Ytterbium perfluorooctanoate [Yb(PFO)3]: a novel and efficient catalyst for the synthesis of tetrahydrobenzo[a]xanthene-11-ones under microwave irradiation
Chereddy
Syama Sundar
,
Kunda Uma
Maheswara Rao
,
Nemallapudi
Bakthavatchala Reddy
,
Mudumala Veera
Narayama Reddy
,
Sthanikam
Siva Prasad
and
Cirandur
Suresh Reddy
*
Department of Chemistry, Sri Venkateswara University, Tirupati-517 502, A.P., India. E-mail: csrsvu@gmail.com; Fax: +91-877 2289555; Tel: +91-9849694958
First published on 12th April 2012
Abstract
Ytterbium perfluorooctanoate [Yb(PFO)3] was found to be an efficient catalyst for the microwave assisted synthesis of tetrahydrobenzo[a]xanthene-11-ones by the one-pot condensation of 2-naphthol, aldehydes and 1,3-dicarbonyl compound under solvent-free conditions. The major advantages of this novel method are short reaction time, high yields, reusability of the catalyst and the absence of any volatile and hazardous organic solvents.
Introduction
Xanthenes, especially benzoxanthenes, synthesis has gained much attention in recent times due to their wide range of therapeutic properties such as antibacterial,1 antiviral,2 anti-inflammatory activities3 and because they act as sensitizers in photodynamic therapy for destroying the tumor cells.4 Further, these heterocyclic compounds were used as dyes,5 antagonists for the paralyzing action of zoxazolamine,6 pH-sensitive fluorescent materials for visualization of biomolecules7 and in laser technology.8 A number of xanthene dyes are extracted naturally from soil and from plants such as Indigofera longeracemosa.9 A variety of reagents and catalysts10–20 have been reported for synthesis of benzoxanthenes. However, the reported methods have drawbacks such as low yields, prolonged reaction times and requirement of toxic solvents and catalysts. Therefore, introduction of a new, efficient and environmentally benign method for preparation of xanthene derivatives is highly imperative.In recent times, exploration of solvent-free reactions has gained importance due to several advantages such as experimental simplicity, less energy requirement, and almost quantitative reactivity of the substrates due to intimacy of the reagents.21 Kabachnik–Fields,22 Horner–Wadsworth–Emmons23 and Michaelis–Arbuzov24 reactions were successfully carried out under these conditions. Improving the substrates reactivity and product formation efficiency by energising the reactions with microwave irradiation is another development in organic green chemical synthesis. Microwave-assisted organic synthesis is characterized by spectacular accelerations in many reactions as a consequence of three dimensional heating of the reaction mass, which cannot be produced by classical heating.25 Simple workup, high yields, improved selectivity and clean reaction pathways are additional advantages with microwave-assisted preparation of organic compounds. Indeed, even reactions that do not occur by conventional heating can be effectively performed using microwaves.26 The combination of microwave irradiation and solvent-free procedures offers an easier approach for organic synthetic transformations.27
The use of rare-earth compounds as catalysts has also attracted tremendous interest due to their unique catalytic activities, low toxicity, stability, and easy handling.28,29 They are more widely used as powerful replacements for the conventional Lewis acid catalysts in various synthetic processes.30,31 Among them, ytterbium perfluorooctanoate [Yb(PFO)3] has invoked enormous interest as a green and potential Lewis acid catalyst to construct C–C, P–C and C–heteroatom bonds in various reactions.32–34 It has received considerable attention due to its low toxicity, cost effectiveness, air and water compatibility, ease of handling, recyclability, experimental simplicity and remarkable ability to suppress side reactions in acid sensitive substrates.
As part of our continued interest in the development of new synthetic methodologies,35–37 herein we report the first procedure for the synthesis of benoxanthenes in the presence of Yb(PFO)3 as catalyst by using microwave irradiation under solvent-free conditions. Comparable yields were observed with short reaction time.
Results and discussion
Initially we have synthesiszed tetrahydrobenzo[a]xanthen-11-ones by the one step one-pot condensation of aldehydes, 2-naphthol and cyclic 1,3-dicarbonyl compound in the presence of Yb(PFO)3 as catalyst by microwave irradiation under solvent-free conditions at 80 °C (Scheme 1).![Yb(PFO)3 catalyzed neat synthesis of tetrahydrobenzo[a]xanthenes-11-ones.](/image/article/2012/CY/c2cy20041d/c2cy20041d-s1.gif) | ||
| Scheme 1 Yb(PFO)3 catalyzed neat synthesis of tetrahydrobenzo[a]xanthenes-11-ones. | ||
First, the optimisation of temperature required for the reaction of 2-naphthol, 4-methoxybenzaldehyde and cyclic 1,3-dicarbonyl compound in the presence of Yb(PFO)3 was standardised by carrying out the reaction at different temperatures ranging from 40 to 120 °C for different periods of time by conventional heating (Table 1). It was found that above and below 80 °C and 2 hours of time, the yield of the product was low. Only at 80 °C and with 2 hours of reaction time, the highest product yield of 64% was observed (Table 1, entry 3). Thus it was proved that 80 °C is the optimised temperature required for effecting this reaction by conventional heating. However, when the same reaction was carried out by microwave irradiation at 80 °C, the reaction was complete in 5 min with 93% yield (Table 1, entry 6).
This reaction proceeded sluggishly in the absence of Yb(PFO)3 catalyst under microwave irradiation and offered only 40% yield of the product (Table 2, entry 12). The yield was greatly affected by the amount of catalyst loaded. When 1, 2, 5 and 10 mol% of the catalyst was used, the yields varied from 51, 75, 93 and 93% respectively (Table 2, entries 8–12). Therefore, 5 mol% of Yb(PFO)3 was sufficient and use of excessive catalyst had no impact either on the rate of the reaction or on the compound yield.
| Entry | Solvents | Yb(PFO)3 (mol%) | Time (min) | Yielda (%) |
|---|---|---|---|---|
| a Isolated yield. b Catalyst was reused three times. | ||||
| 1 | DMF | 10 | 30 | 39 |
| 2 | DCE | 10 | 30 | 45 |
| 3 | EtOH | 10 | 30 | 54 |
| 4 | DMSO | 10 | 30 | 49 |
| 5 | CH3CN | 10 | 30 | 65 |
| 6 | Toluene | 10 | 30 | 63 |
| 7 | Water | 10 | 30 | — |
| 8 | Neat | 10 | 5 | 93 |
| 9b | Neat | 5 | 5 | 93, 91, 90 |
| 10 | Neat | 2 | 5 | 75 |
| 11 | Neat | 1 | 5 | 51 |
| 12 | Neat | 0 | 30 | 40 |
Examination of the recyclability of the catalyst showed that it can be reused three times without loss of activity (Table 2, entry 9). To improve the yields further and to make the process green, the reaction was run in different solvents and without a solvent (Table 2). It was found that the reaction was complete in just 5 min and gave 93% yield under neat conditions. Thus it was established that 5 mol% of Yb(PFO)3 and 5 min of microwave irradiation under solvent-free conditions are the optimized conditions for the effective completion of this reaction.
After optimization of experimental conditions, to study the generality of this procedure, the reactivity of a variety of electronically divergent aromatic aldehydes with 5,5-dimethyl-1,3-cyclohexanedione and β-naphthol was examined, and the results are summarized in Table 3.
| Product (4) | R | Yielda (%) | mp (°C) |
|---|---|---|---|
| a Isolated yield. | |||
| a | 3-F C6H4 | 92 | 156–158 |
| b | 4-Cl C6H4 | 90 | 180–182 |
| c | 3-OH C6H4 | 91 | 240–242 |
| d | 4-OH C6H4 | 90 | 223–225 |
| e | 4-OMe C6H4 | 93 | 204–206 |
| f | 2-OMe C6H4 | 90 | 163–165 |
| g | 4-NO2 C6H4 | 89 | 178–180 |
| h | 3-NO2 C6H4 | 84 | 168–170 |
| i | 2-NO2 C6H4 | 87 | 223–225 |
| j | 4-CH3 C6H4 | 90 | 176–178 |
| k | 2-Furfuryl | 88 | 170–172 |
| l | 2-Thienyl | 90 | 176–178 |
| m | 3-Indolyl | 88 | 202–204 |
| n |

|
91 | 254–256 |
| o |
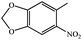
|
90 | 178–180 |
It was observed that aromatic aldehydes carrying both electron-withdrawing and electron-donating groups reacted successfully within 5 min and gave the corresponding tetrahydro-benzo[a]xanthen-11-ones in 84–93% yields. The chemical structures of all the products were fully characterized by 1H NMR, 13C NMR and mass spectral data.
Finally, efficacy of Yb(PFO)3 was compared with other catalysts reported earlier for the synthesis of tetrahydro-benzo[a]xanthen-11-ones. As demonstrated in Table 4, Yb(PFO)3 is found to be equally or even more efficient catalyst for this reaction in terms of yield and reaction time.
| Entry | Catalyst | Reaction conditions | Time (min h−1) | Yield (%) | Ref. |
|---|---|---|---|---|---|
| 1 | Sr(OTf)2 | CH2Cl2/80 °C | 5 h | 89 | 10 |
| 2 | H14[NaP5W30O110] | Neat/120 °C | 1.5 h | 90 | 11 |
| 3 | InCl3 | Neat/120 °C | 30 min | 88 | 12 |
| 4 | Caro's acid–SiO2 | Neat/80 °C | 30 min | 90 | 13 |
| 5 | CeCl3·7H2O | MeOH/50 °C | 2 h | 91 | 14 |
| 6 | TTAB | H2O/RT | 2.5 h | 89 | 15 |
| 7 | pTSA | [bmim]/80 °C | 3.5 h | 83 | 16 |
| 8 | HY zeolite | Neat/80 °C | 1 h | 93 | 17 |
| 9 | Proline triflate | H2O/80 °C | 2.5 h | 88 | 18 |
| 10 | TCT | Neat/80 °C | 30 min | 93 | 20 |
| 11 | CAN | DCM + EtOH/US/26 °C | 2 h | 87 | 19 |
| 12 | Yb(PFO)3 | Neat/80 °C /MWI | 5 min | 93 | This work |
Experimental
Method and apparatus
All reagents were purchased from Aldrich and used without further purification. The Yb(PFO)3 was prepared according to the literature procedure.38 NMR spectra were recorded on a Jeol JNM ECP 400 NMR instrument at room temperature in CDCl3 using TMS as an internal standard. EI-Mass spectra were obtained on a JEOL GCMATE II GC-MS spectrometer at SAIF IIT-Madras, Chennai. Melting points were determined on a Mel-Temp apparatus and were uncorrected.General procedure for the synthesis of tetrahydro-benzo[a]xanthen-11-ones
The mixture of β-naphthol (1 mmol), aldehyde (1 mmol), cyclic 1,3-dicarbonyl compound (1 mmol) and [Yb(PFO)3] (5 mol%) was taken in a vessel and irradiated at 80 °C for 5 min. The completion of the reaction was confirmed by TLC. After methylene dichloride (10 mL) was added, the mixture was stirred and filtered to separate the catalyst which was dried under vacuum and reused. The solvent from the filtrate was evaporated and the crude product was recrystallised from ethanol to afford the pure tetrahydro-benzo[a]xanthen-11-one.Spectral data for novel compounds
Conclusions
An efficient and green chemical methodology has been developed for the synthesis of tetrahydrobenzo[a]xanthene-11-ones by a single step one-pot reaction of aldehyde, β-naphthol and cyclic 1,3-dicarbonyl compound in the presence of Yb(PFO)3 by microwave irradiation under solvent-free conditions. The advantages of this novel method over other existing methods are less reaction time, higher yields, easy product purification and reusability of the catalyst. These merits make it a viable procedure for the large scale commercial synthesis of xanthene derivatives.Acknowledgements
The authors express their grateful thanks to Prof. C. D. Reddy, Department of Chemistry, Sri Venkateswara University, Tirupati, for his helpful discussions and also thank DRDO, New Delhi, for providing financial assistance (ERIP/ER/1103894M/01/1346).Notes and references
- T. Hideo, Chem. Abstr., 1981, 95, 80922b Search PubMed , Jpn. Tokkyo Koho J. P. 56005480, 1981.
- A. Bekaert, J. Andrieux and M. Plat, Tetrahedron Lett., 1992, 33, 2805–2806 CrossRef CAS.
- K. Chibale, M. Visser, D. V. Schalkwyk, P. J. Smith, A. Saravanamuthu and A. H. Fairlamb, Tetrahedron, 2003, 59, 2289–2296 CrossRef CAS.
- A. Zarei, A. R. Hajipour and L. Khazdooz, Dyes Pigm., 2010, 85, 133–138 CrossRef CAS.
- A. Banerjee and A. K. Mukherjee, Stain Technol., 1981, 56, 83–85 CAS.
- G. Saint-Ruf, A. De and H. T. Hieu, Bull. Chim. Ther., 1972, 7, 83–86 Search PubMed.
- C. G. Knight and T. Stephenes, Biochem. J., 1989, 258, 683–687 CAS.
- M. Dabiri, M. Baghbanzadeh, M. S. Nikcheh and E. Arzroomchilar, Bioorg. Med. Chem. Lett., 2008, 18, 436–438 CrossRef CAS.
- P. J. A. Licudine, M. K. Kawate and Q. X. Li, J. Agric. Food Chem., 1997, 45, 766–773 CrossRef.
- J. Li, W. Tang, L. Lu and W. Su, Tetrahedron Lett., 2008, 49, 7117–7120 CrossRef CAS.
- M. M. Heravil, H. Alinejhad, K. Bakhtiari, M. Saeedi, H. A. Oskooiel and F. F. Bamoharram, Bull. Chem. Soc. Ethiop., 2011, 25, 399–406 Search PubMed.
- G. C. Nandi, S. Samai, R. Kumar and M. S. Singh, Tetrahedron, 2009, 65, 7129–7134 CrossRef CAS.
- N. Karimi, H. A. Oskooie, M. M. Heravi and L. Tahershamsi, Synth. Commun., 2011, 41, 307–312 CrossRef CAS.
- M. Kidwai, A. Jahan and N. K. Mishra, C. R. Chim., 2012, 15, 324–330 CrossRef CAS.
- P. V. Shinde, A. H. Kategaonkar, B. B. Shingate and M. S. Shingare, Beilstein J. Org. Chem., 2011, 7, 53–58 CrossRef CAS.
- J. M. Khurana and D. Magoo, Tetrahedron Lett., 2009, 50, 4777–4780 CrossRef CAS.
- V. Rama, K. Kanagaraj and K. Pitchumani, Tetrahedron Lett., 2012, 53, 1018–1024 CrossRef CAS.
- J. Li, L. Lu and W. Su, Tetrahedron Lett., 2010, 53, 2434–2437 CrossRef.
- S. Sudha and M. A. Pasha, Ultrason. Sonochem., 2012 DOI:10.1016/j.ultsonch.2012.02.002 (in press).
- Z. H. Zhanz, P. Zhanz, S. H. Yang, H. J. Wang and J. Deng, J. Chem. Sci, 2010, 122, 427–432 CrossRef.
- P. J. Walsh, H. Li and C. A. de Parrodi, Chem. Rev., 2007, 107, 2503–2545 CrossRef CAS.
- P. Thirumurugan, A. Nandakumar, N. Sudha Priya, D. Muralidaran and P. T. Perumal, Tetrahedron Lett., 2010, 51, 5708–5712 CrossRef CAS.
- Y. Z. Jin, N. Yasuda and J. Inanaga, Green Chem., 2002, 4, 498–500 RSC.
- B. Kaboudin and M. S. Balakrishna, Synth. Commun., 2001, 31, 2773–2776 CrossRef CAS.
- A. de la Hoz, M. P. Prieto, M. Rajzmann, A. de Cozar, A. Diaz-Ortiz, A. Moreno and F. P. Cossio, Tetrahedron, 2008, 64, 8169–8176 CrossRef CAS.
- A. de la Hoz, A. Diaz-Ortiz and A. Moreno, Chem. Soc. Rev., 2005, 34, 164–178 RSC.
- V. Polshettiwar and R. S. Varma, Acc. Chem. Res., 2008, 41, 629–639 CrossRef CAS.
- G. A. Molander, Chem. Rev., 1992, 92, 29–68 CrossRef CAS.
- G. Bartoli, M. Bosco, E. Marcantoni, F. Nobili and L. Sambri, J. Org. Chem., 1997, 62, 4183–4184 CrossRef.
- S. Kobayashi, M. Sugiura, H. Kitagawa and W. Lam, Chem. Rev., 2002, 102, 2227–2302 CrossRef CAS.
- L. M. Wang, L. Hu, H. Chen, Y. Sui and W. Shen, J. Fluorine Chem., 2009, 130, 406–409 CrossRef CAS.
- J. Tang, L. Wang, W. Wang, L. Zhang, S. Wu and D. Mao, J. Fluorine Chem., 2011, 132, 102–106 CrossRef CAS.
- M. Wu, J. Yu, W. Zhao, J. Wu and S. Cao, J. Fluorine Chem., 2011, 132, 155–159 CrossRef CAS.
- M. V. Narayana Reddy, J. Kim and Y. T. Jeong, J. Fluorine Chem., 2011, 135, 155–158 CrossRef.
- Ch. Syama Sundar, D. Srinivasulu, S. K. Nayak and C. Suresh Reddy, Phosphorus, Sulfur Silicon Relat. Elem., 2012, 187, 523–534 CrossRef.
- N. Bakthavatchala Reddy, Ch. Syama Sundar, C. Radha Rani, K. U. Maheswara Rao, S. K. Nayak and C. Suresh Reddy, Arabian J. Chem., 2012 DOI:10.1016/j.arabjc.2011.07.025 (in press).
- K. U. Maheswara Rao, B. S. Krishna, N. Bakthavatchala Reddy, Ch. Syama Sundar, S. K. Nayak and C. Suresh Reddy, Catal. Sci. Technol., 2011, 1, 1665–1670 Search PubMed.
- L. Wang, J. Han, J. Sheng, H. Tian and Z. Fan, Catal. Commun., 2005, 6, 201–204 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
