Photocatalyst activation by intrinsic stimulation in TiO2–BaTiO3
Sarmiza E.
Stanca
*,
Robert
Müller
,
Matthias
Urban
,
Andrea
Csaki
,
Frank
Froehlich
,
Christoph
Krafft
,
Jürgen
Popp
and
Wolfgang
Fritzsche
*
Institute of Photonic Technology, Albert-Einstein-Straße 9, 07745 Jena, Germany. E-mail: sarmiza.stanca@ipht-jena.de; wolfgang.fritzsche@ipht-jena.de
First published on 25th April 2012
Abstract
We report a nanoparticulate composite TiO2–BaTiO3 film which exhibits an increased antibacterial photocatalytic activity under visible light. The pure BaTiO3, TiO2 or their mixture do not attain a significant photocatalytic capacity under visible light. However, when these oxides are simultaneously synthesized under controlled conditions the resulting crystals exhibit a high catalytic effect. The effect of this material on microorganism lysis is studied and the mechanisms for the observed damage are investigated. Attenuated total reflection Fourier transform infrared spectroscopy provides the evidence of chemical changes (formation of carbonyl and carboxylic groups) in the cell membranes under visible light by TiO2–BaTiO3 but not by TiO2. Cyclic voltammetry demonstrates that peroxidation occurs in the absence of UV light and in the presence of TiO2–BaTiO3 but not in the presence of TiO2 alone. Atomic force microscopy reveals the morphological changes of the cells in this process.
1. Introduction
Antibacterial surface coating is an efficient way to suppress microbial infections. Often a coating based on TiO2 is utilized because its photocatalysis has been demonstrated to be a competent mechanism for bacteria and fungi peroxidation.1–8 Upon insufficient illumination the antibacterial photocatalytic ability of TiO2 is inhibited.1 Therefore, TiO2 catalytic activity was improved by its combination with silver,9–11 silver oxide,12 apatite13,14 or carbon nanotubes.15 These efforts showed only modest progress, because they have different mode of actions and thereby complicating the applicability. Here, as an alternative, we report a nanoparticulate composite TiO2–BaTiO3 film whose antibacterial photocatalytic activity is improved under visible light. The pure BaTiO3, TiO2 or their simple mixture do not show a significant photocatalytic effect under visible light. However, when these oxides are simultaneously synthesized under controlled conditions (see Section 2.1) a high catalytic effect is observed for the resulting crystals under visible light; this effect is studied on the bacterial strain Staphylococcus aureus (see Section 3.3).A particular energy level structure in this composite enables electron flow by using lower energy photons that can excite the electrons from the valence to the conduction band.16,17 The photoexcited catalyst transfers electrons and energy into a ground state molecule, thereby generating a sensitized photoreaction.1,11 The efficiency of this reaction can be amplified by enlarging the catalyst surface.18–21 This leads to the light concentration in subwavelength volumes and, consequently, the generated energy transfer can be coupled with the electron oscillators of the nanoparticles surface. They are essentially implicated in electron exchange for energy transfer.1,20
2. Materials and methods
All chemicals were purchased from Sigma-Aldrich (Taufkirchen, Germany, puriss p.a.), except where otherwise mentioned.2.1. Particle synthesis
TiO2–BaTiO3 particles were prepared by an adapted glass crystallization method.22 A glass of composition 50BaO–25B2O3–25TiO2 (mol%) was melted in air at ≈1400 °C in a Pt-crucible. The raw materials were: BaCO3, H3BO3 and TiO2. The melt was quenched between two rotating steel rollers with a quenching rate on the order of 104 K s−1 to obtain amorphous flakes. The flakes were heat treated at 480 °C for one hour to achieve the crystallization of particles in the matrix. The powder was isolated by dissolving the complete matrix with hot diluted acetic acid and afterwards rinsed with water and dried. This annealing temperature led partly to the formation of a mixture of BaTiO3 and TiO2. The sample had a predominant fraction (≈75%) of very fine TiO2-particles (anatase crystallite size < 10 nm) and a minor fraction of large BaTiO3-particles (crystallite size: ca. 100 nm). A specific surface area of the powder of approximately 260 m2 g−1 was measured by a Brunauer - Emmett - Teller (BET) method using the physical adsorption of gas molecules on a solid surface.Although in our non-annealed quenched and cooled glass titanium oxide could not be detected by X-ray diffraction (XRD) we cannot exclude a small amount of TiO2-seeds.
2.2. TiO2–BaTiO3 modified quartz slide
100 μL of TiO2–BaTiO3 dispersion (0.1 mg mL−1 in 1 M acetic acid) were spread on a “fused quartz” piece (1 × 2 cm) cut from a polished wafer provided by Siegert Consulting e.K., Aachen, Germany. The XRD spectra of the substrate identified SiO2 cristobalite structure. For simplicity reasons we call it quartz slide. The coated slides were heated up at a rate of 5 °C min−1 to 1200 °C and cooled down at a rate of 1 °C min−1 to room temperature.2.3. Bacteria and culture conditions
The microorganisms (Staphylococcus aureus RN1HG) were purchased from the Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH. They were sub-cultured and maintained on nutrient agar. To prepare the bacterial culture for photocatalysis experiments, well isolated colonies of the same morphological type were lifted from the nutrient agar Plate-Count-Agar (PCA, Merck, Germany), inoculated into 100 mL nutrient broth and placed in an incubator set at 37 °C and 100 rpm. After 18 h incubation, the bacterial growth was monitored at 600 nm by optical means. The bacterial cells were collected by centrifugation at 4000 rpm for 10 min, then washed and re-suspended in sterile distilled water and finally adjusted to give a cell density of approximately 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 colony forming units (cfu) per mL.
000 colony forming units (cfu) per mL.
2.4. Attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR)
The ATR-FTIR spectra were measured with a Varian FTIR-Spectrometer 670 coupled to a microscope 620 which was equipped with a Germanium ATR accessory (Agilent, USA). Spectra were the results of 64 scans with a resolution of 4 cm−1 in the spectral range 700–4000 cm−1. Specimens for ATR-FTIR were prepared by spreading 0.5 mL of the bacterial dispersion on TiO2 or TiO2–BaTiO3 modified quartz slides (1 × 2 cm). Regions of interest were selected by visual inspection. Then, contact with the ATR crystal was established while monitoring the absorbance. When the absorbance of the quartz band near 1025 cm−1 reached 0.5 to 0.6, the ATR-FTIR spectrum was recorded. The contact area had a diameter of approximately 90 μm. The IR spectra were corrected for residual spectral contributions of water vapour and CO2, and a linear baseline was subtracted.2.5. Raman spectroscopy
Raman spectra were recorded using a Confocal Raman Microscope (WITec GmbH, Ulm, Germany) equipped with a Zeiss EC Epiplan 50×/0.95 objective. The continuous 785 nm laser radiation of 100 mW power was coupled into the microscope by a wavelength-specific single mode optical fiber.2.6. Electrochemistry
Cyclic voltammetry was performed using a computer-assisted potentiostat (Yokogawa 7651 Programmable DC Source, Keithley 2001 Multimeter), connected to an electrochemical cell equipped with a Pt plate, an Ag/AgCl electrode and a quartz–TiO2–BaTiO3 working electrode. For measurements the electrodes were immersed in 1 mL of test dispersion at room temperature and poised at the desired value of the applied potential. The data acquisition has been completed with an in house written program.2.7. Atomic force microscopy
All atomic force micrographs were obtained using a MultimodeIM Nanoscope III (Digital Instrument's, Santa Barbara, CA, USA) instrument equipped with contact mode tips (Park Scientific Instruments Model No. HWMS-06AU). Images were processed using Gwyddion open source software (http://gwyddion.net/).2.8. Spectrophotometry
UV-Vis spectra were obtained with a Jasco V-670 spectrometer (Hachioji, Tokyo, Japan) using plastic cuvettes (Brand GmbH, Wertheim, Germany). A 1 mL volume of bacterial suspensions was placed in a spectrophotometer cuvette holder. TiO2–BaTiO3 coated quartz slides were placed vertically in the dispersion. The reaction vessel was covered with a loose fitting lid and stirred throughout. Samples of the solution were taken in triplicate every 15 min. For the transmittance measurements the modified composite quartz slide was positioned in the light beam by the instrument holder.2.9. X-Ray diffraction
The X-ray diffraction analysis has been performed with an X'pert Pro Instrument (PANanalytical, Almelo, Netherlands) with CuKα radiation. The Scherrer equation was used for determination of the crystallite sizes.2.10. Energy dispersive X-ray (EDX)
EDX measurements were performed with a JSM-6300F from JEOL (Tokyo, Japan) with an INCAPentaFET-x3 detector from Oxford instruments (Abingdon, UK). For light element analysis the open-window mode was used.3. Results and discussions
As a preparation route for TiO2–BaTiO3 we utilized the glass crystallization method (GCM).22 GCM allows preparing phase mixtures of particles much more homogeneous than possible with ceramic preparation methods since both phases are formed simultaneously. Through the crystal growth the excess of TiO2 produces also the vacancies in BaTiO3.23 During the synthesis at high temperature the oxygen can evaporate creating the oxygen vacancies with +2 charges in the composite structure. An oxygen vacancy can trap one or two electrons leading to lower levels in the band gap by 0.2 eV.23The TiO2–BaTiO3 powder was physically characterized (Section 3.1) and utilized for film preparation (Section 2.2). The film was further physically characterized (Section 3.2) and investigated for its antibacterial activity using spectroscopic, electrochemical and morphological methods (Section 3.3).
3.1. Physical characterization of the TiO2–BaTiO3 powder
The TiO2–BaTiO3 powder was examined by scanning electron microscopy (SEM, Fig. 1a and b), energy dispersive X-ray (Fig. 1c), X-ray diffraction (Fig. 1d) and Raman spectroscopy (Fig. 1e–g). SEM investigations with secondary as well as backscattered electrons allow discrimination between areas of higher and lower Ba content. The areas of high Ba content can be observed in both SE and BSE modes of the scanning electron micrographs (Fig. 1a and b). However, the areas of high TiO2 content can only be observed in SE mode (Fig. 1a). The EDX analysis of the powder differentiates also areas of high and low content of Ba (Fig. 1c) and indicates a low proportion of BaTiO3 in the powder. A superposition of the Ba and Ti lines by EDX complicates the analysis of the Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti ratio; although a mean atomic ratio for Ba
Ti ratio; although a mean atomic ratio for Ba![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ti of 1
Ti of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 was approximated (Fig. 1c). This analysis confirms the composite structure of the particles of 75% TiO2 and 25% BaTiO3.
3 was approximated (Fig. 1c). This analysis confirms the composite structure of the particles of 75% TiO2 and 25% BaTiO3.
 | ||
| Fig. 1 The physical characterization of the TiO2–BaTiO3 powder. (a, b) Scanning electron micrographs. The panels represent the images of the surface using secondary electron (SE) mode (a), and a back scattered electron (BSE) mode (b) of the same area. (c) EDX pattern. (d) XRD pattern. The peaks “1”and “2” indicate BaTiO3 and TiO2, respectively. (e–g) Raman spectra of the TiO2, BaTiO3, TiO2–BaTiO3 synthesized, respectively, obtained at 785 nm of 75 mW excitation in 5 s integration time. | ||
Raman spectroscopy analysis was utilized to verify the structure of the nanocomposite. Direct Raman spectra of the pure BaTiO3, pure TiO2, as reference, and synthesized TiO2–BaTiO3 powders are shown in Fig. 1e–g. The spectra were collected through an area of 1 μm2 in 5 s under 785 nm laser excitation. The TiO2 spectrum has a series of Raman bands at 145, 395, 520, and 640 cm−1. The BaTiO3 spectrum presents the Raman bands at 300, 520, and 720 cm−1. Although the spectra of synthesized TiO2–BaTiO3 are dominated by TiO2 bands one can identify the signals from BaTiO3 near 300 and 720 cm−1 (Fig. 1g). The small deviation of the bands in the case of the TiO2–BaTiO3 powder (Fig. 1g) from the band values of each constituent alone (Fig. 1e and f) can be attributed to a change in the crystal lattice or to a new structure formed by the heating effect. For example, at high temperatures during the crystal growth, the oxygen vacancies can electrostatically associate with metal ions creating a cluster like structure.23
3.2. Physical characterisation of the TiO2–BaTiO3 nanoparticulate layer
The physical characterization of the nanoparticulate film is presented in Fig. 2. The atomic force microscopy (AFM) and scanning electron microscopy (SEM) images (Fig. 2a–d) illustrate the homogeneity of this film immobilized on a quartz slide. As a carbon coating effect the grains seem to be larger than those presented in the AFM image, however the film maintains its nanoparticulate character. A roughness value of 32.3 ± 2 nm is indicated by the AFM profile. The difference in brightness between SE and BSE images, BSE being darker, proves the dominance of the TiO2. The XRD pattern of the film shows all three components of the system: BaTiO3 (1), TiO2 (2) and SiO2 (3) (Fig. 2e). The EDX diagram is dominated by the signals of SiO2 and TiO2 however it confirms the presence of BaTiO3 (Fig. 2f). | ||
| Fig. 2 Physical characterization of the nanoparticulate film (TiO2–BaTiO3). (a) AFM image; (b) AFM profile along the line in panel (a); (c) SEM image; (d) BSE image of the same area; (e) XRD pattern. The peaks “1”, “2” and “3” indicate BaTiO3, TiO2, and SiO2, respectively. (f) EDX pattern. (g) Transmittance spectra of the film at room temperature. | ||
Practically, the band gap of the composite layer was estimated by the mean of the transmission spectroscopy.24 The transmittance measurements were recorded at a rate of 100 nm min−1. The transmittance spectra of both quartz and TiO2–BaTiO3–quartz were plotted in the same diagram (Fig. 2g). We observed a significant decrease in transmittance of the photocatalytic layer in the visible domain. The minimum of the transmittance of the TiO2–BaTiO3 was identified at 520 nm (Fig. 2g). The band gap energy was calculated according to the following relation: E = hcλ−1, where h = 4.135 × 10−15 eV s, c = 2.99 × 108 m s−1 and λ represents the wavelength of transmittance minimum. The value of the band gap energy estimated at the minimum of the film transmittance was 2.37 ± 0.05 eV. This low value suggests a reorganisation of the energetic structure, most probably in intermediated states.
3.3. The study of bacterial lysis on TiO2–BaTiO3 nanoparticulate film
The complex mechanisms of organic compounds peroxidation catalysed by TiO2 are already extensively documented. The most rational is the pathway of highly reactive hydroxyl radicals formation by oxidation of water molecules attached to the TiO2 excited surface (Scheme 1).1,11 These radicals propagate the peroxidation of the molecules in their environment. | ||
| Scheme 1 The proposed mechanism of the catalyst activation. | ||
The study of bacterial lysis on TiO2–BaTiO3 nanoparticulate film by attenuated total reflection Fourier transform infrared spectroscopy (ATR-FTIR), cyclic voltammetry and AFM is further reported. ATR-FTIR experiments provide the evidence for the changes in cell membranes as onset of bacterial lysis. Cyclic voltammetry demonstrates that the peroxidation propagates in the presence of TiO2–BaTiO3 and AFM records the morphological changes in this process.
The ATR-FTIR spectra in the range of 1250–4000 cm–1 of the bacteria on TiO2 (a) and TiO2–BaTiO3 (b) layer set aside in the dark (black traces) and for 2 hours in the presence of daylight (grey traces) are presented in Fig. 3a and b. The penetration depth of the IR radiation is larger than the thickness of the bacteria. Therefore, spectral contributions of the quartz substrate dominate below 1250 cm−1 and substrate bands at 1877 and 2000 cm−1 are evident. Although the absorbance is only in the order of 0.01 important information about the bacteria can be obtained.25 The amide bands in IR spectra of bacteria on TiO2 without and after exposure to daylight are found at 1531, 1656 and 3300 cm−1 (amide II, amide I and amide A, respectively). The amide I and II bands are sensitive to the conformation of proteins. The absence of variations indicates that the bacteria are not affected. Changes of the amide A band that are partly due to baseline variations are not significant. The band centred near 2942 cm−1 is assigned to CH2/CH3 valence vibrations. The spectra of bacteria on TiO2–BaTiO3 without light exposure show a shift of the amide II band towards 1539 cm−1, an increase of the amide II/amide I ratio, and more intense bands at 1441, 1729, 2850 and 2920 cm−1. The changes in the amide bands point to changes in the protein conformation. The more intense bands are assigned to CH2 deformation (1441 cm−1), CH2 valence vibrations (2850 and 2920 cm−1) and ester moieties (1729 cm−1). These bands are consistent with lipids. The profiles of amide I and II bands changed significantly after 2 hours daylight exposure due to peroxidation. A decrease in the absorbance at 1649, 1544, and 1441 cm−1 occurred with a concomitant broadening. These spectral changes have been linked to the formation of the aldehydes during the breakdown of hydroperoxides and CO stretching vibrations during the formation of carboxy-groups.3 Two IR signatures were found for bacteria after peroxidation. One IR spectrum (light grey) shows higher lipid content. Another IR spectrum (dark grey) shows lower lipid content compared with the spectrum before peroxidation (black). This could be explained by different levels of lipid accumulation in bacteria on TiO2–BaTiO3.
The peroxidation of bacteria, essentially expressed by the oxidative current, has been electrochemically examined. The cyclic voltammogram (CV) from −1 to 1 V of the bacteria dispersed in PBS pH 7.0 recorded on a TiO2 quartz substrate at a scan rate of 50 mV s−1versus Ag/AgCl shows reversible oxidation and reduction peaks close to 0 V (Fig. 3c). Instead, in the CV of a similar dispersion recorded on a TiO2–BaTiO3–quartz substrate illustrates the presence of the broad oxidation peaks which can be attributed to catalytic peroxidation of bacteria (Fig. 3d). The constancy of the complementary current intensity peaks of barium ions at −0.6 V and 0.9 V, respectively, demonstrates the electrode's stability during the electrochemical approach.
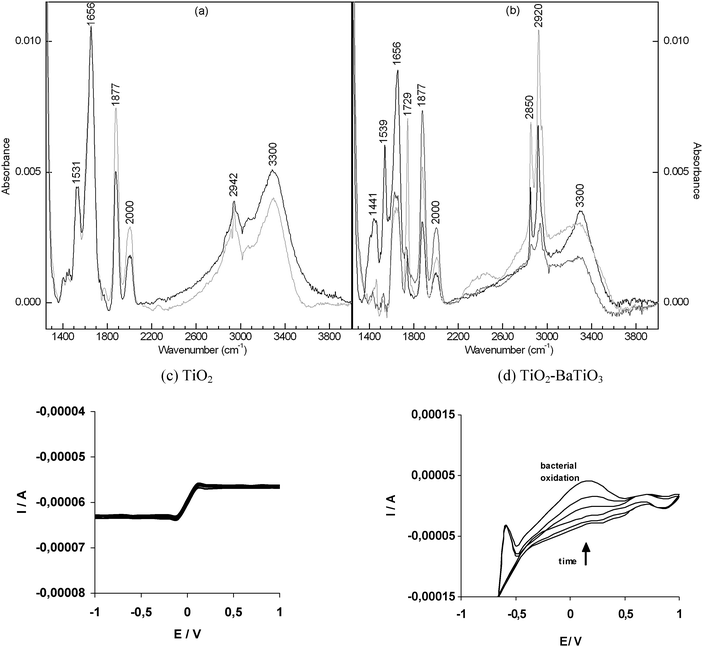 | ||
| Fig. 3 The evidence for bacterial peroxidation. (a) The ATR-FTIR spectra of the bacteria spread on the TiO2 layer set aside for 2 hours in the absence of light (black) and in the presence of daylight (grey). (b) Similar to (a) with bacteria immobilized on TiO2–BaTiO3 in the absence of light (black) and in the presence of daylight (light and dark grey). (c) The evolution of cyclic voltammograms of the bacteria in PBS buffer in the presence of TiO2 recorded in daylight. (d) Similar to (c) in the presence of TiO2–BaTiO3. The irreversible peaks of oxidation are observable from −0.5 to 0.5 V vs. Ag/AgCl. | ||
The evolution of the CV in time indicates the existence of a chemical reaction with electrochemically active intermediates from −0.5 V to +0.5 V. The gradual increase in the amplitude of the oxidation wave and the absence of the complementary reduction peaks indicate that the electrochemical process is irreversible. The oxidation current further increases even if the concentration of bacteria does not change. This instant intensity increase could be explained by a high catalytic activity of TiO2–BaTiO3 upon the bacteria peroxidation. The broad oxidation wave from −0.5 to 0.5 V does not appear in the CV of PBS alone at the same electrode. The influence of TiO2–BaTiO3 on bacteria oxidation has been proven by running the cyclic voltammetry of the bacterial dispersion on the quartz substrate devoid of TiO2–BaTiO3. TiO2–BaTiO3 considerably increased the oxidation wave compared with the background current recorded in its absence. However, the intermediates appearing during the peroxidation cannot be identified by this technique.
The bacterial survival kinetics shows a decrease in the number of cells from 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CFU mL−1 at time zero to 6000 CFU mL−1 on TiO2 (Table 1) and to 50 CFU mL−1 on TiO2–BaTiO3 (Table 1) after 2 h irradiation. Control experiments in the absence of the photocatalyst on the quartz under light irradiation showed a bacterial survival of 10
000 CFU mL−1 at time zero to 6000 CFU mL−1 on TiO2 (Table 1) and to 50 CFU mL−1 on TiO2–BaTiO3 (Table 1) after 2 h irradiation. Control experiments in the absence of the photocatalyst on the quartz under light irradiation showed a bacterial survival of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 CFU mL−1 at 2 h. If the bacterial killing is determined by lysis then the AFM images reported above agree with the survival kinetics showing that the bacteria concentration significantly decreases within 2 h under visible light irradiation on the TiO2–BaTiO3 layer.
000 CFU mL−1 at 2 h. If the bacterial killing is determined by lysis then the AFM images reported above agree with the survival kinetics showing that the bacteria concentration significantly decreases within 2 h under visible light irradiation on the TiO2–BaTiO3 layer.
| Sample | Substrate | CFU mL−1 before light exposure | CFU mL−1 after visible light exposure |
|---|---|---|---|
| 1 | TiO2 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
≅6000 |
| 2 | TiO2–BaTiO3 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
≅50 |
| 3 | Quartz slide (control) | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
≅10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
The FTIR spectra (Fig. 3a and b) show that after 2 h photocatalysis the bacterial proteins and lipids were not completely oxidized. The AFM images (Fig. 4) show significant morphological transformation of the bacteria after 2 h irradiation. These structural modifications could be attributed to the changes induced in the bilayers of the membrane. According to the AFM micrographs the photodegradation of bacteria is achieved by low energy photons in the presence of TiO2–BaTiO3. The images of the bacteria immediately after immobilization on TiO2 (Fig 4a) and after 2 hours exposure under UV light (Fig 4e) prove a morphological change with an evident reduced size of the bacteria. Similar results were obtained with the microorganisms immobilized on TiO2–BaTiO3 even under daylight exposure (Fig 4b and f). The micrographs of bacteria on TiO2 after 2 hours exposure under dark and daylight conditions do not show notable changes (data not shown).
 | ||
| Fig. 4 Antibacterial effect of TiO2–BaTiO3. (a–d) The AFM micrographs at low resolution (a, b) and high resolution (c, d) of the bacteria immobilized on quartz-glass modified with TiO2 and TiO2–BaTiO3, respectively; the AFM profiles are presented in the case of the high magnification images. (e, f) The images of the area indicated in (a) and (b), respectively, recorded after 2 hours of exposure. Scale bar: 5 μm (white), 500 nm (black). | ||
4. Conclusions
The synthesis of the TiO2–BaTiO3 composite and its catalytic effect on bacterial lysis are reported here. A particular electronic energetic structure in the composite permits pumping of electrons in the conduction band after excitation by visible light. The composite structure was identified by XRD, EDX and Raman spectroscopy. The band gap value of 2.37 ± 0.05 eV was measured by transmission spectroscopy. The peroxidation of microorganisms was demonstrated by ATR-FTIR and electrochemical studies. The morphological change of the bacteria in the peroxidation process was visualized by AFM. The experiments run with pure TiO2 under similar conditions exhibited significantly reduced efficiency compared to TiO2–BaTiO3.Acknowledgements
We thank Dr Jan Dellith and Christa Schmidt for EDX and XRD analysis, Hanna Steinmetz for thermal treatment of the samples and Christa Schmidt for XRD measurements.References
- A. L. Linsebigler, G. Lu and J. T. Yates, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- K. P. Kuhn, I. F. Chaberny, K. Massholder, M. Stickler, V. W. Benz, H.-G. Sonntag and L. Erdinger, Chemosphere, 2003, 53, 71 CrossRef CAS.
- V. A. Nadtochenko, A. G. Rincon, S. E. Stanca and J. Kiwi, J. Photochem. Photobiol., A, 2004, 169, 131 CrossRef.
- L. Caballero, K. A. Whitehead, N. S. Allen and J. Verran, J. Photochem. Photobiol., A, 2009, 202, 92 CrossRef CAS.
- T. P. T. Cushnie, P. K. J. Robertson, S. Officer, P. M. Pollard, C. McCullagh and J. M. C. Robertson, Chemosphere, 2009, 74, 1374 CrossRef.
- T. P. T. Cushnie, P. K. J. Robertson, S. Officer, P. M. Pollard, R. Prabhu, C. McCullagh and J. M. C. Robertson, J. Photochem. Photobiol., A, 2010, 216, 290 CrossRef CAS.
- F. Chen, X. Yang, H. K. C. Mak and D. W. T. Chan, Build. Environ., 2010, 45, 1747 CrossRef.
- S.-Y. Lu, D. Wu, Q.-L. Wang, J. Yan, A. G. Buekens and K.-F. Cen, Chemosphere, 2011, 82, 1215 CrossRef CAS.
- Y. Liu, X. Wang, F. Yang and X. Yang, Microporous Mesoporous Mater., 2008, 114, 431 CrossRef CAS.
- G. Li, H. Liu, H. Zhao, Y. Gao, J. Wang, H. Jiang and R. I. Boughton, J. Colloid Interface Sci., 2011, 358, 307 CrossRef CAS.
- H. A. Foster, I. B. Ditta, S. Varghese and A. Steele, Appl. Microbiol. Biotechnol., 2011, 90, 1847 CrossRef CAS.
- M. Grandcolas, J. Yeb and N. Hanagata, Mater. Lett., 2011, 65, 236 CrossRef CAS.
- W. Kangwansupamonkon, V. Lauruengtana, S. Surassmo and U. Ruktanonchai, Nanomed.: Nanotechnol., Biol. Med., 2009, 5, 240–249 CrossRef CAS.
- C. Hu, J. Guo, J. Qu and X. Hu, Appl. Catal., B, 2007, 73, 345 CrossRef CAS.
- O. Akhavan, M. Abdolahad, Y. Abdi and S. Mohajerzadeh, Carbon, 2009, 47, 3280 CrossRef CAS.
- M. L. Moreira, M. F. C. Gurgel, G. P. Mambrini, E. R. Leite, P. S. Pizani, J. A. Varela and E. Longo, J. Phys. Chem. A, 2008, 112, 8938 CrossRef CAS.
- Nature Photonics, Technology Conference Report, Tokyo, 19–21 October 2010, p. 6.
- Y. Ju-Nam and J. R. Lead, Sci. Total Environ., 2008, 400, 396 CrossRef CAS.
- I. A. Kartsonakis, P. Liatsia, I. Danilidisa, D. Bouzareloub and G. Kordas, J. Phys. Chem. Solids, 2008, 69, 214 CrossRef CAS.
- C. W. H. Dunnill, Z. A. Aiken, J. Pratten, M. Wilson, D. J. Morgan and I. P. Parkin, J. Photochem. Photobiol., A, 2009, 207, 244 CrossRef CAS.
- H.-C. Joo, Y.-J. Lima, M.-J. Kima, H.-B. Kwona and J.-H. Han, Appl. Surf. Sci., 2010, 257, 741 CrossRef CAS.
- K. Kusumoto, T. Sekiya and Y. Murase, Mater. Res. Bull., 1993, 28, 461 CrossRef CAS.
- Photorefractive Materials and their Applications I, ed. P. Günther and J. P. Huignard, Springer, Heidelberg, 1988, pp. 441–462, (Topics in Applied Physics), vol. 61, ch. 7 Search PubMed.
- C. Kittel, Introduction to Solid State Physics, John Wiley & Sons Ltd, USA, 8th edn, 2005, pp. 185–252, (Semiconductor crystals; Fermi Surfaces and Metals), ch. 8–9 Search PubMed.
- D. Naumann, Infrared Spectroscopy in Microbiology in Encyclopedia of Analytical Chemistry, ed. R. A. Meyers, John Wiley & Sons Ltd, Chichester, 2000, pp. 102–131 Search PubMed.
| This journal is © The Royal Society of Chemistry 2012 |
