethyl cellulose (CMC) in water were a very efficient and stable catalyst in the aqueous-phase catalytic process for the selective hydrogenation of acetylene under mild reaction conditions. This work demonstrates the possibility of developing an aqueous-phase catalytic process using a durable and environmentally benign catalyst.
Supported palladium catalysts have attracted much attention during the last few decades because they are widely used for the selective hydrogenation of acetylene in ethylene-rich streams in the modern petrochemical industry.1–5 Generally, the ethylene stream usually contains small amounts of acetylene (0.5–2% by volume). To achieve polymer-grade ethylene, the so-called front-end hydrogenation process and tail-end hydrogenation process are two commercial gas selective hydrogenation processes at present, and it is generally required that the acetylene content should be less than 5 ppm.1
Industrially, catalytic performance of supported palladium catalysts strongly depends on the morphology and dispersion of Pd particles. Pd active-sites isolation is the principal objective to use porous oxide supports. Although porous oxide supports can provide a large surface area to form small Pd particles, porous oxide supports generally are undesirable. Because narrow and long pores might hinder the transport of reactants and products. Therefore, in the tail-end hydrogenation process, supported palladium catalysts have low stability due to the formation of plenty of oligomers (green oil) on the solid catalyst surfaces. Although much progress has been made, it is unlikely that further great progress in the stability of supported palladium catalysts can be realized in a fixed-bed reactor.6–11 The deposition of oligomers on the catalysts results in deactivation during the hydrogenation process. Therefore, regeneration of the catalysts may be required every one to three months, and an oxygenation step or a burn step at 371–455 °C is needed to remove carbonaceous deposits from the catalysts to clean the supports and re-expose palladium active-sites. Periodic regeneration of the catalysts is undesirable because the purging process results in environmentally undesirable emissions.10 Any attempt at minimizing waste and implementing sustainable process is underway.
Recently, Pd–Ga intermetallic compounds12–16 and palladium(0) nanoparticles in an ionic liquid phase17 have been investigated deeply in order to overcome the drawbacks of supported palladium catalysts. However, for green and sustainable chemistry, Pd(0) nanoparticles (PdNPs) homogeneously dispersed in water appear to be desirable to overcome the drawbacks of supported palladium catalysts. Therefore, it is compelling to synthesize monodisperse PdNPs with semi-natural water soluble cellulose (Scheme 1). Moreover, transition metal–organic hybrid composites can also facilitate the development of advanced materials for catalysis in optimized interphases.18–27 Herein we report our first step to develop an aqueous-phase catalytic process for the selective hydrogenation of acetylene with monodisperse water soluble PdNPs as catalyst.
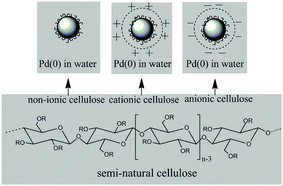 | ||
| Scheme 1 Schematic elucidation of PdNPs stabilized by the three types of semi-natural cellulose in water. | ||
Natural cellulose as a bioresource has recently attracted much attention because of its potential applications.28–33 The materials of semi-natural cellulose are derived from natural cellulose. Semi-natural cellulose such as non-ionic cellulose (hydroxyethyl cellulose), cationic cellulose (polyquaternium-10), and anionic cellulose (sodium carboxymethyl cellulose) are water-soluble soft materials, which are renewable, inexpensive, nontoxic, and linear biopolymer matrices in aqueous media. Accordingly, each of them has its special functional group, which can coordinate with metal nanoparticles and form an excellent synergistic effect to obtain stable metal nanoparticles.34–36 However, few studies have so far been reported on the synthesis of PdNPs with semi-natural cellulose. In this work, three types of semi-natural water soluble cellulose have been used to synthesize PdNPs which were used as catalyst for the selective hydrogenation of acetylene in water.
As can be seen in Fig. 1, PdNPs stabilized by semi-natural cellulose in water do not have any surface plasmon band due to the very small particle size.37,38 The UV-visible spectrum of the Pd(II) ions in aqueous solution shows an absorption band at 380 nm because of a d–d transition before reduction. It can be observed that the absorption peak disappears after reduction (Fig. 1). After the reduction, the broad bands appeared in a wide wavelength region, which indicated that PdNPs were formed.37,38 The colour of the solutions turned from pale yellow to dark brown which also indicates the reduction of Pd(II) ions (Fig. 2).
 | ||
| Fig. 1 UV-visible spectra: (a) an aqueous solution of Pd(II) ions, (b) PdNPs stabilized by HEC in water, (c) PdNPs stabilized by polyquaternium-10 in water, (d) PdNPs stabilized by CMC in water. | ||
 | ||
| Fig. 2 Samples: (a) an aqueous solution of Pd(II) ions, (b) PdNPs stabilized by HEC in water, (c) PdNPs stabilized by polyquaternium-10 in water, (d) PdNPs stabilized by CMC in water. | ||
The catalytic reactions in water were investigated for the selective hydrogenation of acetylene in ethylene-rich streams (Table 1). The catalysts in entries 1–3 are PdNPs stabilized by hydroxyethyl cellulose (HEC), polyquaternium-10, and sodium carboxymethyl cellulose (CMC), respectively. The results from entries 1–3 showed that the catalytic performance of PdNPs is in accordance with the particles size and dispersion (Fig. 3). The transmission electron microscopy (TEM) characterization showed that the catalyst PdNPs stabilized by CMC (Table 1, entry 3) obviously had the smallest particle size and the best dispersion (Fig. 3c). In contrast, the catalyst PdNPs stabilized by HEC (Table 1, entry 1) formed larger particles (Fig. 3a) and the catalyst PdNPs stabilized by polyquaternium-10 (Table 1, entry 2) agglomerated and linked together (Fig. 3b). Fig. 3 shows that the average particle size increased from 2.1 to 3.9 nm and the deviation from the mean diameter is about ±0.4 nm. Meanwhile, the catalyst PdNPs stabilized by CMC produced nearly monodisperse palladium nanoparticles (Fig. 3c).
| Entry | T/°C | Catalyst | Conv.b (%) | Sel.c (%) |
|---|---|---|---|---|
| a Reaction conditions: the total gas flow rate was 12 mL min−1 and the stirring speed was 300 rpm. b Acetylene conversion, calculating formula was based on ref. 7. c Ethylene selectivity, calculating formula was based on ref. 39. d The gas ethylene-rich stream contains 0.45% C2H2, H2/C2H2 = 3.5. e Literature data from ref. 39. f The gas ethylene-rich stream contains 0.45% C2H2, H2/C2H2 = 1.5. g Literature data from ref. 11. | ||||
| 1d | 40 | PdNPs stabilized by HEC | 65 | 29 |
| 2d | 40 | PdNPs stabilized by polyquaternium-10 | 53 | 60 |
| 3d | 40 | PdNPs stabilized by CMC | 100 | 50 |
| 4e | 40 | Pd–Ti/SiO2 | 93 | 11 |
| 5f | 80 | PdNPs stabilized by CMC | 89 | 84 |
| 6g | 80 | Pd/Zn-modified-α-Al2O3 | 70 | 68 |
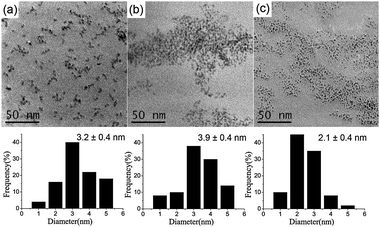 | ||
| Fig. 3 TEM images and size distributions of PdNPs after reaction: (a) PdNPs stabilized by HEC, (b) PdNPs stabilized by polyquaternium-10, (c) PdNPs stabilized by CMC. | ||
We focused our work on the catalyst PdNPs stabilized by CMC because the catalyst PdNPs stabilized by CMC had the best catalytic activity and selectivity for the selective hydrogenation of acetylene in the aqueous-phase catalytic process among the three types of the PdNPs catalysts. A high-resolution TEM (HRTEM) experiment was used to detect the nature of the surface structure of PdNPs stabilized by CMC. The HRTEM image (Fig. 4) shows two kinds of Pd(111) and Pd(200) lattice spacings. The selective area electron diffraction (SAED) pattern obtained when acquiring the HRTEM image reveals a face-centered cubic (fcc) structure corresponding to the indexed planes of the crystals of Pd(0) (111), (200), (220), (311). In the presence of CMC, the (111) and (200) lattice spacings of PdNPs are a little larger than those of the bulk Pd(0) (2.25 Å, 1.95 Å). Similar phenomena have been reported by Lamber et al.40 and Lu et al.41 One of the possible reasons is that relaxing atoms of PdNPs surfaces interact strongly with the surrounding hydroxyl group or the carboxyl group.35 Meanwhile, the hydroxyl group or the carboxyl group of the CMC molecules not only anchored to the PdNPs surfaces but also increased the electron binding energy of PdNPs.15,35 The binding energy of PdNPs stabilized by CMC is higher (335.5 eV) than that of metallic Pd(0) (335.0 eV) (Fig. S1, ESI†). It is close to the binding energy of the Pd–C phase (335.6 to 335.7 eV), which is a carbon-containing stable phase on the palladium surface of solid catalysts. The Pd–C phase is selective in alkyne hydrogenation. The modified binding energy eventually made the palladium nanoparticles bind acetylene strongly and ethylene weakly. This resulted in their good selectivity.15,42–44 On the other hand, CMC as a carboxylate polyelectrolyte in water provides electrostatic and steric stabilization to PdNPs. As catalyst, PdNPs stabilized by CMC are homogeneously dispersed in water. Moreover, PdNPs stabilized by CMC maintained a good colloidal stability with a zeta-potential value of −31.5 mV (Fig. S2, ESI†). This resulted in their high activity and good stability under mild reaction conditions.
 | ||
| Fig. 4 High-resolution TEM image of PdNPs stabilized by CMC and the inset shows the selective area electron diffraction (SAED) pattern of these Pd(0) nanoparticles. | ||
In the tail-end hydrogenation process, acetylene must be completely removed from ethylene-rich streams. Therefore, a slight excess of hydrogen is added to the gas feed streams over the quantity required for the H2/C2H2 (mole ratio) to be equal to one. Industrially, the selectivity of the catalyst beds is mainly controlled by the value of H2/C2H2 and temperature.1 In general, ethylene selectivity will decrease as the value of H2/C2H2 increases at the same temperature. For ethylene selectivity, a comparison between the catalyst PdNPs stabilized by CMC and conventional supported Pd catalysts is made. When the value of H2/C2H2 was high (H2/C2H2 ≥ 2), the catalytic performance of a TiO2-modified Pd catalyst supported on SiO2 was reported by Kang et al.39 The catalyst had a low selectivity at 40 °C and the value of H2/C2H2 was 2 (Table 1, entry 4). However, for the catalyst PdNPs in the aqueous-phase catalytic process, when the value of H2/C2H2 was 3.5, it even had a higher selectivity than Pd–Ti/SiO2 at 40 °C (Table 1, entry 3). On the other hand, for a low value of H2/C2H2 (H2/C2H2 ≤ 2), a Pd catalyst supported on Zn-modified α-Al2O3 for the selective hydrogenation of acetylene in ethylene feed stock was investigated by Chinayon et al.11 The value of H2/C2H2 was 1.1 and the result showed a high selectivity at 80 °C (Table 1, entry 6). For the catalyst PdNPs in the aqueous-phase catalytic process, when the value of H2/C2H2 was 1.5, it also had a higher selectivity than Pd/Zn-modified-α-Al2O3 (Table 1, entry 5). It can be seen from entries 3–6 that the catalyst PdNPs stabilized by CMC in water was a more selective catalyst than the conventional supported palladium catalysts for the selective hydrogenation of acetylene at the same temperature.
Acetylene hydrogenation is a typical consecutive reaction because ethylene is produced as an intermediate. Therefore, for conventional supported palladium catalysts, it has been reported that the ethylene selectivity decreases when acetylene conversion gradually increases.39 With respect to the aqueous-phase catalytic process using PdNPs stabilized by CMC, the conversion and the selectivity have a similar relationship in acetylene hydrogenation. As shown in Fig. 5, the ethylene selectivity decreases with the conversion increasing. Finally, the selectivity becomes negative at high acetylene conversion, indicating that there was a net loss of ethylene in the aqueous-phase catalytic process.
 | ||
| Fig. 5 The conversion–selectivity relationship in acetylene hydrogenation on stream catalyzed by PdNPs stabilized by CMC in water. Reaction conditions: the ethylene-rich stream contains 0.45% C2H2, H2/C2H2 = 3.5, the stirring speed was 900 rpm and the total gas flow rate was 160 mL min−1. | ||
We further investigated the selective hydrogenation of acetylene at different temperatures. As can be seen from Table 2 (entries 1–3), the catalyst PdNPs stabilized by CMC in water still kept a high activity. However, its selectivity gradually dropped with the increase in temperature from 50 to 70 °C. To test the catalytic performance of PdNPs stabilized by CMC in water, they were reused for 4 times at different temperatures when the total gas flow rate was increased up to 160 mL min−1 (Table 2, entries 4–7). As the temperature increased, acetylene conversion increased but ethylene selectivity still had a tendency to decrease. The reason was that the side reaction happened more quickly when the temperature rose. Small amounts of C4 hydrocarbons were detected by gas chromatography (GC) when the total gas flow rate was kept at 160 mL min−1 (Fig. S3, ESI†). Meanwhile, no deactivation or green oil was observed for 24 hours. PdNPs stabilized by CMC were stable in water and no precipitation was found for 10 months after reactions (Fig. S4, ESI†). We also carried out the catalytic test for 12 hours on stream and catalyst reuse. After the initial reaction period, PdNPs stabilized by CMC showed stable activity and selectivity (Fig. S5, ESI†). Moreover, TEM images of the catalyst PdNPs stabilized by CMC showed that there were no agglomerations after reactions (Fig. S6, ESI†).
| Entry | T/°C | Conv.b (%) | Sel.c (%) |
|---|---|---|---|
| a PdNPs stabilized by CMC (Table 1, entry 3). b Acetylene conversion, calculating formula was based on ref. 7. c Ethylene selectivity, calculating formula was based on ref. 39. d Reaction conditions: the total gas flow rate was 12 mL min−1; the stirring speed was 300 rpm and the gas feed stream contains 0.45% C2H2, H2/C2H2 = 3.5. e Reaction conditions: the ethylene-rich stream contains 0.45% C2H2, H2/C2H2 = 3.5, the stirring speed was 900 rpm and the total gas flow rate was 160 mL min−1. | |||
| 1d | 50 | 100 | 31 |
| 2d | 60 | 100 | 12 |
| 3d | 70 | 100 | −8 |
| 4e | 40 | 74 | 63 |
| 5e | 50 | 92 | 33 |
| 6e | 60 | 94 | 15 |
| 7e | 70 | 95 | −5 |
In fact, it has been reported that CMC could work as a suitable stabilizer for the stabilization of Pt nanoparticles in aqueous solution when the concentration of CMC ranged from 0.05 wt% to 0.15 wt%.35 The effects of different CMC concentrations on the stability of PdNPs also have been investigated in this work. Experimental results have revealed that PdNPs stabilized by CMC were found to be stable in aqueous solution when the concentration of CMC ranged from 0.03 wt% to 0.12 wt%. Meanwhile, the CMC concentration within this range (from 0.03 wt% to 0.12 wt%) had no great effect on the Pd particle size (Fig. S7, ESI†).
In summary, we have developed an aqueous-phase catalytic process with the catalyst monodisperse water soluble PdNPs. PdNPs stabilized by CMC in the aqueous-phase catalytic process possessed higher selectivity than that of conventional Pd catalyst supported on Al2O3 or SiO2 at the same temperature. PdNPs stabilized by CMC in water were a very effective and stable catalyst for the selective hydrogenation of acetylene under mild reaction conditions. These results provide a new approach for the selective hydrogenation of acetylene in ethylene-rich streams.
Experimental section
Hydroxyethyl cellulose (Mw: 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), polyquaternium-10, and sodium carboxymethyl cellulose (Mw: 90
000), polyquaternium-10, and sodium carboxymethyl cellulose (Mw: 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were supplied by Sigma-Aldrich. Pd(NO3)2 (50 mg mL−1) and feed gas were obtained from Sinopec Beijing Research Institute of Chemical Industry.
000) were supplied by Sigma-Aldrich. Pd(NO3)2 (50 mg mL−1) and feed gas were obtained from Sinopec Beijing Research Institute of Chemical Industry.
In a typical synthesis, a 150 mL aliquot of a 1 mg mL−1 solution of Pd(NO3)2 was added to 350 mL of a 0.06 wt% aqueous solution of soluble semi-natural cellulose. Meanwhile, the pH was adjusted to about 7 by dropwise addition of an aqueous solution of sodium hydroxide (NaOH, 0.5 M). The mixture in a stainless steel autoclave was treated with a hydrogen flow passing through the solution at 40 °C for at least 30 minutes at atmospheric pressure. The stirring speed was 300 rpm. The mixture was subsequently reduced to obtain Pd(0) nanoparticles. For the evaluation of the catalyst, a solution of the freshly prepared palladium(0) nanoparticles stabilized by semi-natural cellulose (200 mL) was placed in a 500 mL stainless steel autoclave. Feed gas was introduced at the bottom of the autoclave and flowed out from the top (1 atm). Sampling is online. When the reaction temperature was kept at a certain centigrade temperature, the reactor outlet products were analyzed at about 2 hours on stream by a gas chromatograph (Agilent 6890) with a FID and a HP-PLOT/Q column (30 m × 0.535 mm × 40.00 μm). The detection limit of the GC (Agilent 6890) is 1 ppm for acetylene.
Characterization: TEM images were recorded by a FEI Tecnai 20 (200 kV) and a high-resolution TEM image was recorded by a JEOL JEM-3010 (200 kV). The sample for TEM characterization was prepared by placing a drop of the obtained solution after reaction on a carbon-coated copper grid, followed by drying in vacuum at room temperature. The particle size distribution was determined by counting 100 particles from TEM images. UV-visible absorption spectra of the solution before and after the reduction of palladium ions were measured by a Thermo Fisher Evolution 300 spectrophotometer.
Acknowledgements
This work was supported by Sinopec Beijing Research Institute of Chemical Industry (01-09ZS0440).Notes and references
- A. Borodziński and G. C. Bond, Catal. Rev. Sci. Eng., 2006, 48, 91 Search PubMed.
- A. Borodziński and G. C. Bond, Catal. Rev. Sci. Eng., 2008, 50, 379 Search PubMed.
- Á. Molnár, A. Sárkány and M. Varga, J. Mol. Catal. A: Chem., 2001, 173, 185 CrossRef.
- M. M. Johnson, D. W. Walker and G. P. Nowack, US Pat., 4404124 1983 Search PubMed.
- C. N. Thanh, B. Didillon, P. Sarrazin and C. Cameron, US Pat., 6054409 2000 Search PubMed.
- R. J. Liu, P. A. Crozier, C. M. Smith, D. A. Hucul, J. Blackson and G. Salaita, Appl. Catal., A, 2005, 282, 111 CrossRef CAS.
- A. Pachulski, R. Schödel and P. Claus, Appl. Catal., A, 2011, 400, 14 CrossRef CAS.
- A. Y. Stakheev and L. M. Kustov, Appl. Catal., A, 1999, 188, 3 CrossRef CAS.
- B. L. Mojet, J. T. Miller, D. E. Ramaker and D. C. Koningsberger, J. Catal., 1999, 186, 373 CrossRef CAS.
- Y. J. Huang, C. F. Shun, L. G. Daniel, E. L. Mohundro and J. E. Hartgerink, US Pat., 5332705 1994 Search PubMed.
- S. Chinayon, O. Mekasuwandumrong, P. Praserthdam and J. Panpranot, Catal. Commun., 2008, 9, 2297 CrossRef CAS.
- J. Osswald, R. Giedigkeit, R. E. Jentoft, M. Armbrüster, F. Girgsdies, K. Kovnir, T. Ressler, Y. Grin and R. Schlögl, J. Catal., 2008, 258, 210 CrossRef CAS.
- J. Osswald, K. Kovnir, M. Armbrüster, R. Giedigkeit, R. E. Jentoft, U. Wild, Y. Grin and R. Schlögl, J. Catal., 2008, 258, 219 CrossRef CAS.
- L. Shao, W. Zhang, M. Armbrüster, D. Teschner, F. Girgsdies, B. Zhang, O. Timpe, M. Friedrich, R. Schlögl and D. S. Su, Angew. Chem., Int. Ed., 2011, 50, 1 CrossRef.
- K. Kovnir, J. Osswald, M. Armbrüster, D. Teschner, G. Weinberg, U. Wild, A. Knop-Gericke, T. Ressler, Y. Grin and R. Schlögl, J. Catal., 2009, 264, 93 CrossRef CAS.
- M. Armbrüster, K. Kovnir, M. Behrens, D. Teschner, Y. Grin and R. Schlögl, J. Am. Chem. Soc., 2010, 132, 14745 CrossRef.
- M. Ruta, G. Laurenczy, P. J. Dyson and L. Kiwi-Minsker, J. Phys. Chem. C, 2008, 112, 17814 CAS.
- E. Lindner, T. Schneller, F. Auer and H. A. Mayer, Angew. Chem., Int. Ed., 1999, 38, 2154 CrossRef.
- B. Jandeleit, D. J. Schaefer, T. S. Powers, H. W. Turner and W. H. Weinberg, Angew. Chem., Int. Ed., 1999, 38, 2494 CrossRef CAS.
- N. E. Leadbeater and M. Marco, Chem. Rev., 2002, 102, 3217 CrossRef CAS.
- C. A. Mcnamara, M. J. Dixon and M. Bradley, Chem. Rev., 2002, 102, 3275 CrossRef CAS.
- T. J. Dickerson, N. N. Reed and K. D. Janda, Chem. Rev., 2002, 102, 3325 CrossRef CAS.
- Z.-L. Lu, E. Lindner and H. A. Mayer, Chem. Rev., 2002, 102, 3543 CrossRef CAS.
- B. L. Cushing, V. L. Kolesnichenko and C. J. OConnor, Chem. Rev., 2004, 104, 3893 CrossRef CAS.
- C. Burda, X.-B. Chen, R. Narayanan and M. A. EI-Sayed, Chem. Rev., 2005, 105, 1025 CrossRef CAS.
- B. J. Gallon, R. W. Kojima, R. B. Kaner and P. L. Diaconescu, Angew. Chem., Int. Ed., 2007, 46, 7251 CrossRef CAS.
- W. J. Stark, Angew. Chem., Int. Ed., 2011, 50, 1242 CrossRef CAS.
- D. Klemm, F. Kramer, S. Moritz, T. Lindström, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 5438 CrossRef CAS.
- D. Klemm, B. Heublein, H.-P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358 CrossRef CAS.
- R. J. Moon, A. Martini, J. Nairn, J. Simonsen and J. Youngblood, Chem. Soc. Rev., 2011, 40, 3941 RSC.
- C. M. Cirtiu, A. F. Dunlop-Brière and A. Moores, Green Chem., 2011, 13, 288 RSC.
- K. Benaissi, L. Johnson, D. A. Walsh and W. Thielemans, Green Chem., 2010, 12, 220 RSC.
- L. Heath and W. Thielemans, Green Chem., 2010, 12, 1448 RSC.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940 CrossRef CAS.
- J. C. Liu, J. Sutton and C. B. Roberts, J. Phys. Chem. C, 2007, 111, 11566 CAS.
- F. He, D. Y. Zhao, J. C. Liu and C. B. Roberts, Ind. Eng. Chem. Res., 2007, 46, 29 CrossRef CAS.
- K. Esumi, R. Isono and T. Yoshimura, Langmuir, 2004, 20, 237 CrossRef CAS.
- E. Coronado, A. Ribera, J. García-Martínez, N. Linares and L. M. Liz-Marzán, J. Mater. Chem., 2008, 18, 5682 RSC.
- J. H. Kang, E. W. Shin, W. J. Kim, J. D. Park and S. H. Moon, J. Catal., 2002, 208, 310 CrossRef CAS.
- R. Lamber, S. Wetjen and N. Jaeger, Phys. Rev. B: Condens. Matter, 1995, 51, 10968 CrossRef CAS.
- P. Lu, J. Dong and N. Toshima, Langmuir, 1999, 15, 7980 CrossRef CAS.
- F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sørensen, C. H. Christensen and J. K. Nørskov, Angew. Chem., Int. Ed., 2008, 47, 9299 CrossRef CAS.
- F. Studt, F. Abild-Pedersen, T. Bligaard, R. Z. Sørensen, C. H. Christensen and J. K. Nørskov, Science, 2008, 320, 1320 CrossRef CAS.
- D. Teschner, Z. Révay, J. Borsodi, M. Hävecker, A. Knop-Gericke, R. Schlögl, D. Milroy, S. D. Jackson, D. Torres and P. Sautet, Angew. Chem., Int. Ed., 2008, 47, 9274 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Stability of the catalyst and the catalyst recycling, detailed analysis of products using GC, XPS data and zeta-potential data. See DOI: 10.1039/c2cy20179h |
| This journal is © The Royal Society of Chemistry 2012 |
