Tuning lipase activity with perfluoro carboxylic acids as additives†
Carlos G.
Acevedo-Rocha
ab and
Manfred T.
Reetz
*ab
aMax-Planck-Institut für Kohlenforschung, Kaiser-Wilhelm-Platz 1, 45470 Mülheim, Germany. E-mail: reetz@mpi-muelheim.mpg.de; Tel: +49 (0)208/306-2002
bFachbereich Chemie, Philipps-Universität-Marburg, Marburg, Germany. Tel: +49 (0)6421-28-25500
First published on 3rd May 2012
Abstract
Perfluoro fatty acids of diverse chain length can be used as additives to tune lipase activity, as documented for the thermostable lipase of the bacterium Thermoanaerobacter thermohydrosulfuricus that showed up to 260% activity enhancement upon incubation with perfluorodecanoic acid.
Lipases (EC 3.1.1.3) are the most common biocatalysts used in biotechnology and organic chemistry, the reason being their broad substrate acceptance for hydrolysing and synthesizing carboxylic acid esters in aqueous and non-aqueous solutions, respectively. As hydrolases, lipases are widely used to degrade fats and oils in the detergent, food, pulp and paper industry while as synthetases they are useful for chemo-, regio- and stereoselective transformations of a wide range of, inter alia, prochiral or chiral alcohols, carboxylic acids, diols, cyano- and chlorohydrins, lactones, amines and diamines.1–10
Although lipase-catalysed reactions often proceed rapidly with high regio- and/or enantioselectivity, this is by far not always the case. Depending upon the nature of the substrate, a given compound may not even be accepted or it may react with unacceptably low rate. In order to solve problems of this kind, directed evolution has emerged as a reliable protein engineering method.11–17 Recent examples are the protein engineering of lipases with enhanced activity and stereoselectivity using advanced directed evolution methods.18–20 Nonetheless, other common strategies primarily employed to enhance lipase activity and sometimes also enantioselectivity include variation of organic solvents,21 immobilization,22 or use of additives including triethylamine, salts and polyethylene glycol,23 crown ethers and cyclodextrins,23,24 non-ionic surfactants,25 solid-state buffers,26 and metal ions.27 The simple addition of substances to lipases may thus increase dramatically their activity, but the outcome regarding enantioselectivity remains uncertain.
Recently, we discovered that the addition of perfluorinated fatty acids of different chain length to the monooxygenase P450-BM3 increases dramatically the catalytic activity towards oxidation of methane and other challenging small alkanes.28 It was speculated that these additives enter the large binding pockets of the enzyme. Whatever the underlying factor may actually be, we wondered whether perfluoro carboxylic acids could function as activating additives in other enzyme-catalyzed reactions. Therefore, we turned our attention to lipases because these are the most widely used biocatalysts in organic synthesis.
As a model enzyme, we chose the recently well-characterized lipase from the thermostable bacterium Thermoanaerobacter thermohydrosulfuricus,29 aka TTL. It not only exhibits reasonable activity at broad ranges of temperature (40–90 °C) and pH (6.5–10), but also displays enhanced activity upon harsh treatments with metals, organic solvents and denaturing agents.
In the present work TTL was expressed in E. coli BL21-Gold(DE3) cells, purified and characterised (see ESI†). To test the influence of several perfluoro substances on lipase activity, TTL was pre-incubated for one hour at 30 °C with several perfluoro carboxylic acids, 1–13, and perfluoro alcohols, 14–18 (Scheme 1).
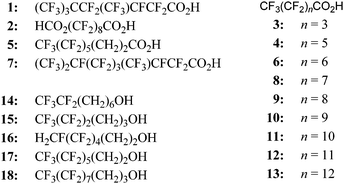 | ||
| Scheme 1 Perfluoro carboxylic acids 1–13 and alcohols 14–18 (for more details regarding name and commercial source, see ESI†). | ||
Next, the activity of TTL was determined using esters of various side-chain lengths, 19–26, by measuring the rate of release of p-nitrophenol, 27, as described in the ESI† (Scheme 2; Table 1).
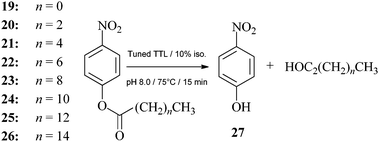 | ||
| Scheme 2 Lipase activity test using p-nitrophenyl esters. | ||
| a Each sample was measured in triplicate and corrected to p-nitrophenol auto-hydrolysis. All absorbance values were then normalized to an absolute value of 1.0 for TTL containing isopropanol as co-solvent but without additive because the effect of isopropanol on TTL was minimal. The effect of the additives and co-solvent on p-nitrophenol auto-hydrolysis in the absence of TTL was negligible. b A negative effect of additives on lipase activity is shown in white (<1.0 fold) background, whereas neutral and positives effects are displayed on light (1.0) and dark (>1.0 fold) grey backgrounds, respectively. |
|---|
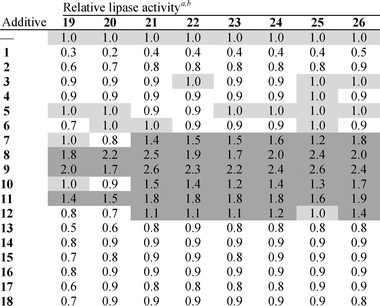
|
As shown in Table 1, it becomes apparent that perfluoro carboxylic acids of medium length, 3–6, and alcohols 14–18 display a minimal or slight negative effect on lipase activity. An even stronger inhibition is provoked by the very short-chained acid 1 or the di-acid 2. Interestingly, the longest perfluoro fatty acid 13 also induces notable inhibition. In sharp contrast, a pronounced activating effect is observed when employing perfluoro acids having medium to long chain lengths (additives 7–12). Thus, positive tuning of TTL strongly depends on the length of the carboxylic acid, perfluoro nonanoic, 8, and decanoic, 9, acids constituting the optimal additives. The contrasting effects of 9 (1.7- to 2.6-fold) and 2 (0.6- to 0.9-fold) reveal that hydrophobicity plays an important role in influencing lipase activity, irrespective of the substrate used. In other words, since the C10 perfluoro di-carboxylic acid 2 and the mono-carboxylic acid 9 acid differ only in the nature of one moiety (HO2C vs. CF3) and not in length, the more pronounced hydrophobicity in 9 is crucial for activity enhancement.
A hallmark of most lipases is the presence of a flexible lid that exposes a catalytic triad in the active site upon activation with lipid aggregates30 or exposure to heat.31 In the case of TTL, it is known that it can be maximally activated at 80 °C for 60 minutes prior to use in catalysis at its optimal temperature of 75 °C.32 Importantly, even after fully activating TTL under these conditions, we noticed that 9 exhibits a similar positive effect on lipase activity, whereas the effect is not observed when using 2 as the additive (data not shown).
It is worth mentioning that the effects reported in Table 1 were recorded using a final concentration of 100 μM 1–18 in relation to 0.25 to 2.5 μg TTL (the shorter the substrate, the less lipase amount was needed). However, in our optimization experiments, we observed that higher concentrations (e.g. 1–10 mM) of perfluoro carboxylic acids 1–13 had a contrasting negative effect on lipase activity, while this effect was minimal and sometimes slightly positive in the case of the perfluoro alcohols 14–18. Therefore, it should be kept in mind that, besides chain length and hydrophobicity, the right concentration of perfluoro fatty acid is necessary to exert a positive effect on lipase activity.
Recently, the use of traditional fatty acids as additives for better immobilization of a lipase in organic solvent was reported.33 The underlying reason for more efficient immobilization as ascertained by activity measurements was not elucidated. We were nonetheless interested in studying the traditional fatty acids as additives in our experiments. Similar or even slightly higher positive effects were observed, although this required the use of 10 times more non-perfluoro additives. However, upon optimal heat treatment (80 °C for 1 hour) and incubation with the non- and fluorinated fatty acids showing the highest effects on TTL activity i.e. undecanoic acid and 9, clear differences were observed. In the case of the normal fatty acid, undecanoic acid, no enhanced rate of reaction was detected, in contrast to perfluoro decanoic acid (data not shown). Therefore, perfluoro carboxylic acids may be useful additives when immobilizing lipases under harsh conditions.
In conclusion, we have discovered that appropriate perfluoro carboxylic acids are useful additives in the quest to enhance the activity of the thermophilic lipase from T. thermohydrosulfuricus. In general, the best perfluoro fatty acids are those that have medium to long (but not too long) chains. Up to 260% rate enhancement was observed. It remains to be seen whether other lipases behave in a similar manner and whether the strategy presented here would also be valid for the inverse synthetic reaction as this could potentially represent an important factor for other applications. In any industrial process, it is also important to consider the benefit–cost ratio of using perfluoro fatty acids.
We thank Dr Marina Royter (Technical University Hamburg) as well as Dr Michael Hoesl (Technical University Berlin) for providing the TTL as protein and plasmid forms. We are also grateful to Dr Uwe Linne (Philipps-Universität-Marburg) for helping us with the mass analyses. This work was supported by the Arthur C. Cope foundation.
Notes and references
- P. Woolley and S. B. Petersen, Lipases: Their Structure, Biochemistry and Application, Cambridge University Press, Cambridge, 1994 Search PubMed.
- R. D. Schmid and R. Verger, Angew. Chem., Int. Ed., 1998, 37, 1608–1633 CrossRef.
- K. E. Jaeger and M. T. Reetz, Trends Biotechnol., 1998, 16, 396–403 CrossRef CAS.
- K. E. Jaeger and T. Eggert, Curr. Opin. Biotechnol., 2002, 13, 390–397 CrossRef CAS.
- M. T. Reetz, Curr. Opin. Chem. Biol., 2002, 6, 145–150 CrossRef CAS.
- U. T. Bornscheuer, C. Bessler, R. Srinivas and S. H. Krishna, Trends Biotechnol., 2002, 20, 433–437 CrossRef CAS.
- R. Gupta, N. Gupta and P. Rathi, Appl. Microbiol. Biotechnol., 2004, 64, 763–781 CrossRef CAS.
- U. T. Bornscheuer and R. J. Kazlauskas, Hydrolases in Organic Synthesis: Regio- and Stereoselective Biotransformations, Wiley-VCH, Weinheim, 2005 Search PubMed.
- M. Salameh and J. Wiegel, Adv. Appl. Microbiol., 2007, 61, 253–283 CrossRef CAS.
- M. L. Verma, W. Azmi and S. S. Kanwar, Acta Microbiol. Immunol. Hung., 2008, 55, 265–294 CrossRef CAS.
- S. Bershtein and D. S. Tawfik, Curr. Opin. Chem. Biol., 2008, 12, 151–158 CrossRef CAS.
- N. J. Turner, Nat. Chem. Biol., 2009, 5, 567–573 CrossRef CAS.
- R. J. Kazlauskas and U. T. Bornscheuer, Nat. Chem. Biol., 2009, 5, 526–529 CrossRef CAS.
- A. V. Shivange, J. Marienhagen, H. Mundhada, A. Schenk and U. Schwaneberg, Curr. Opin. Chem. Biol., 2009, 13, 19–25 CrossRef CAS.
- P. A. Romero and F. H. Arnold, Nat. Rev. Mol. Cell Biol., 2009, 10, 866–876 CrossRef CAS.
- M. T. Reetz, Angew. Chem., Int. Ed., 2011, 50, 138–174 CrossRef CAS.
- M. Baker, Nat. Methods, 2011, 8, 623–626 CrossRef CAS.
- M. T. Reetz, S. Prasad, J. D. Carballeira, Y. Gumulya and M. Bocola, J. Am. Chem. Soc., 2010, 132, 9144–9152 CrossRef CAS.
- S. Prasad, M. Bocola and M. T. Reetz, ChemPhysChem, 2011, 12, 1550–1557 CrossRef CAS.
- H. B. Brundiek, A. S. Evitt, R. Kourist and U. T. Bornscheuer, Angew. Chem., Int. Ed., 2011, 51, 412–414 CrossRef.
- M. Matsumoto, K. Kida and K. Kondo, J. Chem. Technol. Biotechnol., 2001, 76, 1070–1073 CrossRef CAS.
- F. X. Malcata, H. Reyes, H. Garcia, C. Hill and C. Amundson, J. Am. Oil Chem. Soc., 1990, 67, 890–910 CrossRef CAS.
- F. Theil, Tetrahedron, 2000, 56, 2905–2919 CrossRef CAS.
- Y. Mine, K. Fukunaga, K. Itoh, M. Yoshimoto, K. Nakao and Y. Sugimura, J. Biosci. Bioeng., 2003, 95, 441–447 CAS.
- Y. Y. Liu, J. H. Xu and Y. Hu, J. Mol. Catal. B: Enzym., 2000, 10, 523–529 CrossRef CAS.
- M. Quiros, M. C. Parker and N. J. Turner, J. Org. Chem., 2001, 66, 5074–5079 CrossRef CAS.
- T. Okamoto, E. Yasuhito and S. Ueji, Org. Biomol. Chem., 2006, 4, 1147–1153 CAS.
- F. E. Zilly, J. P. Acevedo, W. Augustyniak, A. Deege, U. W. Hausig and M. T. Reetz, Angew. Chem., Int. Ed., 2011, 50, 2720–2724 CrossRef CAS.
- M. Royter, M. Schmidt, C. Elend, H. Hobenreich, T. Schafer, U. T. Bornscheuer and G. Antranikian, Extremophiles, 2009, 13, 769–783 CrossRef CAS.
- R. Verger, Annu. Rev. Biophys. Bioeng., 1976, 5, 77–117 CrossRef CAS.
- P. Rathi, S. Bradoo, R. K. Saxena and R. Gupta, Biotechnol. Lett., 2000, 22, 495–498 CrossRef CAS.
- M. G. Hoesl, C. G. Acevedo-Rocha, S. Nehring, M. Royter, C. Wolschner, B. Wiltschi, N. Budisa and G. Antranikian, ChemCatChem, 2011, 3, 213–221 CrossRef CAS.
- T. K. Ozturk and A. Kilinc, J. Mol. Catal. B: Enzym., 2010, 67, 214–218 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Chemicals, protein expression, purification and analytical characterization; spectrophotometric lipase assay. See DOI: 10.1039/c2cy20173a |
| This journal is © The Royal Society of Chemistry 2012 |
