Aqueous biphasic hydrogenation of benzene catalyzed by ruthenium complex of trisulfonated tris(biphenyl)phosphine
Aasif A.
Dabbawala
a,
Hari C.
Bajaj
*a,
Hervé
Bricout
b and
Eric
Monflier
b
aDiscipline of Inorganic Materials and Catalysis, Central Salt and Marine Chemicals Research Institute, Council of Scientific and Industrial Research, G. B. Marg, Bhavnagar 364002, Gujarat, India. E-mail: hcbajaj@csmcri.org; Fax: +91-278-2566970; Tel: +91-278-2471793
bUniversité d'Artois, Unité de Catalyse et Chimie du Solide (UCCS), UMR CNRS 8181, Faculté des Sciences Jean Perrin, Rue Jean Souvraz, SP 18-62307 Lens Cedex, France
First published on 2nd July 2012
Abstract
Ruthenium-catalyzed aqueous biphasic hydrogenation of benzene was investigated using the water-soluble ligand trisulfonated tris(biphenyl)phosphine (BiphTS). The ruthenium/BiphTS system exhibited an efficient catalytic activity and effectively hydrogenated benzene to cyclohexane with 100% selectivity under mild conditions. The effect of reaction temperature, partial pressure of hydrogen, ligand/Ru molar ratio, benzene/Ru molar ratio, non-ionic surfactants, thiophene concentration and reusability of catalyst were investigated systematically for the hydrogenation of benzene. A considerably high TOF (up to 8000 h−1) was obtained in the presence of mass transfer promoters (such as non ionic surfactants). The Ru/BiphTS was easily recovered from the product by a simple phase separation and recycled.
1. Introduction
The hydrogenation of benzene to cyclohexane is one of the imperative industrially performed hydrogenation reactions. Millions of tonnes of cyclohexane is produced by hydrogenation of benzene per year and about 90% of world's production of cyclohexane is used for manufacturing nylon-6 and nylon-6,6.1–4 The hydrogenation of benzene has significant importance in the petroleum industry and also for environmental protection because of their known toxic properties as stern environmental regulations have been established worldwide.1–4 Conventionally, hydrogenation of benzene occurs under drastic temperatures and pressures over a metal-supported heterogeneous catalyst, such as Rh/Al2O3 or RANEY® nickel and have critical problems such as (i) requires careful control of temperature, pressure and residence time in order to achieve quantitative conversion of benzene, as hydrogenation of benzene is a strongly exothermic reaction, (ii) the formation of methylcyclopentane with other by-products obtained by isomerization and hydrocracking side reactions due to the high reaction temperature, (iii) the low stability of heterogeneous catalysts e.g., nickel catalysts which require extremely pure benzene feed stocks with less than 1 ppm sulfur in order to remain effective in the liquid phase.5 Thus, in recent years, there have been many efforts in the development of proficient catalyst systems for hydrogenation of benzene under mild conditions.6–20 The main aim of these efforts has been to lower effluents, waste and increase energy efficiency. As a consequence, various catalyst systems have been developed during the last two decades for selective hydrogenation of benzene under mild conditions; supported noble metal nanoparticles,6–8 colloidal noble metal nanoparticles or noble metal complexes catalysts are generally used as catalysts.9–25 Along with an increase in the catalyst efficiency, the separation of precious catalyst being dissolved in the reaction media is also important as the loss of catalyst is not acceptable due to the economical and environmental considerations. To combine the advantages of homogeneous and heterogeneous catalysts such as easy separation of reactants, products and catalyst as well as high activity and selectivity, several approaches have been developed using alternative reaction media such as aqueous, fluorous, ionic liquid and supercritical fluid instead of using classical organic solvents.26,27 In particular, aqueous biphasic catalysis has attracted interest due to the increasing environmental constraints which combines the use of water and easy catalyst recovery and recycling by simple phase separation,27 as water is a non-toxic, non-inflammable, safe, inexpensive, abundantly available and ‘green’ solvent. Moreover, this aqueous–organic biphasic approach has proven its commercial feasibility in the hydroformylation of propene developed by Ruhr Chemie–Rhone Poulenc.26,27 Nevertheless, there are only a few effective reports on the hydrogenation of benzene catalyzed by transition metal complexes modified with phosphine ligands in aqueous–organic two-phase systems.15–25Among the various water-soluble ligands, the trisodium salt of tris(m-sulfophenyl)phosphine P(3-C6H4SO3Na)3, (TPPTS) is one the most commonly used ligand due to its excellent coordinating ability and solubility in water.26 However, the synthesis of TPPTS is intricate because of the use of fuming sulfuric acid and long reaction time (76 h), which leads to the formation of phosphine oxide as an impurity.28 In this context, we have synthesized the sodium salt of trisulfonated tris(biphenyl)phosphine (BiphTS), as a water-soluble ligand.29 The synthesis conditions (Scheme 1) are less severe (concentrated H2SO4, 98%) than those used for phenyl-containing phosphines (oleum 65%). The synthesis of BiphTS then emerged to be less expensive and more accessible than TPPTS. Thus, for environmentally benign hydrogenation process and the key role of ruthenium in the hydrogenation, the application of BiphTS as a ligand in ruthenium-catalyzed hydrogenation of benzene has been investigated under aqueous biphasic conditions (Scheme 1). In order to reduce mass transfer limitations resulting from the low solubility of benzene in water (1.1 g L−1), non-ionic surfactants were added above their critical micelle concentration (CMC). Hydrogenation reactions were carried out under mild conditions and the catalyst was recycled.
 | ||
| Scheme 1 (a) Synthesis of TPPTS; (b) synthesis of BiphTS and aqueous biphasic hydrogenation of benzene catalyzed by Ru/BiphTS. | ||
2. Results and discussions
A Ru/BiphTS catalytic system examined for aqueous biphasic hydrogenation of benzene was derived in situ by mixing RuCl3·3H2O and water-soluble ligand BiphTS with particular molar ratio. Hydrogenation of benzene was studied in detail using the Ru/BiphTS complex in the absence and presence of non-ionic surfactants. The effect of reaction temperature, partial pressure of hydrogen, ligand/Ru molar ratio, substrate/Ru molar ratio, non-ionic surfactants and reusability of catalyst were investigated systematically for the hydrogenation of benzene. Only one product of benzene hydrogenation, i.e., cyclohexane was formed under the studied experimental conditions.The hydrogenation of benzene was first carried out using the Ru/BiphTS complex with a BiphTS to Ru molar ratio of 5 at 60 °C and 2 MPa hydrogen pressure. The influence of the reaction temperature and partial pressure of hydrogen was then examined. Table 1 presents the effect of reaction temperature and hydrogen pressure on conversion and selectivity in the hydrogenation of benzene catalyzed by the Rh/BiphTS complex. At a reaction temperature of 60 °C, BiphTS/Ru = 5 and 2 MPa hydrogen partial pressure, the conversion of benzene was 10% with a turnover frequency (TOF) of 200 h−1 (Table 1, entry 1). The conversion was accelerated by a factor of 4.5 when the temperature was increased from 60 °C to 120 °C. The conversion of benzene increased from 10 to 45% on increasing the temperature up to 120 °C with a TOF of 900 h−1 and 100% selectivity for cyclohexane (Table 1, entries 1–5).
| Entry | Temp. (°C) | Press. (MPa) | Conv. (%) | S cyclohexane (%) | TOFb (h−1) |
|---|---|---|---|---|---|
| a Reaction conditions: Substrate/Ru = 2000, RuCl3·3H2O = 0.011 mmol, BiphTS/Ru = 5; decane = 100 mg, water = 15 mL, reaction time = 60 min. b Moles of cyclohexane formed per mole of Ru per hour. | |||||
| 1 | 60 | 2.0 | 10 | 100 | 200 |
| 2 | 70 | 2.0 | 15 | 100 | 300 |
| 3 | 80 | 2.0 | 23 | 100 | 460 |
| 4 | 100 | 2.0 | 37 | 100 | 740 |
| 5 | 120 | 2.0 | 45 | 100 | 900 |
| 6 | 80 | 3.0 | 31 | 100 | 620 |
| 7 | 80 | 4.0 | 40 | 100 | 800 |
| 8 | 80 | 5.0 | 55 | 100 | 1100 |
| 9 | 80 | 6.0 | 62 | 100 | 1240 |
To examine the influence of hydrogen partial pressure on the Rh/BiphTS-catalyzed aqueous biphasic hydrogenation of benzene, a series of hydrogenation experiments were carried out by varying the hydrogen partial pressure over the range of 2 to 6 MPa at 80 °C with ligand/Ru molar ratio of 5 (Table 1). The conversion of benzene to cyclohexane increased on increasing the partial pressure of hydrogen. The conversion of benzene was increased from 23 to 62% with an increased partial pressure of hydrogen from 2 to 6 MPa while the TOF increased from 460 to 1240 h−1 (Table 1). The increase in the conversion of benzene at higher partial pressure of hydrogen is mainly due to enhanced oxidative addition of hydrogen on the metal (Ru) sites of the catalyst, which in turn increased the concentration of hydrogen available for benzene hydrogenation.
2.1. Effect of BiphTS/Ru molar ratio
Hydrogenation of benzene catalyzed by Ru/BiphTS was influenced by the molar ratio of BiphTS to RuCl3·3H2O precursor. The effect of addition of BiphTS was studied at 80 °C, 3.0 MPa hydrogen pressure and benzene/Ru molar ratio of 2000. The ligand BiphTS to RuCl3·3H2O molar concentration was varied from 2 to 12 (Fig. 1). The conversion of benzene was observed to increase with an increased BiphTS/Ru mmol ratio from 2 to 4. The highest catalytic activity (TOF = 780 h−1) was obtained at a ligand/Ru molar ratio of 4 (Fig. 1). Whereas with an increase in ligand BiphTS to Ru molar ratio from 4 to 12, the conversion of benzene dramatically decreases from 39 to 16% with a TOF value of only 320 h−1. This lower catalytic activity at higher ligand/Ru molar ratio could be due to the enhanced stability of the active catalytic species with the increase of ligand concentration and a competition between the free ligand and the interaction of benzene for coordination with Ru center. To confirm the role of BiphTS ligand in the ruthenium-catalyzed hydrogenation of benzene, one experiment for benzene hydrogenation was also carried out with RuCl3·3H2O without addition of BiphTS ligand. No conversion of benzene was observed under the employed reaction conditions which clearly indicates the role of the ligand in ruthenium-catalyzed hydrogenation of benzene.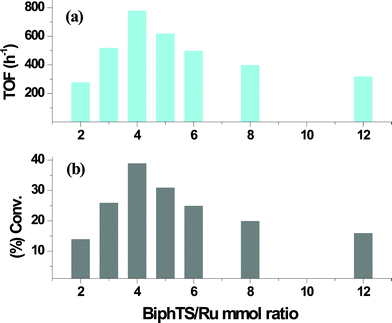 | ||
| Fig. 1 Effect of BiphTS/Ru molar ratio on (a) activity and (b) conversion in the hydrogenation of benzene catalyzed by Ru/BiphTS. Reaction conditions: Substrate/Ru = 2000, RuCl3·3H2O = 0.011 mmol, decane = 100 mg, water = 15 mL, Temp. = 80 °C, pH2 = 3.0 MPa, reaction time = 60 min. | ||
2.2. Effect of substrate/Ru molar ratio
To examine the effect of substrate/Ru molar ratio on the hydrogenation of benzene catalyzed by the Ru/BiphTS system, hydrogenation experiments were carried out by varying the benzene to RuCl3·3H2O ratio from 2000 to 8000 at 80 °C, 3.0 MPa hydrogen pressure and a ligand/Ru molar ratio of 4 (Fig. 2). | ||
| Fig. 2 Effect of substrate/Ru molar ratio in the hydrogenation of benzene catalyzed by Ru/BiphTS. Reaction conditions: RuCl3·3H2O = 0.011 mmol, BiphTS/Ru = 4, decane = 100 mg, water = 15 mL, Temp. = 80 °C, pH2 = 3.0 MPa, reaction time = 60 min. | ||
The conversion of benzene was observed to decrease significantly on increasing the substrate to Ru molar ratio. At 2000 benzene to Ru molar ratio, 39% conversion of benzene was observed in 1 h which decreased to 12% on increasing the benzene to Ru molar ratio to 8000. However, the catalytic activity of benzene hydrogenation increases with an increase of benzene to Ru molar ratio. The catalytic activity increased to give TOF values from 780 up to 1020 h−1 (Fig. 2).
2.3. Effect of non-ionic surfactants
In order to improve the solubility of benzene in aqueous media, the non-ionic surfactants such as polyoxyethylene(23)lauryl ether (Brij-35) and polyoxyethylene(4)lauryl ether (Brij-30) have been used.25 In present study, use of such non-ionic surfactants was thus attempted to overcome the problem of low solubility of benzene in aqueous medium during hydrogenation reaction. It was observed that the addition of the non-ionic surfactants such as Brij-30, Brij-35, and Brij-56 has an appreciable effect on the hydrogenation benzene (Table 2) catalyzed by Ru/BiphTS under aqueous biphasic conditions.| Entry | Non-ionic surfactant (mmol L−1) | Temp. (°C) | Press. (MPa) | Conv. (%) | TOFb (h−1) |
|---|---|---|---|---|---|
| a Reaction conditions: Benzene/Ru = 6000, RuCl3·3H2O = 0.011 mmol, BiphTS/Ru = 4, decane = 100 mg, water = 15 mL, reaction time = 60 min. b Moles of cyclohexane formed per mole of Ru per hour. c Reaction time = 45 min. | |||||
| 1 | — | 80 | 3.0 | 17 | 1020 |
| 2 | (Brij-30) (5.0) | 80 | 3.0 | 38 | 2280 |
| 3 | (Brij-30) (11.0) | 80 | 3.0 | 56 | 3360 |
| 4 | (Brij-35) (11.0) | 80 | 3.0 | 50 | 3000 |
| 5 | (Brij-56) (11.0) | 80 | 3.0 | 52 | 3120 |
| 6 | (Brij-30) (11.0) | 100 | 3.0 | 71 | 4260 |
| 7 | (Brij-30) (11.0) | 120 | 3.0 | 92 | 5520 |
| 8 | (Brij-30) (11.0) | 120 | 4.0 | 100 | 6000 |
| 9c | (Brij-30) (11.0) | 120 | 5.0 | 90 | 7200 |
| 10c | (Brij-30) (11.0) | 120 | 6.0 | 100 | 8000 |
The results showed that the concentration of surfactant plays an important role in increasing the conversion of benzene. With the addition of surfactant Brij-30 with a concentration of 5 mmol L−1, 38% conversion of benzene was observed in 1 h which increased to 56% on increasing the concentration of (Brij-30) to 11 mmol L−1 (Table 2). On further increase in the concentration of Brij-30, formation of an emulsion was observed. The addition of other non-ionic surfactants such as Brij-35 and Brij-56 also increased the conversion of benzene; however, high conversion of benzene was achieved with Brij-30 at 11 mmol L−1 concentration. The increase in the conversion of benzene with an addition of non-ionic Brij surfactants may be due to enhanced interaction of benzene and catalyst at the core of the micelle as addition of surfactant increases the interlayer area and the solubility of the substrate in water by forming micelles.30 The surfactants supplement the catalytic species on the surface and dissolve the benzene in the inner hydrophobic part of the micelle and consequently increase the contact and the coordination of benzene with the catalytic species. It was also observed that the separation of aqueous and organic phases after the completion of reaction occurs within an hour, helps to recover and recycle the catalysts from products.
The effect of temperature and hydrogen partial pressure on conversion and selectivity were also investigated in the presence of surfactant Brij-30 (11 mmol L−1) by varying temperature (80 to120 °C) and hydrogen pressure (3.0 to 6.0 MPa). At 3.0 MPa hydrogen pressure, with an increase in temperature from 80 to 120 °C, benzene conversion increased from 56 to 92% with an increase in the TOF from 3360 to 5520 h−1. The hydrogenation of benzene catalyzed by Ru/BiphTS in presence of the non-ionic surfactant Brij-30 system was then studied at different hydrogenation pressures, keeping the temperature at 120 °C (Table 2). At 3.0 MPa hydrogen pressure and 120 °C, the conversion of benzene to cyclohexane was 92%. The TOF was observed to increase on increasing the partial pressure of hydrogen. The complete conversion of benzene to cyclohexane was obtained at 4 MPa H2 pressure and 120 °C. With an increase in the H2 pressure from 4 to 6 MPa, the complete conversion of benzene was achieved in 45 min with TOF of 8000 h−1.
The main purpose of using an aqueous biphasic system is the easy recycling of the catalyst and separation of product. Therefore, catalyst recycling experiments were carried out using the Ru/BiphTS catalyst in the presence 11 mmol L−1 of surfactant Brij-30. The reusability of catalyst was studied up to four cycles at 80 °C and 3 MPa hydrogen pressure. For reusability experiments, the aqueous catalyst phase of the biphasic system was separated from the upper organic layer by decantation which was then recycled by adding benzene into the recovered aqueous phase containing catalyst and surfactant (Fig. 3). No significant loss in the conversion of benzene and selectivity of cyclohexane were observed up to four cycles. However, the conversion of benzene decrease slightly from 56 to 49% up to fourth cycle with the decrease in the TOF from 3360 to 2940 h−1 due to handling loss of catalyst suggesting that the catalyst is reusable up to four cycles under the employed reaction conditions (Fig. 3). These reusability experiments also confirmed that the leaching of ruthenium from aqueous phase to organic phase is low.
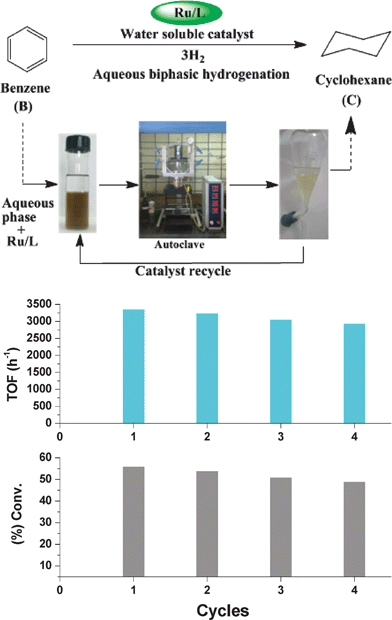 | ||
| Fig. 3 Recycling of Ru/BiphTS catalyst for the hydrogenation of benzene. Reaction conditions: Benzene/Ru = 6000, RuCl3·3H2O = 0.011 mmol, BiphTS/Ru = 4, decane = 100 mg, Brij-30 = 11 mmol L−1, water = 15 mL, Temp. = 80 °C, pH2 = 3.0 MPa, reaction time = 60 min. | ||
In order to check the stability and the catalytic activity of Ru/BiphTS system in the presence of sulfur-containing compound and to confirm whether the nature of Ru/BiphTS system is homogeneous or colloidal, control hydrogenation experiments were performed in the presence of thiophene (as a sulfur source) by varying the thiophene concentration from 1 to 9 ppm (Fig. 4) and mercury (1 mL) at 80 °C and 3 MPa pressure of hydrogen. Both methods are well-known to determine the nature of the Ru-based catalyst19,31 as thiophene and Hg poison through efficient adsorption on the surface of transition metal catalysts. In the case of thiophene, the conversion of benzene was decreased to some extent on increasing the concentration of sulfur from 1 to 5 ppm. However, upon further increase in the concentration of thiophene from 5 to 9 ppm, the conversion of benzene and TOF decreased considerably (Fig. 4).
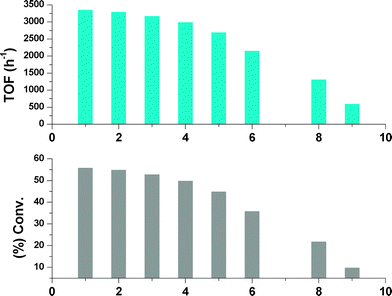 | ||
| Fig. 4 Effect of thiophene concentration on the hydrogenation of benzene. Reaction conditions: Benzene/Ru = 6000, RuCl3·3H2O = 0.011 mmol, BiphTS/Ru = 4, decane = 100 mg, Brij-30 = 11 mmol L−1, water = 15 mL, Temp. = 80 °C, pH2 = 3.0 MPa, reaction time = 60 min. | ||
We also performed one hydrogenation experiment in presence of mercury; the conversion of benzene decreased noticeably from 56 to 2%. These precise results in the presence of thiophene and mercury indicated that the nature of Ru/BiphTS is colloidal, similar to the reported Ru/TPPTS system.19 These results also indicated that ligand BiphTS stabilises the colloids Ru particles in the aqueous solution which is supplemented by surfactant, resulting in the high activity in the presence of surfactant and supports reusability of the catalyst.
The catalytic activity of the Ru/BiphTS system was compared with that of a Ru/TPPTS system under similar reaction conditions (Temp. 80 °C, pH 2, 3.0 MPa and Benzene/Ru = 6000) in the absence and presence of 11.0 mmol L−1 Brij-30 surfactant. In the absence of any surfactant and with a TPPTS/Ru molar ratio of 4, the conversions obtained with the in situ prepared Ru/TPPTS and Ru/BiphTS catalysts were 12% and 17%, respectively. In the presence of 11.0 mmol L−1 Brij-30 surfactant, the Ru/TPPTS yielded 45% conversion of benzene whereas Ru/BiphTS exhibited 56%. The catalytic activity of the Ru/BiphTS system was also compared with the catalytic activities of ruthenium-based catalysts reported in the literature for hydrogenation of benzene to cyclohexane under aqueous biphasic conditions (Table 3). These results clearly demonstrate the efficiency of the Ru/BiphTS system over Ru-based catalytic systems reported in the literature.
| Catalyst system | Temp. (°C) | Press. (MPa) | TOF (h−1) | Ref. |
|---|---|---|---|---|
| Ru/BiphTS | 80 | 3.0 | 1020 | Present work |
| Ru/BiphTS/Brij-30 | 120 | 6.0 | 8000 | |
| Ru3(CO)11TPPTS | 120 | 9.0 | 45 | 15 |
| (η6-C6H6)2Ru2Cl4 | 90 | 6.0 | 1998 | 16 |
| RuCl2(TPPTS)3 | 150 | 1.0 | 0.6 | 17 |
| Ru(η6-C10H14)(pta)Cl2 | 90 | 6.0 | 170 | 18 |
| [H4Ru4(η6-C6H6)][BF4]2 | 90 | 6.0 | 352 | 18 |
| Ru(η6-C10H14)(TPPTS)Cl2 | 90 | 6.0 | 488 | 19 |
| [Ru(η5-C5H5)Cl(TPPDS)2] | 105 | 9.65 | 65 | 20 |
| Ru/BISBI | 100 | 5.0 | 16.9 | 21 |
| Ru/BDPX | 100 | 5.0 | 29.3 | 21 |
| Ru/BDNA | 100 | 5.0 | 56.9 | 21 |
| Ru/dppm | 100 | 4.5 | 100 | 22 |
| [Ru4(η6-C6H6)4(OH)4]4+ | 60 | 6.0 | 606 | 23 |
3. Experimental
The ligand synthesis was performed using standard Schlenk techniques under nitrogen atmosphere. The water-soluble ligand BiphTS was prepared according to a procedure previously reported.26 The purity of BiphTS was carefully controlled. In particular, 1H and 31P NMR analysis indicated that less than 1% of its oxide was present. Tetrahydrofuran (THF) was distilled from Na–benzophenone prior to use. Ethanol (99.5%) procured from Baroda Chemicals Industries Ltd, India was used as received. Benzene was purchased from Sigma–Aldrich Chemicals, USA and used as received. RuCl3·3H2O and non-ionic surfactants used in reactions were purchased from Merck India. Methanol, chloroform, sulfuric acid (98%, AR), tridecane, and trioctylamine were from S. d. Fine Chemicals, India. Distilled and deionized water was used in all experiments. The hydrogen gas (99.9%) used was from Hydro Gas India Pvt. Ltd., India.3.1. Instrumentation
All the catalytic hydrogenation reactions were performed in a 100 mL stainless steel autoclave reactor (EZE–Seal Reactor) with controlling unit supplied by Autoclave Engineers, USA having provisions for stirring, optimization of temperature, pressure and sample withdrawing. 31P NMR measurements were performed using D2O as solvent and 85% H3PO4 as an external reference, on Bruker Avance DPX 500 MHz FT-NMR. IR spectra were recorded using nujol mull and KBr pellet on a Perkin-Elmer spectrum GX FT-IR system in the range 4000–400 cm−1 with a resolution of 4 cm−1. Elemental analysis has been performed on a Perkin-Elmer, 2400 C, H, N, S/O analyzer. Atomic Absorption Spectra were recorded using a Shimadzu AA-680/G V-5 spectrometer. The reaction products were analyzed with a Shimadzu GC-17A gas chromatograph (GC) with flame ionization detector (FID) having 5% diphenyl and 95% dimethyl siloxane capillary column (60 m length, 0.25 mm diameter). Column temperature was initially kept at 50 °C for 5 min and then raised to 200 °C at 10 °C min−1. Nitrogen was used as a carrier gas (1.2 mL min−1). n-Decane was used as internal standard.3.2. Catalytic hydrogenation reaction
In a typical hydrogenation experiment, the required amount of RuCl3·3H2O, ligand BiphTS, substrate, n-decane and water were charged into the stainless steel autoclave 100 mL reactor. The reactor was fitted air tight. The reactor was flushed with nitrogen followed by flushing with hydrogen twice at room temperature after which reactor was pressurized with the desired hydrogen pressure and brought to the reaction temperature. The reaction was initiated by stirring (900 rpm); after the desired reaction time, the stirring was stopped and reactor was cooled down to room temperature, depressurized, flushed with N2 and opened. The separating funnel was used to separate the organic–aqueous layer to collect product (organic layer) for GC analysis.In the case of surfactants, a typical hydrogenation experiment was conducted as follows: the required amount of RuCl3·3H2O, ligand BiphTS and surfactants were first dissolved in water. The resulting catalytic aqueous solution was charged into the reactor. Then, substrate with n-decane (internal standard) was transferred into the aqueous catalytic solution. The reactor was fitted air tight. The reactor was flushed with nitrogen followed by flushing with hydrogen twice at room temperature. After these operations, the reactor was pressurized with the desired hydrogen pressure and brought to the desired reaction temperature. The reaction was initiated by stirring (900 rpm). After a preset reaction time, the stirring was stopped, reactor was cooled down to room temperature, depressurized, flushed with N2 and opened. The aqueous and organic phases were separated by decantation and finally, the organic phase was collected for GC analysis. For reusability of catalyst, after decantation, the recovered aqueous phase was reloaded in autoclave with surfactant and substrate as described above.
4. Conclusions
The Ru/BiphTS system effectively catalyzed hydrogenation of benzene and demonstrated the high potential of efficient green aqueous phase organometallic catalysis as benzene is difficult to reduce with high selectivity under mild conditions. The investigated results in presence of thiophene and mercury indicated that the nature of Ru/BiphTS is colloidal as it poisoned by sulfur and mercury, similar to the reported Ru/TPPTS system. The ligand BiphTS stabilises the colloids in aqueous solution which is supplemented by surfactant resulting in to the high activity in the presence of surfactant and supports reusability of the catalyst. The interesting features of the BiphTS ligand and its efficiency in Ru-catalyzed hydrogenation reaction could improve the scope of aqueous biphasic methodology.Acknowledgements
Authors are thankful to the analytical discipline & centralized instrument facility of the institute for assistance with analyses and CSIR for financial support under CSIR Network Programme NWP 010. AAD thanks to CSIR, New Delhi, for the award of Senior Research Fellowship.Notes and references
- (a) J. F. L. Page, Applied Heterogeneous Catalysis: Design, Manufacture, Use of Solid Catalysts, Technip, Paris, 1987, p. 291 Search PubMed; (b) M. Sanati, B. Harrysson, M. Faghihi, B. Gevert and S. Jaras, Catalysis, Royal Society of Chemistry, Cambridge, 2002, vol. 16, ch. 1, p. 1 Search PubMed.
- A. Stanislaus and B. H. Cooper, Catal. Rev. Sci. Eng., 1994, 36, 75–123 CAS.
- K. Weissermel and H.-J. Arpe, Industrial Organic Chemistry, Wiley- VCH, Weinheim, fourth edn, 2003 Search PubMed.
- K. B. Sidhpuria and P. A. Parikh, Bull. Catal. Soc. India, 2004, 3, 67–71 Search PubMed.
- R. L. Augustine, Heterogeneous Catalysis for the Synthetic Chemist, Marcel Dekker, New York, 1996 Search PubMed.
- (a) K. B. Sidhpuria, P. A. Parikh, P. Bahadur, B. Tyagi and R. V. Jasra, Catal. Today, 2009, 141, 12–18 CrossRef CAS; (b) K. B. Sidhpuria, P. A. Parikh, P. Bahadur and R. V. Jasra, Ind. Eng. Chem. Res., 2008, 47, 4034–4042 CrossRef CAS; (c) K. B. Sidhpuria, H. A. Patel, P. A. Parikh, P. Bahadur, H. C. Bajaj and R. V. Jasra, Appl. Clay Sci., 2009, 42, 386–390 CrossRef CAS.
- (a) M. Zahmakiran and S. Ozkar, Langmuir, 2008, 24, 7065–7067 CrossRef CAS; (b) M. Zahmakıran, T. Kodaira and S. Ozkar, Appl. Catal., B, 2010, 96, 533–540 CrossRef.
- F. Wyrwalski, B. Léger, C. Lancelot, A. Roucoux, E. Monflier and A. Ponchel, Appl. Catal., A, 2011, 391, 334–341 CrossRef CAS.
- A. Nowicki, Y. Zhang, B. Leger, J. Rolland, H. Bricout, E. Monflier and A. Roucoux, Chem. Commun., 2006, 296–298 RSC.
- C. Hubert, A. Denicourt-Nowicki, A. Roucoux, D. Landy, B. Leger, G. Crowyn and E. Monflier, Chem. Commun., 2009, 1228–1230 RSC.
- (a) G. Papadogianakis and R. A. Sheldon, Catalysis, 1997, 13, 114–193 Search PubMed; (b) G. Papadogianakis and R. A. Sheldon, New J. Chem., 1996, 20, 175–185 CAS; (c) G. Verspui, G. Papadogianakis and R. A. Sheldon, Catal. Today, 1998, 42, 449–458 CrossRef CAS.
- (a) A. Chanda and V. V. Fokin, Chem. Rev., 2009, 109, 725–748 CrossRef CAS; (b) D. G. Blackmond, A. Armstrong, Y. Coombe and A. Wells, Angew. Chem., Int. Ed., 2007, 46, 3798–3800 CrossRef CAS.
- (a) J. A. Widegren and R. G. Finke, J. Mol. Catal. A: Chem., 2003, 191, 187–207 CrossRef CAS; (b) J. Schulz, A. Roucoux and H. Patin, Chem.–Eur. J., 2000, 6, 618–624 CrossRef CAS; (c) G. Süss-Fink, M. Faure and T. R. Ward, Angew. Chem., Int. Ed., 2002, 41, 99–101 CrossRef; (d) M. Faure, A. T. Vallina, H. Stoeckli-Evans and G. Süss-Fink, J. Organomet. Chem., 2001, 621, 103–108 CrossRef CAS.
- (a) P. J. Dyson, Dalton Trans., 2003, 2964–2974 RSC; (b) C. M. Hagen, L. Vieille-Petit, G. Laurenczy, G. Süss-Fink and R. G. Finke, Organometallics, 2005, 24, 1819–1831 CrossRef CAS; (c) C. M. Hagen, J. A. Widegren, P. M. Maitlis and R. G. Finke, J. Am. Chem. Soc., 2005, 127, 4423–4432 CrossRef CAS.
- D. J. Ellis, P. J. Dyson, D. G. Parker and T. Welton, J. Mol. Catal. A: Chem., 1999, 150, 71–75 CrossRef CAS.
- E. G. Fidalgo, L. Plasseraud and G. Süss-Fink, J. Mol. Catal. A: Chem., 1998, 132, 5–12 CrossRef.
- D. U. Parmar, S. D. Bhatt, H. C. Bajaj and R. V. Jasra, J. Mol. Catal. A: Chem., 2003, 202, 9–15 CrossRef CAS.
- P. J. Dyson, D. J. Ellis, W. Henderson and G. Laurenczy, Adv. Synth. Catal., 2003, 345, 216–221 CrossRef CAS.
- P. J. Dyson, D. J. Ellis and G. Laurenczy, Adv. Synth. Catal., 2003, 345, 211–215 CrossRef CAS.
- T. Suárez, A. Guzmán, B. Fontal, M. Reyes, F. Bellandi, R. R. Contreras, P. Cancines, G. León and L. Rojas, Transition Met. Chem., 2006, 31, 176–180 CrossRef.
- L. Zhang, L. Wang, X. Yan Ma, R. X. Li and X. J. Li, Catal. Commun., 2007, 8, 2238–2242 CrossRef CAS.
- C. Daguenet and P. J. Dyson, Catal. Commun., 2003, 4, 153–157 CrossRef CAS.
- P. J. Dyson, K. Russell and T. Welton, Inorg. Chem. Commun., 2001, 4, 571–573 CrossRef CAS.
- (a) P. Baricelli, G. Morfes and D. E. Páez, J. Mol. Catal. A: Chem., 2001, 176, 1–10 CrossRef CAS; (b) P. J. Baricelli, J. López, E. Lujano and F. López Linares, J. Mol. Catal. A: Chem., 2002, 186, 57–63 CrossRef CAS.
- C. Vangelis, A. Bouriazos, S. Sotiriou, M. Samorski, B. Gutsche and G. Papadogianakis, J. Catal., 2010, 274, 21–28 CrossRef CAS.
- B. Cornils and W. A. Herrmann, Aqueous-Phase Organometallic catalysis–Concept and Application, John Wiley & Sons, Chichester, 1996 Search PubMed.
- D. J. Cole-Hamilton, Science, 2003, 299, 1702–1706 CrossRef CAS.
- H. Gulyas, A. Szöllosy, B. E. Hanson and J. Bakos, Tetrahedron Lett., 2002, 43, 2543–2546 CrossRef CAS.
- M. Ferreira, H. Bricout, F. Hapiot, A. Sayede, S. Tilloy and E. Monflier, ChemSusChem, 2008, 1, 631–636 CrossRef CAS.
- (a) E. Monflier, P. Bourdauducq, J. L. Couturier, J. Kervennal and A. Mortreux, Appl. Catal., A, 1995, 131, 167–178 CrossRef CAS; (b) E. Monflier, P. Bourdauducq, J. L. Couturier, I. Suisse, J. Kervennal and A. Mortreux, Catal. Lett., 1995, 34, 201–212 CrossRef CAS.
- L. J. Simon, J. G. van Ommen, A. Jentys and J. A. Lercher, J. Catal., 2001, 201, 60–69 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |

