Biofilms and their engineered counterparts: A new generation of immobilised biocatalysts
Michael
Winn
a,
Joanne M.
Foulkes
a,
Stefano
Perni
b,
Mark J. H.
Simmons
*b,
Tim W.
Overton
*b and
Rebecca J. M.
Goss
*a
aSchool of Chemistry, University of East Anglia, Norwich Research Park, Norwich, NR4 7TJ, UK. E-mail: r.goss@uea.ac.uk
bSchool of Chemical Engineering, University of Birmingham, Birmingham, B15 2TT, UK. E-mail: m.j.simmons@bham.ac.uk; t.w.overton@bham.ac.uk
First published on 14th May 2012
Abstract
The robust nature of biofilms makes them medicinally difficult to treat, however this same property renders them an attractive method for protecting and immobilising enzymes for biotransformation. Although biofilms consisting of a consortium of different microbial species have been routinely used in water purification for many decades, there are few reported examples of single species biofilms being harnessed for industrial applications. The potential of using tailored single species biofilms in order to catalyse a biotransformation of choice is attractive; we reflect upon recent advances in the use and generation of such platforms, from both biological and process engineering viewpoints.
Introduction
The advantages of using biocatalysts for chemical synthesis are well recognised and there are many examples of their incorporation in chemical processes.1 A recent review lists over 125 purified enzyme or whole cell biocatalyst mediated reactions that are currently utilised for industrial applications.2,3 The advantages of using purified enzymes over whole cells include improved enzyme-substrate access and the elimination of unwanted side reactions that can be caused by the interference of additional enzymes present inside cells. However disadvantages include instability when isolated; enzymes can be readily degraded by proteolysis, denatured by extremes of temperature, pH and ion concentration, and generally have low tolerance to shear stress and organic solvents. This, to an extent, limits the number of useful biocatalytic enzymes available to industry, as activity usually relies on maintaining enzyme structure. It is therefore important to develop robust enzymes with useful applications.Enzyme stability can be improved by increasing the structural rigidity by directed evolution and/or multipoint immobilisation (see Mateo 2007 and Hernandez 2011 for recent reviews);4,5 immobilised enzyme biocatalysts can also be more easily removed from reaction vessels, recycled or used in continuous flow reactors. However, the consequences of this increased rigidity may be poorer catalytic performance as enzymes often go through rapid and precise changes in structure during catalysis. Too much conformational stability can restrict flexibility and therefore activity.6 In some cases immobilisation may actually be advantageous to catalytic activity or site-directed mutagenesis applied to combat any negative affects but the consequences of immobilising a specific enzyme are hard to predict and many passes of directed evolution may be required for any improvements to be seen.
In industry, whole cell biocatalysts predominate as they present a number of advantages for large scale processes.3,7 Cells can protect enzymes from harsh external reaction conditions, thus maintaining structure and activity without the need to explore and optimise an immobilisation strategy. Preparation of whole cell biocatalysts is simple, not requiring lengthy protein purification procedures, and more complicated, multi-step syntheses that require multiple enzymes can all be contained inside single cells and expensive cofactors can be recycled by internal cellular mechanisms.8 Potential challenges of whole cell biocatalysts when compared to purified enzymes include increased mass transfer limitations and unwanted side reactions. In addition, although cells are able to protect enzymes from harsh reaction conditions, these harsh conditions may be toxic to cells. In some cases, biphasic systems have been used to great effect and the development of ionic solvents has minimised the problem of organic solvent toxicity but with added expense (for recent reviews see Murphy 2012 and Zhao 2010).9,10
Whole cells can also be immobilised inside calcium alginate beads for continuous flow processes,11 this however reduces the mass to catalytic activity ratio and requires intricate preparation steps which add to the cost. As a result the vast majority of whole cell catalysed biotransformation reactions are still performed as batch processes.2 An alternative immobilisation method makes use of the natural phenomenon of biofilms. Because of their increased antibiotic resistance and mechanical toughness biofilms are generally perceived as being problematic, both medically and in industry, for example in reactor fouling.23,24 However, recent studies have shown that these characteristics make biofilms an attractive biocatalytic platform as they can potentially withstand much harsher conditions than planktonic cells.25
Biofilms
Biofilms are multi-layered communities of microbial cells embedded in extracellular matrixes which develop naturally at solid–liquid and air–liquid interfaces, and are formed by most species of bacteria (Table 1). During biofilm growth, initial cell attachment to a surface is followed by the adhesion of the cells both to the surface and to each other through the production and utilisation of extracellular polymeric substances (EPS) (Fig. 1). Mature biofilms are dynamic structures, adapting to the demands of the local environment. Entrapped cells can disperse depending on the action of shear forces and the availability of nutrients.26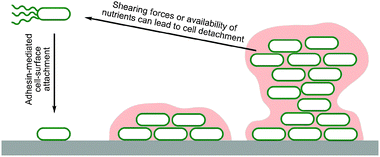 | ||
| Fig. 1 Cartoon illustrating biofilm formation by motile bacteria. Motile bacteria actively travel to a surface and attach via adhesins. Once attached some motility is lost and further adhesins and EPS components are made, generating a matrix. Continued twitching motility can give rise to mushroom shaped colonies and water channels. Variation in local conditions also leads to phenotypic diversification. Entrapped cell dispersion can be triggered by the action of shearing forces and/or the availability of nutrients. | ||
| Microbial species | Use/Function | Reference |
|---|---|---|
| Wild type biofilms | ||
| Thiomonas arsenivorans | Bioremediation of arsenic contaiminated mining effluent | Michel 200712 |
| Zymomonas mobilis | Production of ethanol | Weuster-Botz 199313 |
| Saccharomyces cerevisiae | Kunduru 199614 | |
| Caldicellulosiruptor saccharolyticus | Generation of hydrogen | van-Groenestijn 200915 |
| Clostridium beijerinckii | Production of butanol | Lienhardt 200216 |
| Lactobacillus casei | Production of lactic acid | Demirci 200317 |
| Pseudomonas sp. VLB120ΔC | Transformation of (S)-styrene oxide from styrene | Gross 200718 |
| Gluconobacter oxydans | Production of dihydroxyacetone from glycerol | Hekmat 200719 |
| Recombinant biofilms | ||
| E. coli KO11 | Production of ethanol | Zhou 200820 |
| Acetobacter xylinum | D-amino acid oxidase | Setyawati 200921 |
| E. coli PHL644 | Biotransformation of haloindole into L-halotryptophan | Tsoligkas 201122 |
The EPS consists of a mixture of different biopolymers, including nucleic acids, proteins, and polysaccharides, responsible for a range of diverse functions.27 It is thought that this matrix of EPS together with the lower metabolic rate generally seen within biofilms contributes to their robustness when faced with harsh environments.28 However, EPS can present a potential problem for biotransformations if over-produced. An excess of EPS takes up room in bioreactors, sloughing of this matrix can complicate product purification and EPS can limit mass transfer, decreasing product yield.2 In one study, in which the cellular component of an Acetobacter xylinum biofilm grown in a shaken culture was only 25% of the entire biofilm mass, the activity of D-amino acid oxidase (DAAO) was six times lower than that exhibited by self-immobilised cells grown in static culture.21
Biofilms for bioremediation
The increased robustness of biofilms compared to planktonic cells means that mixed species biofilms have found significant industrial application in bioremediation (reviewed by Singh et al. 2006)29 including waste water treatment and the clean-up of sites contaminated with hydrocarbons and heavy metals.30–32 Biofilms of an autotrophic arsenic(III)-oxidizing bacterial population isolated from an arsenic contaminated gold mine, named CAsO1, and a single species isolated from CAsO1, Thiomonas arsenivorans, have been used in As(III)-oxidising biofilm reactors to treat effluent from mining environments.12,33 CAsO1 is particularly suitable for this, as its optimum activity is at the low pH found in such environments. Strikingly, oxidation by this biofilm was not inhibited by concentrations as high as 1 g L−1 of As(III).33 Mixed species biofilms used for bioremediation such as this are usually formed from bacterial samples collected from contaminated environments and as such display increased tolerance to the particular challenging conditions required. Common types of biofilm reactors used in this industry include the stirred-tank reactor, the trickling filter reactor, the rotating-disk reactor, the membrane biofilm reactor, the fluidized-bed reactor, and the airlift reactor. In both stirred-tank and airlift reactors, the biofilms may be grown onto fixed or mobile supports. Reactors vary between their energy requirements, shear stresses on the biofilms, whether or not continuous or semi-continuous processes are required, and the risk of fouling.34Single-species biofilms as biocatalysts
Whilst commercial processes involving biofilms use a consortia of several species of microbe,2,35 there are a number of studies which have investigated the use of single-species biofilms. For the generation of biofuels, Zymomonas mobilis and Saccharomyces cerevisiae biofilms have been used for the production of ethanol.13,14 These studies mirror the current development of continuous beer brewing and maturation processes using immobilised yeast (reviewed by Verbelen et al. 2006).36 Bacterial single-species biofilms have also been developed; the thermophilic anaerobic bacterium Caldicellulosiruptor saccharolyticus has been used for the generation of hydrogen.15 Production of high levels of butanol has been achieved using a Clostridium beijerinckii biofilm reactor,16,37 and also of lactic acid using Lactobacillus casei in a plastic-composite-support biofilm reactor.38 The benefits of using biofilms in these cases has as much to do with the ease of biofilm formation and low operational cost compared to immobilising whole cells or developing robust free enzymes as it does with the increased robustness of the biofilm to high concentrations of potentially toxic products produced during the reactions.The generation of industrially useful chemicals has been demonstrated using a Pseudomonas sp. VLB120ΔC biofilm for the production of (S)-styrene oxide (2). with a yield of 16 g Laq−1 day−1,18 as a model reaction to demonstrate prolonged asymmetric epoxide activity (see Scheme 1). In addition to protecting the cells from the toxic product, the immobilised nature of the biofilm also allowed in situ removal of product throughout the reaction.
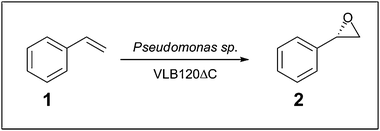 | ||
| Scheme 1 Pseudomonas catalysed biotransformation of (S)-styrene oxide (2) from styrene (1).18 | ||
The process was stable for 55 days, using silicone tubing as the substratum in the bioreactor. Later experiments increased the yield to a maximum of 70 g Laq−1 day−1, with the process appearing to select for a biofilm-forming phenotype.39
The production of the fine chemical dihydroxyacetone (4) from glycerol (3) by Gluconobacter oxydans in a fed-batch process was increased when using self-immobilised cells compared with planktonic cells (Scheme 2).19 However, only about 65% of the cells were immobilised, probably due to the strictly aerobic culture requirements of G. oxydans.
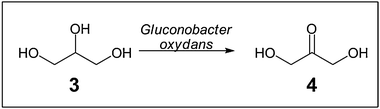 | ||
| Scheme 2 Conversion of glycerol (3) into dihydroxyacetone (4) mediated by a Gluconobacter oxydans biofilm.19 | ||
Recombinant biofilms
In spite of the wide use of recombinant strains in whole cell catalysed biotransformations there are currently very few reports of recombinant strains of bacteria being utilised to generate a specific enzyme and catalyse desired biotransformation reactions within biofilms. The first reported study involved the use of a recombinant ethanol-producing strain of E. coli KO11;20 when used in planktonic form the yield dropped to 60% of the theoretical maximum after only 8–9 days. When immobilised on glass microspheres, this rose to >70% for up to 40 days. A second study used Acetobacter xylinum,21 which forms a biofilm at the air/liquid interface, producing an EPS matrix in the form of cellulose nanofibres. Using this technique a biofilm of A. xylinum transformed with the D-amino acid oxidase (DAAO) gene of Rhodosporidium toruloides was grown in a shaken culture. In the case of DAAO a whole cell solution is advantageous as the hydrogen peroxide produced during the reaction may inactive isolated enzymes. However this biofilm showed only 10% of the activity of a crude cell extract, and was six times less active than self-immobilised cells grown in static culture.21 Although the immobilised cells saw diminished activity the benefits of using a biofilm system included improved thermal and operational stability and easy retrieval of the catalyst for repeated use.The most recent study to use recombinant cells in a biofilm demonstrated the conversion of haloindoles and serine to L-halotryptophans (7) (see Scheme 3) using the biofilm-forming strain E. coli PHL644.22 The strain was transformed with the tryptophan synthase genes from Salmonella enterica sv Typhimurium, and artificially engineered biofilms were produced by spin-coating E. coli cultures onto glass slides which were then left to mature for 7 days in minimal media. The engineered biofilms matured more rapidly than naturally-formed biofilms, adhering more firmly to the glass substrate, and had formed mushroom structures, pores and channels by day 6 (see Fig. 2).40 Higher conversions were obtained than with cell-free-lysate, immobilised enzyme, or planktonic cells at twice the biomass, and in three sequential biotransformations for the production of 5-chloroindole, no loss of activity was observed. Although these initial investigations were conducted in batch the authors note that the immobilised nature of the biofilm and the lack of observed drop in yield after recycling the biofilm opens up the possibility for conducting these types of biotransformation reaction as a component of flow chemistry.
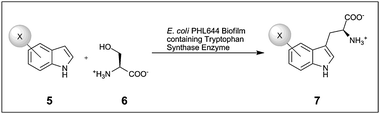 | ||
| Scheme 3 Tryptophan synthase mediated biotransformation of L-halotryptophan (7) from haloindole (5) and L-serine (6) catalysed by recombinant E. coli PHL644 biofilms. X = F, Cl or Br.22 | ||
 | ||
| Fig. 2 Environmental scanning electron micrograph of an engineered E. coli biofilm on day 6 after attachment, showing the presence of EPS (white fibrous material) and the development of mushroom structures, pores and channels.22 | ||
In these last two cases the stated goal was to produce a general, easily formed and adaptable biofilm platform, composed of a recombinant organism, designed to be able to perform a range of required biotransformations.
Future prospects
Biotransformations are becoming increasingly important components of commercial and academic syntheses enabling a greening of processes and cheaper more expeditious access to fine chemicals and pharmaceuticals. The drive to run such processes in flow raises fresh challenges. The use of biofilms for biotransformation adds an extra dimension to the current choice of biocatalytic systems (typically soluble enzyme, immobilised enzyme or whole cells) and so offers a new alternative when considering the design and development of process pathways. Biofilms confer stability providing a robust catalytic platform in which the enzyme is immobilised, stabilised and protected from exposure to denaturing solvents, toxic by-products, shear stress and thermal extremes. The decreased cost represented by biofilm formation compared to traditional methods of cell immobilisation also makes them suitable for large scale processes.The possibility of engineering biofilms from recombinant single species provides an attractive platform for plug-and-play biocatalysis, despite the mass transfer problems encountered by some workers. It could be envisioned that metabolic engineering and synthetic biology tools currently used to develop novel enzymatic reactions and metabolic routes within cells could be expanded to encompass biofilm catalysts, allowing development of multiple platform technologies to respond to challenging requirements in biocatalysis. In particular, biofilm catalysts could be envisioned to assist in the development of novel biotransformations involving highly toxic solvents, substrates or products using well-studied and genetically tractable organisms such as E. coli without the time-consuming necessity of isolating novel organisms that have resistance to the reaction conditions. In the last few years there has been a surge of interest in the potential of biofilms to perform biotransformation reactions,22,41 and engineered biofilms in particular appear to offer a low cost, high efficiency alternative to traditional fed-batch processes, and should be sufficiently flexible regarding the generation of a range of fine chemicals to warrant further study in this area.
Notes and references
- T. Hudlicky and J. W. Reed, Chem. Soc. Rev., 2009, 38, 3117–3132 RSC.
- B. Rosche, X. Z. Li, B. Hauer, A. Schmid and K. Buehler, Trends Biotechnol., 2009, 27, 636–643 CrossRef CAS.
- A. Liese, K. Seelbach and C. Wandrey, Industrial Biotransformations, Wiley-VCH, 2006 Search PubMed.
- C. Mateo, J. M. Palomo, G. Fernandez-Lorente, J. M. Guisan and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2007, 40, 1451–1463 CrossRef CAS.
- K. Hernandez and R. Fernandez-Lafuente, Enzyme Microb. Technol., 2011, 48, 107–122 CrossRef CAS.
- G. N. Somero, Annu. Rev. Physiol., 1995, 57, 43–68 CrossRef CAS.
- A. J. J. Straathof, S. Panke and A. Schmid, Curr. Opin. Biotechnol., 2002, 13, 548–556 CrossRef CAS.
- Y. Ni and R. R. Chen, Biotechnol. Bioeng., 2004, 87, 804–811 CrossRef CAS.
- C. D. Murphy, Org. Biomol. Chem., 2012, 10, 1949–1957 CAS.
- H. Zhao, J. Chem. Technol. Biotechnol., 2010, 85, 891–907 CrossRef CAS.
- J. K. Park and H. N. Chang, Biotechnol. Adv., 2000, 18, 303–319 CrossRef CAS.
- C. Michel, M. Jean, S. Coulon, M. C. Dictor, F. Delorme, D. Morin and F. Garrido, Appl. Microbiol. Biotechnol., 2007, 77, 457–467 CrossRef CAS.
- D. Weuster-Botz, A. Aivasidis and C. Wandrey, Appl. Microbiol. Biotechnol., 1993, 39, 685–690 CrossRef CAS.
- M. R. Kunduru and A. L. Pometto, Journal of Industrial Microbiology and Biotechnology, 1996, 16, 249–256 CAS.
- J. W. van Groenestijn, J. S. Geelhoed, H. P. Goorissen, K. P. M. Meesters, A. J. M. Stams and P. A. M. Claassen, Biotechnol. Bioeng., 2009, 102, 1361–1367 CrossRef CAS.
- J. Lienhardt, J. Schripsema, N. Qureshi and H. Blaschek, Appl. Biochem. Biotechnol., 2002, 98–100, 591–598 CrossRef CAS.
- A. Demirci, J. C. Cotton, A. L. Pometto, K. R. Harkins and P. N. Hinz, Biotechnol. Bioeng., 2003, 83, 749–759 CrossRef CAS.
- R. Gross, B. Hauer, K. Otto and A. Schmid, Biotechnol. Bioeng., 2007, 98, 1123–1134 CrossRef CAS.
- D. Hekmat, R. Bauer and V. Neff, Process Biochem., 2007, 42, 71–76 CrossRef CAS.
- B. Zhou, G. J. O. Martin and N. B. Pamment, Biotechnol. Bioeng., 2008, 100, 627–633 CrossRef CAS.
- M. I. Setyawati, L.-J. Chien and C.-K. Lee, Biochem. Eng. J., 2009, 43, 78–84 CrossRef CAS.
- A. N. Tsoligkas, M. Winn, J. Bowen, T. W. Overton, M. J. H. Simmons and R. J. M. Goss, ChemBioChem, 2011, 12, 1391–1395 CrossRef CAS.
- J. W. Costerton, P. S. Stewart and E. P. Greenberg, Science, 1999, 284, 1318–1322 CrossRef CAS.
- N. Qureshi, B. A. Annous, T. C. Ezeji, P. Karcher and I. S. Maddox, Microb. Cell Fact., 2005, 4, 24 CrossRef No pp given.
- X. Z. Li, J. S. Webb, S. Kjelleberg and B. Rosche, Appl. Environ. Microbiol., 2006, 72, 1639–1644 CrossRef CAS.
- P. Stoodley, K. Sauer, D. G. Davies and J. W. Costerton, Annu. Rev. Microbiol., 2002, 56, 187–209 CrossRef CAS.
- H.-C. Flemming and J. Wingender, Nat. Rev. Micro., 2010, 8, 623–633 CAS.
- G. G. Anderson and G. A. O'Toole, Curr. Top. Microbiol. Immunol., 2008, 322, 85–105 CrossRef CAS.
- R. Singh, D. Paul and R. K. Jain, Trends Microbiol., 2006, 14, 389–397 CrossRef CAS.
- T. Iwamoto and M. Nasu, J. Biosci. Bioeng., 2001, 92, 1–8 CAS.
- W. Zhang, E. Bouwer, L. Wilson and N. Durant, Water Sci. Technol., 1995, 31, 1–14 CrossRef CAS.
- P. Singh and S. S. Cameotra, Biochem. Biophys. Res. Commun., 2004, 319, 291–297 CrossRef CAS.
- F. Battaglia-Brunet, M. C. Dictor, F. Garrido, C. Crouzet, D. Morin, K. Dekeyser, M. Clarens and P. Baranger, J. Appl. Microbiol., 2002, 93, 656–667 CrossRef CAS.
- K.-C. Cheng, A. Demirci and J. Catchmark, Appl. Microbiol. Biotechnol., 2010, 87, 445–456 CrossRef CAS.
- B. Stubblefield, K. Howery, B. Islam, A. Santiago, W. Cardenas and E. Gilbert, Appl. Microbiol. Biotechnol., 2010, 86, 1941–1946 CrossRef CAS.
- P. Verbelen, D. De Schutter, F. Delvaux, K. Verstrepen and F. Delvaux, Biotechnol. Lett., 2006, 28, 1515–1525 CrossRef CAS.
- N. Qureshi, P. Karcher, M. Cotta and H. Blaschek, Appl. Biochem. Biotechnol., 2004, 114, 713–721 CrossRef.
- A. Demirci, J. C. Cotton, A. L. Pometto, K. R. Harkins and P. N. Hinz, Biotechnol. Bioeng., 2003, 83, 749–759 CrossRef CAS.
- R. Gross, K. Lang, K. Bühler and A. Schmid, Biotechnology and Bioengineering, 2010, 105, 705–717 CAS.
- A. N. Tsoligkas, J. Bowen, M. Winn, R. J. M. Goss, T. W. Overton and M. J. H. Simmons, Colloids Surf., B, 2012, 89, 152–160 CrossRef CAS.
- X. Z. Li, B. Hauer and B. Rosche, Appl. Microbiol. Biotechnol., 2007, 76, 1255–1262 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
