Pd@aluminium foil: a highly efficient and environment-friendly “tea bag” style catalyst with high TON†
Lei
Fan
,
Rong
Yi
,
Lei
Yu
*,
Yulan
Wu
,
Tian
Chen
and
Rong
Guo
School of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, P. R. China. E-mail: yulei@yzu.edu.cn; Fax: +86-514-8797-5244
First published on 9th March 2012
Abstract
A tea bag catalyst, Pd@Al foil, was prepared simply by heating cheap aluminium foil in a xylene solution of Pd(OAc)2. This material showed high catalytic ability and good cycling ability. The reaction conditions proved to be mild and environment-friendly. It shows good potential in catalysis.
Transition metal-catalyzed reactions are one of the most potent tools in organic synthesis. Being able to construct many useful skeletons precisely and efficiently, the transition metal-catalyzed reactions are now widely applied in academic research as well as industry production. Meanwhile, many of them have become classical name-reactions, including Kumada,1 Negishi,2 Suzuki,3 Stille,4 Hiyama,5 Heck,6 Sonogashira7 reactions and so on. However, in these reactions, environment-unfriendly organic solvents and phosphorus ligands are usually required. Soluble homogeneous catalysts are one-off and cause waste. These drawbacks counteract the applications much. Hence, for a long time, chemists are making great efforts to solve these problems. Many references have reported the ligand-free reactions8 and there are also many examples that are carried out in non-toxic solvents.9 However, they often need some special catalysts, which are hard to prepare.
Catalytic ability of metal nanoparticles has attracted chemists' great attention during the past decade.10 Previous investigations revealed that metal nanoparticles are excellent catalysts and in many cases the reaction could be well catalyzed in the absence of phosphorus ligands. Moreover, as heterogeneous catalysts, metal nanoparticles could be separated by filtration or centrifugation, and reused in the next cycle. However, if there are indiscerptible impurities in the reaction system, it is hard to separate the catalysts by filtration or centrifugal separation. Therefore, we have developed a novel style of recyclable catalysts, which are much easier to separate after reaction.
Tea bags are convenient drinks. Since tea leaves are encapsulated in a paper bag, they could be removed easily after being used. Therefore, illumed by tea bags, we propose to design a novel style of catalyst. This kind of catalyst is designed to be uploaded on a mass of solid and then be sent into the reaction liquid. After reaction, it could be removed easily from the system and reused in the next cycle (see Fig. 1). Due to the similarity to the tea bags, we would like to call this kind of catalyst a “tea bag” style catalyst. Herein, we report a simple and economic material, Pd@Al foil, which is called a “tea bag” style catalyst. It could be prepared by putting Al foil into a Pd(OAc)2 solution, so it is simple and convenient. Al foil is common and this catalyst could be used for multiple times, so it is economic. The most important thing is that it has excellent catalytic ability.
 | ||
| Fig. 1 A “tea bag” style catalyst. | ||
The material was prepared just by heating Al foil (1 cm × 1 cm, area) in a xylene solution of Pd(OAc)2. After rinsing with deionized water and alcohol sequentially, it was then examined for its catalytic ability. It was very surprising to find that the Pd coated Al foil could catalyze the Suzuki–Miyaura cross-coupling well. The reaction conditions were mild and the yield was extremely high (Scheme 1). The catalyst was also effective in large scale preparation. When a 10-times scale (5 mmol) reaction was catalyzed by one piece of Pd@Al foil (1 cm × 1 cm, area), diphenyl could be obtained in 93% yield. X-Ray fluorescence spectra analysis proved the existence of the plated palladium surface, which was obviously the effective catalyst (see ESI†). Further, inductively coupled plasma mass spectrometry (ICP-MS) showed that there was 49 μg of Pd on each Al foil. Thus, the TON of this catalyst could reach 104 (mol mol−1) at the maximum.
 | ||
| Scheme 1 Pd@Al foil catalyzed Suzuki–Miyaura cross-coupling on 0.5 mmol scale. | ||
To exclude the possibility that the reaction was catalyzed by ambient factors (e.g. palladium pollution of equipment, solvent, Al foil or base), a blank reaction was carried out. When phenyl iodide, phenylboronic acid and K2CO3 were heated in alcohol in the presence of untreated Al foil, no reaction occurred (Scheme 2).
 | ||
| Scheme 2 Blank reaction in the presence of untreated Al foil. | ||
In order to investigate the composition of the obtained catalyst, a series of methods were used in characterization. An X-ray photoelectron spectroscopy (XPS) experiment was used to confirm the valence of the Al on the surface. The binding energies of the Al2p emission lines indicate the presence of Al3+ species. Al2p photoelectrons from zerovalent, metallic Al are known to appear at the binding energies between 71 and 72 eV, while Al2p emission from the Al3+ valence state is observed at binding energy around 75 eV. Therefore, the observed Al2p binding energy value of 74.4 eV in this work (Fig. 2) indicated that the Al foil surface was covered by an Al2O3 layer. The spectra also indicated that Pd(0) existed on the surface (335.0 eV, Pd3d5; 340.3 eV, Pd3d3) (Fig. 3).11 Pd(0) was reduced from Pd(OAc)2 by Al foil in the catalyst preparation step.
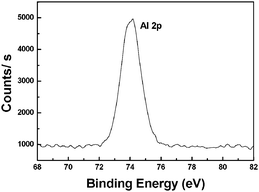 | ||
| Fig. 2 X-Ray photoelectron spectra for Al of the catalyst surface. | ||
 | ||
| Fig. 3 X-Ray photoelectron spectra for Pd of the catalyst surface. | ||
Moreover, the field emission scanning electron microscopy (FE-SEM) images provided more interesting information. By comparison of the FE-SEM images of the Pd@Al foil before and after reactions, it is easy to find that the surface appearance changed much (Fig. 4). The rearrangement to the twig-like loosened surface could increase the surface area and may enhance the catalytic ability (Fig. 4b and c). The change in the surface appearance was probably due to the slow eroding of aluminium under weak basic conditions, followed by the aggregation, self-assembly, and adsorption. The apropos weak basic condition was the key point. We once tried to carry out the reaction in water to achieve a more environment-friendly condition. However, no reaction was observed. This failed result was probably due to the stronger basic conditions (K2CO3 is soluble in water. In contrast, its solubility in alcohol is very low.), which eroded the aluminium surface quickly and made the palladium atoms to fall off, aggregate and lose activity. Moreover, no reaction occurred with toluene as solvent. When Al was replaced with other metals, such as copper and silver, the material did not have any catalytic ability.
 | ||
| Fig. 4 FE-SEM images of Pd@Al foil before (image a, 50 nm) and after (image b and c, 50 nm and 30 nm respectively) reactions. | ||
The Pd@Al foil material was also a recyclable catalyst. After reaction, it can be conveniently removed just by a pair of forceps and then put in the next cycle. It worked well even after more than 10 cycles (Table 1).
Then, the application scope of this catalyst was carefully examined.12 Besides phenyl iodide, the cross-coupling of phenyl bromide also provided diphenyl in high yields (Table 2, entries 2–5). However, when phenyl chloride was employed, no reaction was observed (Table 2, entry 6), probably due to the stronger C–Cl bond energy. In most cases, the yields were very high. Both electron-donating and electron-withdrawing substituent groups were tolerated on the aryl ring (Table 2, entries 7–15). When a nitro substituted substrate was employed, the yield decreased sharply, probably due to the by-reactions of the nitryl group in the reductive reaction conditions (Table 2, entry 16). This catalyst was also effective on more complex substrates (Scheme 3). However, if o-methyliodobenzene or o-methoxyiodobenzene were used, no reaction occurred. Just like other heterogeneous catalyses, if there is a substituent at the o-position, it is difficult for the reaction to occur due to the strong steric effects.
| Entry | Ar1 | Ar2 | X | Yield of 3b (%) |
|---|---|---|---|---|
| a 0.5 mmol of Ar2I, 0.75 mmol of Ar1B(OH)2 and 1 mmol of base were employed. 3 mL of EtOH was employed. The reaction was monitored by TLC (eluent: petroleum ether). b Isolated yields. | ||||
| 1 | C6H5 | C6H5 | I | 100 (3a) |
| 2 | C6H5 | C6H5 | Br | 98 (3a) |
| 3 | C6H5 | p-CH3C6H4 | Br | 86 (3b) |
| 4 | p-CH3C6H4 | C6H5 | Br | 90 (3b) |
| 5 | C6H5 | p-CHOC6H4 | Br | 83 (3c) |
| 6 | C6H5 | C6H5 | Cl | N.R. |
| 7 | C6H5 | p-CH3C6H4 | I | 100 (3b) |
| 8 | C6H5 | m-CH3C6H4 | I | 97 (3d) |
| 9 | C6H5 | p-CH3OC6H4 | I | 100 (3e) |
| 10 | C6H5 | p-C2H5OC6H4 | I | 91 (3f) |
| 11 | C6H5 | p-HOC6H4 | I | 96 (3g) |
| 12 | p-CH3C6H4 | C6H5 | I | 96 (3b) |
| 13 | p-CH3OC6H4 | C6H5 | I | 97 (3e) |
| 14 | m-CH3OC6H4 | C6H5 | I | 98 (3h) |
| 15 | m-ClC6H4 | C6H5 | I | 100 (3i) |
| 16 | m-NO2C6H4 | C6H5 | I | 15 (3j) |
 | ||
| Scheme 3 The cross-coupling reaction on halo-olefins. | ||
In conclusion, we have successfully developed a novel “tea bag” style catalyst, Pd@Al foil, which could be easily prepared by heating the cheap Al foil in a Pd(OAc)2 solution. The application conditions of this catalyst were mild. Considering the phosphorus ligand-free conditions and the harmless alcohol solvent, this catalyst is very friendly to the environment. Moreover, this catalyst could be easily separated after reaction and could be reused for many times. All of these advantages show that it is an excellent catalyst.
This work was supported by the Priority Academic Program Development of Jiangsu Higher Education Institutions, the Natural Scientific Foundation of Jiangsu Province (No. BK2010321), the National Natural Scientific Foundation of China (No. 21001091, 20633010 and 20773106), University Natural Scientific Foundation of Jiangsu Province (No. 09KJB150014), the 45th Post-doctoral foundation of China (No. 20090451249); the opening laboratory fund of Jiangsu Province (K100027); and the opening laboratory fund of Zhejiang Province (100061200138). We thank Prof. Hongliang Yu and Miss Lingfeng Ren for help in this research.
Notes and references
- (a) K. Tamao, Y. Kiso, K. Sumitani and M. Kumada, J. Am. Chem. Soc., 1972, 94, 9268 CrossRef CAS; (b) K. Tamao, J. Organomet. Chem., 2002, 653, 23 CrossRef CAS; (c) K. Tamao and N. Miyaura, Top. Curr. Chem., 2002, 219, 1 CrossRef CAS; (d) E. Negishi, J. Organomet. Chem., 2002, 653, 34 CrossRef CAS; (e) S. I. Murahashi, J. Organomet. Chem., 2002, 653, 27 CrossRef CAS; (f) R. Martin and S. L. Buchwald, J. Am. Chem. Soc., 2007, 129, 3844 CrossRef CAS; (g) D. Gauthier, S. Beckendorf, A. T. Lindhardt and T. Skrydstrup, J. Org. Chem., 2009, 74, 3536 CrossRef CAS.
- (a) E. Negishi, A. O. King and N. Okukado, J. Org. Chem., 1977, 42, 1821 CrossRef CAS; (b) E. Negishi and D. E. Van Horn, J. Am. Chem. Soc., 1977, 99, 3168 CrossRef CAS; (c) A. O. King, E. Negishi, F. J. Jr. Villani and A. Jr. Silveria, J. Org. Chem., 1978, 43, 358 CrossRef CAS; (d) E. Negishi, Handbook of Organopalladium Chemistry for Organic Synthesis, Wiley, New York, 2002, vol. 1, p. 229 Search PubMed; (e) C. Wang, T. Tobrman, Z. Q. Xu and E. Negishi, Org. Lett., 2009, 11, 4092 CrossRef CAS; (f) A. T. Lindhardt and T. Skrydstrup, J. Org. Chem., 2009, 74, 135 CrossRef CAS; (g) G. Zhou, P. Ting, R. Aslanian and J. J. Piwinski, Org. Lett., 2008, 10, 2517 CrossRef CAS.
- (a) N. Miyaura and A. Suzuki, Chem. Commun., 1979, 866 RSC; (b) S. Kotha, K. Lahiri and D. Kashinath, Tetrahedron, 2002, 58, 9633 CrossRef CAS; (c) M. Y. Zhu and G. W. Diao, J. Phys. Chem. C, 2011, 115, 24743 CrossRef CAS; (d) C. W. Yang, K. Chanda, P. H. Lin, Y. N. Wang, C. W. Liao and M. H. Huang, J. Am. Chem. Soc., 2011, 133, 19993 CrossRef CAS; (e) S. Horikoshi, A. Osawa, M. Abe and N. Serpone, J. Phys. Chem. C, 2011, 115, 23030 CAS.
- (a) D. Milstein and J. K. Stille, J. Am. Chem. Soc., 1978, 100, 3636 CrossRef CAS; (b) P. Espinet and A. M. Echavarren, Angew. Chem., Int. Ed., 2004, 43, 4704 CAS; (c) D. B. Pacardo, M. Sethi, S. E. Jones, R. R. Naik and M. R. Knecht, ACS Nano, 2009, 3, 1288 CrossRef CAS; (d) M. Bernechea, E. Jesus, C. L. Mardomingo and P. Terreros, Inorg. Chem., 2009, 48, 4491 CrossRef CAS.
- (a) Y. Hatanaka and T. Hiyama, J. Org. Chem., 1988, 53, 918 CrossRef CAS; (b) L. D. Pachón, M. B. Thathagar, F. Hartl and G. Rothenberg, Phys. Chem. Chem. Phys., 2006, 8, 151 RSC; (c) A. Gordillo, E. de Jesús and C. L. Mardomingo, Chem. Commun., 2007, 4056 RSC; (d) A. Balanta, C. Godard and C. Claver, Chem. Soc. Rev., 2011, 40, 4973 RSC; (e) C. L. Sun, B. J. Li and Z. J. Shi, Chem. Commun., 2010, 46, 677 RSC.
- (a) R. F. Heck, J. Am. Chem. Soc., 1968, 90, 5518 CrossRef CAS; (b) M. Weck and C. W. Jones, Inorg. Chem., 2007, 46, 1865 CrossRef CAS; (c) M. Bradshaw, J. L. Zou, L. Byrne, K. S. Iyer, S. G. Stewart and C. L. Raston, Chem. Commun., 2011, 47, 12292 RSC; (d) M. Hyotanishi, Y. Isomura, H. Yamamoto, H. Kawasaki and Y. Obora, Chem. Commun., 2011, 47, 5750 RSC; (e) G. Liu, M. Q. Hou, J. Y. Song, T. Jiang, H. L. Fan, Z. F. Zhang and B. X. Han, Green Chem., 2010, 12, 65 RSC; (f) M. A. R. Meier, M. Filali, J. F. Gohy and U. S. Schubert, J. Mater. Chem., 2006, 16, 3001 RSC.
- (a) K. Sonogashira, Y. Tohda and N. Hagihara, Tetrahedron Lett., 1975, 16, 4467 CrossRef; (b) J. Y. Park, E. J. Park, A. J. Kim, S. A. Park, Y. G. Lee, K. W. Chi, Y. H. Jung and I. S. Kim, J. Org. Chem., 2011, 76, 2214 CrossRef CAS; (c) T. Lauterbach, M. Livendahl, A. Rosellon, P. Espinet and A. M. Echavarren, Org. Lett., 2010, 12, 3006 CrossRef CAS; (d) T. Posset and J. Blümel, J. Am. Chem. Soc., 2006, 128, 8394 CrossRef CAS.
- (a) E. Alacid and C. Nájera, J. Org. Chem., 2008, 73, 2315 CrossRef CAS; (b) E. Sperotto, G. P. M. van Klink, J. G. de Vries and G. van Koten, J. Org. Chem., 2008, 73, 5625 CrossRef CAS; (c) T. M. Razler, Y. Hsiao, F. Qian, R.-L. Fu, R. K. Khan and W. Doubleday, J. Org. Chem., 2009, 74, 1381 CrossRef CAS; (d) J. L. Roger, F. Pozgan and H. Doucet, J. Org. Chem., 2009, 74, 1179 CrossRef CAS; (e) D. Gauthier, S. Beckendorf, T. M. Gogsig, A. T. Lindhardt and T. Skrydstrup, J. Org. Chem., 2009, 74, 3536 CrossRef CAS; (f) Q. Yang and H. Alper, J. Org. Chem., 2010, 75, 948 CrossRef CAS; (g) J. Salvadori, E. Balducci, S. Zaza, E. Petricci and M. Taddei, J. Org. Chem., 2010, 75, 1841 CrossRef CAS; (h) Y. J. Su and N. Jiao, Org. Lett., 2009, 11, 2980 CrossRef CAS.
- For a monograph please see: C.-J. Li and T.-H. Chan, Comprehensive Organic Reactions in Aqueous Media, Wiley, 2nd edn, 2007, and references therein Search PubMed.
- (a) V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 951 CrossRef CAS; (b) V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 1697 CrossRef CAS; (c) J. H. Park, S. Y. Kim, S. M. Kim and Y. K. Chung, Org. Lett., 2007, 9, 2465 CrossRef CAS; (d) J. H. Park, E. Kim and Y. K. Chung, Org. Lett., 2008, 10, 4719 CrossRef CAS; (e) J. Han, Y. Liu and R. Guo, J. Am. Chem. Soc., 2009, 131, 2060 CrossRef CAS; (f) V. Calo, A. Nacci, A. Monipoli and P. Cotugno, Angew. Chem., Int. Ed., 2009, 48, 6101 CrossRef CAS; (g) D. Astruc, F. Lu and J. R. Aranzaes, Angew. Chem., Int. Ed., 2005, 44, 7852 CrossRef CAS; (h) R. Dey, K. Chattopadhyay and B. C. Ranu, J. Org. Chem., 2008, 73, 9461 CrossRef CAS; (i) L. Adak, K. Chattopadhyay and B. C. Ranu, J. Org. Chem., 2009, 74, 3982 CrossRef CAS; (j) B. C. Ranu, K. Chattopadhyay and L. Adak, Org. Lett., 2007, 9, 4595 CrossRef CAS; (k) J. Liu, Y. Deng, H. B. Wang, H. Zhang, G. X. Yu, B. B. Wu, H. Zhang, Q. Li, T. B. Marder, Z. Yang and A. W. Lei, Org. Lett., 2008, 10, 2661 CrossRef CAS; (l) J. Liu, H.-B. Wang, H. Zhang, X.-J. Wu, H. Zhang, Y. Deng, Z. Yang and A.-W. Lei, Chem.–Eur. J., 2009, 15, 4437 CrossRef CAS.
- K. Dumbuya, K. Christmann and S. L. M. Schroeder, Langmuir, 2007, 23, 5386 CrossRef CAS.
- Preparation of the catalyst Pd@Al foil. Under a nitrogen atmosphere, 5 pieces of Al foil (1 cm × 1 cm, area) were stirred in a solution of Pd(OAc)2 (5.3 mg) in xylene (15 mL) at 100 °C for 3 h. Then, these materials were rinsed with deionized water and alcohol. Each of the pieces could be used as catalyst in Suzuki cross coupling reactions as mentioned in the article. Typical procedure for the Suzuki–Miyaura cross-coupling catalysed by the Pd@Al foil. Phenylboronic acid (117 mg, 0.75 mmol), K2CO3 (138 mg, 1.0 mmol) and a piece of Pd@Al foil were added into a Schlenk tube. Under a nitrogen atmosphere, a solution of iodobenzene (0.5 mmol) in alcohol (3 mL) was injected by a syringe. The mixture was heated at 60 °C without stirring. The reaction was monitored by TLC (eluent: petroleum ether). When the reaction terminated, 5 mL of water was added and the liquid was extracted by ether (5 mL × 3). The combined organic layer was then washed by saturated NaCl solution (5 mL × 2) and dried by a little anhydrous MgSO4. Evaporation of the solvent provided the almost pure (examined by 1H NMR) product biphenyl. The product could also be further purified by column chromatography (eluent: petroleum ether). Compound3a: White solid, mp 68–69 °C. IR (KBr): 3045, 2980, 2870, 1945, 1876, 1696, 1647, 1480, 1429, 1370, 1347, 1307, 1171, 1142, 1112, 1089, 1075, 1027, 1000, 903, 729, 697 cm−1. 1H NMR (600 MHz, CDCl3, TMS): δ 7.33–7.34 (m, 2H), 7.41–7.44 (m, 4H), 7.58–7.59 (m, 4H) ppm. Known product.13.
- (a) http://riodb01.ibase.aist.go.jp/sdbs/cgi-bin/direct_frame_top.cgi ; (b) W.-J. Zhou, K.-H. Wang and J.-X. Wang, Adv. Synth. Catal., 2009, 351, 1378 CrossRef CAS; (c) W. E. Truce, D. P. Tate and D. N. Burdge, J. Am. Chem. Soc., 1960, 82, 2872 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Preparation of the catalyst, typical procedure for the organic reactions, and the 1H NMR and the relevant spectral data of all the new compounds mentioned in the paper. See DOI: 10.1039/c2cy20132a |
| This journal is © The Royal Society of Chemistry 2012 |


