Cuo@Ag as a highly active catalyst for the selective oxidation of trans-stilbene and alcohols†
Zhengmao
Ye
a,
Lei
Hu
a,
Jiang
Jiang
b,
Jianxin
Tang
c,
Xueqin
Cao
*ad and
Hongwei
Gu
*a
aKey Laboratory of Organic Synthesis of Jiangsu Province, College of Chemistry, Chemical Engineering and Materials Science, Soochow University, Suzhou 215123, China. E-mail: hongwei@suda.edu.cn; xqcao@suda.edu.cn
bi-Lab and Division of Nanobiomedicine, Suzhou Institute of Nano-tech and Nano-Bionics, Chinese Academy of Sciences, Suzhou 215123, China
cJiangsu Key Laboratory for Carbon-Based Functional Materials & Devices, Institute of Functional Nano & Soft Materials Laboratory (FUNSOM), Soochow University, Suzhou, Jiangsu 215123, China
dNational Engineering laboratory for Modern silk, Soochow University, Suzhou 215123, China
First published on 28th March 2012
Abstract
Uniform CuO@Ag nanowires were prepared by reduction of Cu(OAc)2 on the surface of Ag nanowires in ethylene glycol. This novel material shows outstanding catalytic activities in the epoxidation of trans-stilbene and the oxidation of alcohols under mild reaction conditions.
It is well known that selective oxidation is one of the most important methods for the synthesis of chemical intermediates in chemical laboratories and industries.1 In the catalytic systems, traditional oxidants, such as PhIO, NaClO, have been used to accomplish the transformation.2 However, these oxidants are often expensive and tend to be hazardous to handle. Therefore, a sustainable environmentally and industrially friendly oxidant for selective oxidation is thus of great fundamental as well as practical interest. Molecular oxygen, air, hydrogen peroxide and organic peroxides are the preferred oxidants from environmental and industrial points of view and have extensively been used in many selective oxidation reactions.3 Hutchings et al. demonstrated that oxygen is a good oxidant for the oxidation of primary alcohols4 under mild conditions. Our group has found that in the epoxidation of trans-stilbene by molecular oxygen FePt@Cu nanowires as the catalyst show excellent catalytic activity and selectivity.5
Copper-based materials have been extensively studied in recent years due to their excellent properties and potential applications.6 For example, nano-cupric oxide (CuO) particles have been used as a heterogeneous catalyst for oxidation, epoxidation and cross-coupling reactions. Zhou et al.7 proved CuO nanoparticles to be an efficient catalyst for CO oxidation. Chen et al.8 reported that CuO nanoclusters coated with mesoporous SiO2 were highly active catalysts for olefin epoxidation. Punniyamurthy et al.9 found that CuO nanoparticles catalyzed the C–N, C–O and C–S cross coupling reactions in excellent yield. All these results are encouraging, however, the CuO nanoparticles as the catalysts are not stable and the coagulation is frequently unavoidable if no supporter was used.8 Recently, one-dimensional (1D) nanostructures such as wires, rods, belts and tubes have sparked an explosive interest in the development of novel materials.10 In comparison with supported nanoparticles, one of the attractive characteristics of 1D nanostructures is the large surface area, which presents much higher catalytic activity than that of common nanocrystal catalysts in organic catalysis or electrochemical catalysis.11 These 1D nanomaterials can also be easily separated from the liquid phase reaction systems when used as catalysts.5,8,12 Christopher and Linic used Ag nanowires as the catalyst for alkenes epoxidation which showed excellent catalytic activity and selectivity than that of Ag nanoparticles,13 and some metal nanowires have been demonstrated to be effective catalysts without any supporter for the liquid phase reactions and can be recycled many times.12
Inspired by these results, we designed a novel nanomaterial structure using Ag nanowires to carry CuO nanoparticles (CuO@Ag nanowires). This novel nanomaterial was prepared through a facile synthetic route including the synthesis of Ag nanowires (NWs) and subsequent deposition of CuO nanoparticles on them in ethylene glycol (EG). CuO@Ag nanowires show outstanding catalytic activity and selectivity when used as the catalyst in the epoxidation of trans-stilbene with the green oxidant air. At the same time, the CuO@Ag nanowires catalyst also shows excellent catalytic activity in the oxidation of alcohols at low temperature using tert-butyl hydroperoxide (TBHP) as the oxidant.
The process of CuO@Ag nanowire preparation is described in Scheme 1. Firstly, Ag NWs with an average diameter of 40 nm were synthesized following a reported methodology14 and dispersed in 20 mL of EG by magnetic stirring. Then 20 mg of cupric acetate was added and the temperature was raised to 180 °C and the reaction mixture was kept at this temperature for 30 minutes. The reaction mixture was then cooled to room temperature under air. The CuO@Ag NWs were precipitated by adding ethanol and collected by centrifugation. This gray precipitate was washed several times using ethanol and redispersed in water or ethanol for further use.
 | ||
| Scheme 1 Preparation of CuO@Ag nanowires. | ||
The morphologies of the Ag NWs and CuO@Ag NWs were characterized by transmission electron microscopy (TEM). The lengths of the Ag NWs were several micrometres and their diameters were controlled to be in the range of 30–50 nm (Fig. 1A). The TEM image in Fig. 1B shows that a large number of CuO nanoparticles (NPs) were formed and evenly distributed on the surface of the Ag NWs. The Cu signal in the energy dispersive spectrum (EDS) was observed and the ratio of Cu and O atoms was close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which confirms the presence of CuO NPs on the surface of Ag NWs (Area 1). The EDS recorded at different regions indicated that the CuO content in the CuO@Ag nanowires was about 22%. The surface of CuO@Ag NWs was also investigated using EDS and only Cu was observed which confirmed that CuO was distributed on the surface of the Ag NWs (Area 2). To further investigate the structural features of the nanowires, X-ray photoelectron spectroscopy (XPS) was performed and the results are shown in Fig. 1C and D. The survey XPS spectrum of the CuO@Ag NWs showed the presence of copper, silver and oxide without other obvious contaminant species. A small amount of carbon was present and the C 1s peak position (285.0 eV) was used to calibrate the spectrum (Fig. 1C). From the Cu 2p core level XPS spectrum (Fig. 1D), the peaks corresponding to Cu 2p3/2 and Cu 2p1/2 are observed at around 934.5 eV and 954.3 eV, respectively, which match well with the literature values.15 Moreover, the Cu 2p core level spectrum shows several strong satellite peaks, which are characteristic of CuO having a d9 configuration in the ground state.15
1, which confirms the presence of CuO NPs on the surface of Ag NWs (Area 1). The EDS recorded at different regions indicated that the CuO content in the CuO@Ag nanowires was about 22%. The surface of CuO@Ag NWs was also investigated using EDS and only Cu was observed which confirmed that CuO was distributed on the surface of the Ag NWs (Area 2). To further investigate the structural features of the nanowires, X-ray photoelectron spectroscopy (XPS) was performed and the results are shown in Fig. 1C and D. The survey XPS spectrum of the CuO@Ag NWs showed the presence of copper, silver and oxide without other obvious contaminant species. A small amount of carbon was present and the C 1s peak position (285.0 eV) was used to calibrate the spectrum (Fig. 1C). From the Cu 2p core level XPS spectrum (Fig. 1D), the peaks corresponding to Cu 2p3/2 and Cu 2p1/2 are observed at around 934.5 eV and 954.3 eV, respectively, which match well with the literature values.15 Moreover, the Cu 2p core level spectrum shows several strong satellite peaks, which are characteristic of CuO having a d9 configuration in the ground state.15
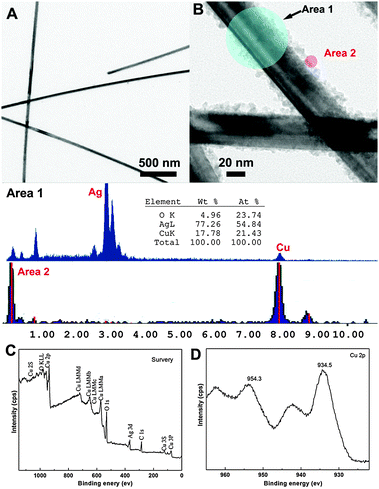 | ||
| Fig. 1 TEM images of (A) Ag NWs and (B) CuO@Ag NWs (inset shows the EDS spectrum of the different areas of the CuO@Ag NWs). XPS analysis of CuO@Ag NWs: (C) survey and (D) Cu 2p. | ||
The epoxidation of trans-stilbene was used to investigate the catalytic activity of CuO@Ag NWs. The reactions were carried out using 0.5 mg of CuO@Ag NWs as the catalyst for 0.25 mmol trans-stilbene epoxidation in air. Table 1 shows the catalytic performance of the CuO@Ag NWs for trans-stilbene epoxidation in different solvents at 100 °C for 24 h. It was interesting to find that the solvent has an important effect on product formation. The efficiency of the solvents was: toluene, DMF < dioxane < isopropylbenzene < o-xylene. o-Xylene was the best solvent and gave the highest conversion rate (>99%) with good selectivity in this reaction (Table 1, entry 5), which agrees with our previous report using FePt@Cu as the catalyst.5 When o-xylene was used as the solvent, 2-methylbenzaldehyde, o-tolylmethanol and 2-methylbenzoic acid were obtained as byproducts from the oxidation of the solvent, which indicates that the solvent was first oxidized and then the substrate.5 The reaction mechanism was proposed as follows: o-xylene reacted firstly with active oxygen to form an unstable free radical intermediate and then a peroxide. The peroxide acted as an oxidant in the stilbene oxidation and the epoxy compound was obtained as the major product (Scheme 2). o-Xylene will be oxidized to o-tolylmethanol (Cycle 1), 2-methylbenzaldehyde (Scheme S1, Cycle 2, ESI†) and 2-methylbenzoic acid (Scheme S1, Cycle 3, ESI†), simultaneously. The products from the oxidation of o-xylene can be detected by GC-MS (Fig. S7, ESI†). We also detected the catalytic activity of our catalyst in DMF, dioxane and toluene and no new product was observed. This observation and the mechanism were similar to that of FePt@Cu5 and Au/TiO216 catalysts, which were used as the catalysts for stilbene oxidation.
 | ||
| Scheme 2 The proposed reaction mechanism (Cycle 1). | ||
The activity of CuO@Ag NWs at different temperatures was also investigated using o-xylene as the solvent. The temperature was a significant factor as well for the conversion and selectivity of the reaction (Table 2). At low temperature (60 °C), almost no reaction occurred with the catalyst. When the temperature was increased to 80 °C, the conversion increased to 8% with high selectivity (100%). 100 °C was found to be the optimal temperature with excellent conversion (>99%) and selectivity (84%). When the temperature was increased to 120 °C, trans-stilbene was quantitatively converted, only with a little decrease in selectivity (78.6%) which is better than that of a Cu based catalyst reported previously.5 From the results of the conversion and the temperature, we can get the Arrhenius plot of the catalyst (Fig. S3, ESI†). The apparent activation energy of CuO@Ag NWs is determined to be 73.8 kJ mol−1 which is larger than that of the reported Co catalyst (66.64 kJ mol−1).17 The high apparent activation energy implies that the catalytic activity of CuO@Ag NWs has a high dependency on temperature; thus, increasing the temperature will increase the trans-stilbene conversion and the reaction rate. This catalyst can also be recycled without a significant loss in activity (Fig. S4, ESI†). The structure of the CuO@Ag NWs after reaction was retained as confirmed by TEM and trace CuO loss was detected in these recycled nanowires (Fig. S5, ESI†).
At the same time, we performed control experiments under the same conditions as above using Ag NWs and “free” CuO nanoparticles as catalysts, to investigate whether they exhibit catalytic activity in trans-stilbene epoxidation. The results showed that using Ag NW catalysts only a 40% conversion with a selectivity of 95% (Fig. S6, ESI†) can be obtained. The yield of the corresponding product from trans-stilbene can reach 80% in the first use of “free” CuO nanoparticles, however, it cannot be recycled for further use because of its aggregation which was also reported in previous work.8 By comparing the catalytic activity and stability of our catalyst with Ag NWs and “free” CuO nanoparticles, it is found that CuO@Ag NWs show higher catalytic activity and stability, showing a synergistic effect.
This catalyst can also be used in other olefin epoxidation reactions under the same reaction conditions. When cis-stilbene was used as the reactant, the conversion and selectivity were 95% and 62.8/23.7 (cis/trans), respectively. When styrene was used as the reactant, the selectivity of styrene oxide was 53.1% with a high conversion (>99%) and we also detected another two products: benzaldehyde (12.3%) and benzoic acid (34.6%).
Furthermore, CuO@Ag NWs are active not only for olefin epoxidation but also for the oxidation of alcohols to the corresponding aldehydes, carboxylic acids or ketones in another catalytic system (TBHP as the oxidant and acetonitrile as the solvent18). We first investigated the catalytic activities of CuO@Ag NWs for benzyl alcohol oxidation using different solvents. All reactions showed a satisfactory yield in 24 h. Moreover, it was found that when using acetonitrile as the solvent, the quantitative conversion with 98.4% selectivity was obtained from benzyl alcohol to benzoic acid (Table S1, entry 1, ESI†). For oxidation of benzyl alcohol, the catalyst could also be easily recycled and reused by simple centrifugation and ethanol washing (Fig. S9, ESI†). By comparing the catalytic activity of Ag NWs and “free” CuO nanoparticles, it was found that this novel nanomaterial showed higher catalytic activity than Ag NWs (50% in yield) and higher selectivity than “free” CuO nanoparticles (30.0% of benzoic acid) (Fig. S10, ESI†).
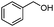
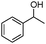
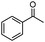
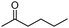
Next, the selective oxidation of various alcohols in the presence of CuO@Ag NW catalysts was investigated. The results obtained are summarized in Table 3. Secondary alcohols, such as 1-phenylethanol, cyclohexanol and hexan-2-ol, were oxidized to the corresponding carbonyl compounds. Oxidation of 1-phenylethanol gave acetophenone with a 100% yield (Table 3, entry 2). Cyclohexanone (80%) was the main product from the oxidation of cyclohexanol (Table 3, entry 3). Hexan-2-one (75%) was the main product from hexan-2-ol oxidation (Table 3, entry 4). The oxidation of 1-hexyl alcohol gave hexanal as the main product with a yield of 18% and a high selectivity (100%) (Table 3, entry 5). Oxidation of primary aliphatic alcohols to the corresponding aliphatic aldehydes is difficult using most heterogeneous catalysts,19 thus the low conversion of 1-hexyl alcohol is understandable because of its low reactivity.
In summary, a novel nanomaterial, CuO@Ag NWs, was synthesized through the reduction of Cu(OAc)2 on the surface of Ag NWs in ethylene glycol solution and the CuO nanoparticles were evenly distributed on the surface of the Ag NWs. This nanomaterial exhibited high catalytic activity and selectivity for selective oxidation of trans-stilbene and alcohols under “green” reaction conditions, and could be recycled several times with good activity. Our new findings will undoubtedly be extended to fabricate new categories of nanomaterials as the catalysts in organic reactions.
H.W.G. acknowledges financial support from the National Natural Science Foundation of China (No. 21003092), the Key Project of Chinese Ministry of Education (No. 211064) and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions. X.Q.C. acknowledges financial support from the National Engineering Laboratory for Modern Silk, Soochow University, China.
Notes and references
- C. D. Pina, E. Falletta, L. Prati and M. Rossi, Chem. Soc. Rev., 2008, 37, 2077 RSC; T. Mallal and A. Baiker, Chem. Rev., 2004, 104, 3037 CrossRef CAS; R. A. Sheldon, I. W. C. E. Arends, G. Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774–781 CrossRef.
- S. H. Wang, B. S. Mandimutsira, R. Todd, B. Ramdhanie, J. P. Fox and D. P. Goldberg, J. Am. Chem. Soc., 2004, 126, 18 CrossRef CAS; X. L. Geng, Z. Wang, X. Q. Li and C. Zhang, J. Org. Chem., 2005, 70, 9610 CrossRef; W. Adam, K. J. Roschmann, C. R. Saha-Moller and D. Seebach, J. Am. Chem. Soc., 2002, 124, 5068 CrossRef.
- P. T. Anastas and J. C. Waner, Green Chemistry: Theory and Practice, New York, 1998, p. 30 CrossRef CAS; T. Punniyamurthy, S. Velusamy and J. Iqbal, Chem. Rev., 2005, 105, 2329 CrossRef CAS; B. S. Lane and K. Burgess, Chem. Rev., 2003, 103, 2457 CrossRef.
- D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Kinght and G. J. Hutchings, Science, 2006, 311, 362 CrossRef CAS.
- L. Hu, L. Y. Shi, H. Y. Hong, M. Li, Q. Y. Bao, J. X. Tang, J. F. Ge, J. M. Lu, X. Q. Cao and H. W. Gu, Chem. Commun., 2010, 46, 8591 RSC.
- M. Basu, A. K. Sinha, M. Pradhan, S. Sarkar, A. Pal and T. Pal, Chem. Commun., 2010, 46, 8785–8787 RSC; L. I. Hung, C. K. Tsung, W. Huang and P. Yang, Adv. Mater., 2012, 22, 1910–1914 CrossRef CAS; L. P. Xu, S. Sithambaram, Y. S. Zhang, C. H. Chen, L. Jin, R. Joesten and S. L. Suib, Chem. Mater., 2009, 21, 1253–1259 CrossRef; H. Zhu, J. Wang and G. Xu, Cryst. Growth Des., 2008, 9, 633 Search PubMed; F. Zhang, A. W. Zhu, Y. P. Luo, Y. Tian, J. H. Yang and Y. Qin, J. Phys. Chem. C, 2010, 114, 19214 Search PubMed; Y. Z. Feng and X. L. Zheng, Nano Lett., 2010, 10, 4762 CrossRef; J. C. Park, J. Kim, H. Kwon and H. Song, Adv. Mater., 2009, 21, 803 CrossRef.
- K. B. Zhou, R. P Wang, B. Q. Xu and Y. D. Li, Nanotechnology, 2006, 17, 3939 CrossRef CAS.
- C. Q. Chen, J. Qu, C. Y. Cao, F. Niu and W. G. Song, J. Mater. Chem., 2011, 21, 5774 RSC.
- S. Jammi, S. Sakthievl, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971 CrossRef CAS.
- B. L. Cademartiri and G. A. Ozin, Adv. Mater., 2009, 21, 1013 CrossRef.
- S. J. Guo, S. Zhang, X. L. Sun and S. H. Sun, J. Am. Chem. Soc., 2011, 133, 15354 CrossRef CAS; B. Hu, K. L. Ding, T. B. Wu, X. S. Zhou, H. l. Fan, T. Jiang, Q. Wang and B. X. Han, Chem. Commun., 2010, 46, 8552 RSC.
- H. Y. Hong, L. Hu, M. Li, J. W. Zheng, X. H. Sun, X. H. Lu, X. Q. Cao, J. M. Lu and H. W. Gu, Chem.–Eur. J., 2011, 17, 8726–8730 CrossRef CAS; L. Hu, X. Q. Cao, J. H. Yang, M. Li, H. Y. Hong, F. Q. Xu, J. F. Ge, L. H. Wang, J. M. Lu, L. Cheng and H. W. Gu, Chem. Commun., 2011, 47, 1303 RSC; M. Li, L. Hu, X. Q. Cao, H. Y. Hong, J. M. Lu and H. W. Gu, Chem.–Eur. J., 2011, 17, 2763 CrossRef.
- P. Christopher and S. Linic, J. Am. Chem. Soc., 2008, 130, 11264 CrossRef CAS.
- Y. G. Sun, B. Gates, B. Mayers and Y. N. Xia, Nano Lett., 2002, 2, 165 CrossRef CAS; Y. G. Sun, Y. D. Yin, B. T. Mayers, T. Herricks and Y. N. Xia, Chem. Mater., 2002, 14, 4736 CrossRef.
- Y. C. Zhang, J. Y. Tang, G. l. Wang, M. Zhang and X. Y. Hu, J. Cryst. Growth, 2006, 294, 278 CrossRef CAS.
- P. Lignier, M. Comotti, F. Schüth, J. L. Rousset and V. Caps, Catal. Today, 2009, 141, 355 CrossRef CAS; P. Lignier, S. Mangematin, F. Morfin, J. L. Rousset and V. Caps, Catal. Today, 2008, 138, 50 CrossRef; P. Lignier, F. Morfin, S. Mangematin, L. Massin, J. L. Rousset and V. Caps, Chem. Commun., 2007, 186 RSC; D. Gajan, K. Guillois, P. Delichère, J. M. Basset, J. P. Candy, V. Caps, C. Copéret, A. Lesage and L. Emsley, J. Am. Chem. Soc., 2009, 131, 14667 CrossRef; M. Boualleg, K. Guillois, B. Istria, L. Burel, L. Veyre, J. M. Basset, C. Thieuleux and V. Caps, Chem. Commun., 2010, 46, 5361 RSC.
- X.-Y. Quek, Q. Tang, S. Hu and Y. Yang, Appl. Catal., A, 2009, 361, 130 CrossRef CAS.
- S. Pande, A. Saha, S. Jana, S. Sarkar, M. Basu, M. Pradhan, A. K. Sinha, S. Saha, A. Pal and T. Pal, Org. Lett., 2008, 10, 5179 CrossRef CAS; I. E. Wachs and R. J. Madix, J. Catal., 1978, 53, 208 CrossRef; T. Mallat and A. Baiker, Catal. Sci. Technol., 2011, 1, 1572 Search PubMed.
- A. Dhakshmamoorthy, M. Alvaro and H. Garcia, ACS Catal., 2011, 1, 48 CrossRef CAS; C. Keresszegi, D. Ferri, T. Mallat and A. Baiker, J. Catal., 2005, 234, 64 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, TEM images of Ag NWs and CuO@Ag NWs. See DOI: 10.1039/c2cy20138k |
| This journal is © The Royal Society of Chemistry 2012 |