Hydrogenated mesoporous TiO2–SiO2 with increased moderate strong Brönsted acidic sites for Friedel–Crafts alkylation reaction†
Sha
Li
a,
Hui
Zhou
a,
Bing
Han
b,
Feng
Deng
b,
Xiaonao
Liu
a,
Liping
Xiao
*a and
Jie
Fan
*a
aKey Lab of Applied Chemistry of Zhejiang Province, Department of Chemistry, Zhejiang University, Hangzhou, China. E-mail: jfan@zju.edu.cn; lpxiao@zju.edu.cn; Web: http://www.chem.zju.edu.cn/jiefan/index.htm Fax: +86 571 87952338; Tel: +86 571 87952338
bWuhan Center for Magnetic Resonance, State Key Laboratory of Magnetic Resonance and Atomic and Molecular Physics, Wuhan Institute of Physics and Mathematics, Chinese Academy of Sciences, Wuhan, China. Tel: +86 027-87198820
First published on 26th January 2012
Abstract
The acidic properties of mesoporous TiO2–SiO2 mixed oxides were evaluated by 31P magic-angle spinning NMR spectroscopy using trimethylphosphine oxide (TMPO) and trimethylphosphine (TMP) as probe molecules. It confirms that mesoporous TiO2–SiO2 has moderate strong Brönsted acid sites. The number of the Brönsted acidic sites can be increased via a high-temperature hydrogenation process, which subsequently improves their catalytic performance in the Friedel–Crafts reaction of anisole and benzyl alcohol.
Mixed metal oxides are of great interest for their applications in heterogeneous catalysis, gas sensor, optics and energy conversion.1 In particular, TiO2–SiO2 mixed oxides have received much attention due to their unique acid/base and reduction/oxidation properties.2,3 TiO2 has exclusively the Lewis acid sites, while the silanol groups on the SiO2 surface are only weakly acidic. However, the nature of the acidic sites on TiO2–SiO2 mixed oxides is still under debate for several decades.4 In 1974, Tanabe et al. proposed that the formation of Brönsted acidity resulted in the catalytic activity,2 which has been supported by a number of reports.5,6 On the other hand, Notari et al. have provided evidence that TiO2–SiO2 mixed oxides of high purity do not have Brönsted acidity.4 Stair and his co-workers found that supported TiO2/SiO2 materials exhibit Lewis acidity but only films containing chloride or carbonyl impurities possessed Brönsted acid sites by using diffuse reflectance infrared Fourier transform spectroscopy and visible Raman spectroscopy with pyridine as a probe molecule.7 The discrepancy among previous results prompts us to re-examine the acidic nature of TiO2–SiO2 mixed oxides using a new method that is diagnostic of Brönsted acidity.
Herein we introduce a nanoparticle assembly system for the preparation of homogeneous, ordered, mesoporous TiO2–SiO2 mixed oxides (denoted mTS). The acidic properties of mTS were characterized by 31P magic-angle spinning NMR spectroscopy using trimethylphosphine oxide (TMPO) and trimethylphosphine (TMP) as probe molecules, which confirms that mTS has moderate strong Brönsted acid sites. Moreover, a high-temperature hydrogenation process has been used to increase the Brönsted acidic sites of mesoporous TiO2–SiO2 mixed oxides. The obtained hydrogenated mTS (H-mTS) has Brönsted acidic sites 2.3 times as much as mTS, and exhibits significantly improved catalytic performance in the Friedel–Crafts (F–C) reaction of anisole and benzyl alcohol which proceeds on strong or moderate strong Brönsted acid sites.8,9
Mesoporous TiO2–SiO2 mixed oxides (mTS-x, x indicates the Ti/Si mole ratio) were synthesized according to our previously reported AcHE method.10 The isolated Ti and Si species in the AcHE solution exhibit distinct particle sizes of 5.9 and 1.6 nm, respectively, but the AcHE solution containing the same total concentration of a mixture of Ti and Si gives a single intermediate size (3.5 nm after 200 min) and growth rate (2.6 × 10−3 nm min−1) shown in the time-resolved dynamic laser light scattering (DLS, see Fig. S1 in ESI†). Such behavior is consistent with the formation of a new mixed-composition nanoparticle precursor in which the Ti and Si are interconnected via oxo/acetate bridges. These preformed precursor particles serve as inorganic building blocks, and are rapidly co-organized with organic amphiphilic block copolymers into ordered mesostructures during the evaporation process. The organic templates were removed by calcination in air at 550 °C to obtain mTS. The hydrogenated mesoporous materials (H-mTS-x) were obtained by thermal treatment at 550 °C in H2/Ar for 12 hours.
The small-angle XRD and TEM analyses confirm that both mTS-1.0 and H-mTS-1.0 have an ordered 2-D hexagonal regularity as shown in Fig. 1a and b. The cell parameter of mTS-1.0 and H-mTS-1.0 samples is 11.4 nm and 11.6 nm, respectively, suggesting that the high-temperature hydrogenation process does not change the mesostructure of mesoporous mixed metal oxides. The WAXRD patterns indicate that the major phase of TiO2 in the mixed oxides is amorphous. Several weak XRD peaks are attributed to the presence of small amount of anatase TiO2 nanocrystals (Fig. S2, ESI†). N2 sorption measurement shows that the obtained isotherms are of type IV with H2 type hysteresis and confirms that the materials have typical mesoporous networks. The pore size distribution of both mTS-1.0 and H-mTS-1.0 is about 6.2 nm (Fig. S3, ESI†).
 | ||
| Fig. 1 (a) SAXRD patterns, (b) TEM images, (c) EPR spectra and (d) DRUV-vis spectra of mTS-1.0 and H-mTS-1.0. | ||
The successful Ti(IV)-to-Ti(III) reduction via the high-temperature hydrogenation process is verified by H2-TPR (Hydrogen-Temperature Programmed Reduction) analysis and electron paramagnetic resonance spectroscopy (EPR). The H2-TPR profile reveals that the reduction reaction takes place at a temperature around 500 °C (Fig. S4, ESI†). The choice of 550 °C as the hydrogenation temperature not only ensures the Ti(IV)-to-Ti(III) reduction but also renders less loss of surface hydroxyl groups from thermal condensation. The generation of Ti(III) species after the hydrogenation process is witnessed by EPR analysis. As shown in Fig. 1c, an obvious EPR signal at g = 1.982 is observed for H-mTS-1.0, indicating the existence of Ti(III) species.11,12 The pulse H2-titration experiment was performed to quantify the amount of reduced Ti species in H-mTS-1.0. It reveals that 35% of Ti4+ atoms in mTS-1.0 were reduced.
The Ti(IV)-to-Ti(III) reduction also leads to a significant change in sample color (Fig. 1d). We observe a shift in the onset of absorption in the H-mTS material, from the ultraviolet (UV) to visible light after hydrogenation, accompanied by a dramatic color change: from white to black. The change in optical properties is related to the reduction of TiO2, which substantially broadens the tails of electronic states extending into the otherwise forbidden band gap and consequently enhances their visible-light absorption.13
The acidic properties of mTS were evaluated by 31P magic-angle spinning NMR spectroscopy using trimethylphosphine oxide (TMPO) and trimethylphosphine (TMP) as probe molecules.14,15 The TMPO probe molecule is more sensitive to Brönsted sites with close acid strengths compared with other molecules, while TMP is effective to discriminate Brönsted and Lewis acid sites on various solid acids and determine the concentration of acidic sites.14 A peak at δ = 62.7 ppm of mTS-1.0, in the 31P NMR spectrum with TMPO as probe molecules indicates that the acidic sites of mTS-1.0 are moderate strong Brönsted acid sites.16 The other two peaks at 57.1 ppm and 44.2 ppm of mTS-1.0 are ascribed to TMPO adsorbed on Lewis acid sites and physisorbed TMPO respectively (Fig. 2a). The 31P NMR signal (using TMP as a probe molecule) at δ = −5.8 ppm and −35.5 ppm is used to relatively quantitate the concentration of Brönsted and Lewis acid sites,15 respectively. As shown in Fig. 2b, it is concluded that both types of acidic sites exist in mTS-1.0 and H-mTS-1.0. There is no obvious difference in the concentration of Lewis acid sites between two samples. The 31P NMR signal at δ = −5.8 ppm corresponds to TMPH+ species. It suggests that the Brönsted acid sites of TiO2–SiO2 are much stronger than –OH groups of pure metal oxides (TiO2, SiO2, ZrO2, and Al2O3). The weak acidic –OH groups of these pure oxides cannot protonate TMP molecules (giving rise to TMPH+ and a 31P signal at −5.8 ppm) and only interact with TMP through hydrogen bonding, generating a 31P NMR signal around −50 to −60 ppm.17 The concentration of Brönsted acid sites in H-mTS-1.0 is 2.3 times as high as that of mTS-1.0. The significantly enhanced concentration of Brönsted acid sites of H-mTS-1.0 via hydrogenation also excludes possible origin of Brönsted acid sites from chloride or carbonyl impurities.
 | ||
| Fig. 2 (a) 31P NMR of mTS-1.0 using TMPO as a probe molecule; (b) 31P NMR of mTS-1.0 and H-mTS-1.0 using TMP as a probe molecule. | ||
The acidic properties are also verified by pyridine adsorption IR spectra (Fig. S5, ESI†). Both spectra of mTS-1.0 and H-mTS-1.0 show the adsorption bands at 1605 cm−1 and 1446 cm−1 which correspond to pyridine interaction with Lewis acid sites. Besides, a peak at 1541 cm−1 attributed to adsorbed pyridine bound to a Brönsted acid site is also observed.18,19 These facts confirm that both mTS-1.0 and H-mTS-1.0 mixed oxides not only possess Lewis acidity but also Brönsted acid sites, which is consistent with 31P NMR results.
The existence of the moderate strong Brönsted acid sites in mTS-1.0 and H-mTS-1.0 is further confirmed by the fact that they can catalyze the F–C reaction of anisole and benzyl alcohol (BA for short). The reaction is well known to be proceeded on strong or moderate strong Brönsted acid sites.8,9 The non-mesoporous materials (Ti/Si = 0.1, 0.5 and 1.0) show negligible catalytic activity (less than 0.5% benzyl alcohol conversion after 3 h reaction). In stark contrast, both mTS-1.0 and H-mTS-1.0 are active catalysts for the F–C reaction (Fig. 3a). After 1 hour reaction, the conversion of benzyl alcohol for H-mTS-1.0 and mTS-1.0 is 24% and 12%, respectively. The improved performance of H-mTS-1.0 is confirmed in the wide temperature range of 130 °C–154 °C (Fig. S6, ESI†). The increased concentration of Brönsted acid sites in H-mTS-1.0 is credited to its enhanced catalytic activity in the F–C reaction.
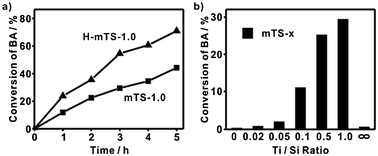 | ||
| Fig. 3 (a) The BA conversion vs. reaction time in the F–C reaction using mTS-1.0 and H-mTS-1.0 as the catalysts. (b) The BA conversion (3 h) as the function of Ti/Si ratio when using mTS-x as the catalysts. | ||
The Ti/Si mole ratio plays a very important role in determining the acidic property of mTS materials. Plotting the BA conversion (3 h) versus the Ti/Si ratio (Fig. 3b) for each mTS-x catalysts reveals a strong catalytic activity dependence on the Ti/Si ratio. Generally, the activity increases with an increase in the Ti/Si ratio of mTS-x over a wide range of x = 0.05–1.0. There is a threshold Ti/Si ratio (x = 0.02–0.05), before which no obvious activity is observed and after which the activity increases rapidly with an increase in the Ti/Si ratio. This onset Ti/Si ratio is the threshold for the formation of Brönsted acid sites. Both Tanabe and K-D models proposed that only six-coordinated Ti species, [Ti(IV)O6], can generate Brönsted acid sites in silica-rich TiO2–SiO2 mixed oxides.2,16,20,21 The DRUV-vis spectra of mTS-x show that [Ti(IV)O6] species appear when the Ti/Si ratio is larger than 0.05 (Fig. S7 ESI†). Both F–C activity and DRUV-vis measurements suggest that Brönsted acid sites only form when the Ti/Si ratio is higher than the threshold ratio (0.05) and [Ti(IV)O6] species develop in mixed metal oxides.
The increased Brönsted acid sites in H-mTS can also be explained by Tanabe model (Fig. S8, ESI†). After hydrogenation, [Ti(IV)O6] is reduced to [Ti(III)O6] that increases the charge difference from −2 to −3. The increased charge difference is neutralized by H+ from the hydrogenation process, which produces the added Brönsted acid sites in H-mTS-1.0.
Conclusion
In summary, we have successfully synthesized mesoporous titania–silica materials with moderate strong Brönsted acid sites. NMR characterization brings new light on the understanding of the acidic nature of these old materials. The correlation of their Brönsted acid catalytic activity with the Ti/Si ratio of the materials excludes possible origin of Brönsted acid sites from chloride or carbonyl impurities. More importantly, the way to increase the acidic sites via hydrogenation provides a rational process to regulate their acidic property and corresponding catalytic performance.We thank financial support from the National Science Foundation of China (20873122, 21003106, and J0830413), Science and Technology Department of Zhejiang Province (2009C11141) and the Fundamental Research Funds from the Central Universities (2011QNA3005). J.F. thanks Dr Z. Wang for IR measurement.
Notes and references
- J. L. G. Fierro, Metal Oxides: Chemistry and Applications, CRC Press, Boca Raton, 2006 Search PubMed.
- M. Itoh, H. Hattori and K. Tanabe, J. Catal., 1974, 35, 225–231 CrossRef CAS.
- C. Perego, A. Carati, P. Ingallina, M. A. Mantegazza and G. Bellussi, Appl. Catal., A, 2001, 221, 63–72 CrossRef CAS.
- B. Notari, R. J. Willey, M. Panizza and G. Busca, Catal. Today, 2006, 116, 99–110 CrossRef CAS.
- J. Ren, Z. Li, S. S. Liu, Y. L. Xing and K. C. Xie, Catal. Lett., 2008, 124, 185–194 CrossRef CAS.
- B. Bonelli, M. Cozzolino, R. Tesser, M. Di Serio, M. Piumetti, E. Garrone and E. Santacesaria, J. Catal., 2007, 246, 293–300 CrossRef CAS.
- J. L. Lu, K. M. Kosuda, R. P. Van Duyne and P. C. Stair, J. Phys. Chem. C, 2009, 113, 12412–12418 CAS.
- C. Tagusagawa, A. Takagaki, A. Iguchi, K. Takanabe, J. N. Kondo, K. Ebitani, S. Hayashi, T. Tatsumi and K. Domen, Angew. Chem., Int. Ed., 2010, 49, 1128–1132 CrossRef CAS.
- C. Tagusagawa, A. Takagaki, A. Iguchi, K. Takanabe, J. N. Kondo, K. Ebitani, T. Tatsumi and K. Domen, Chem. Mater., 2010, 22, 3072–3078 CrossRef CAS.
- J. Fan, S. W. Boettcher and G. D. Stucky, Chem. Mater., 2006, 18, 6391–6396 CrossRef CAS.
- R. Bal, K. Chaudhari, D. Srinivas, S. Sivasanker and P. Ratnasamy, J. Mol. Catal. A: Chem., 2000, 162, 199–207 CrossRef CAS.
- K. Y. Jung, S. B. Park and S. K. Ihm, Appl. Catal., B, 2004, 51, 239–245 CrossRef CAS.
- X. B. Chen, L. Liu, P. Y. Yu and S. S. Mao, Science, 2011, 331, 746–750 CrossRef CAS.
- H. L. Zhang, H. G. Yu, A. M. Zheng, S. H. Li, W. L. Shen and F. Deng, Environ. Sci. Technol., 2008, 42, 5316–5321 CrossRef CAS.
- J. Yang, M. J. Zhang, F. Deng, Q. Luo, D. L. Yi and C. H. Ye, Chem. Commun., 2003, 884–885 RSC.
- A. Zheng, S.-J. Huang, S.-B. Liu and F. Deng, Phys. Chem. Chem. Phys., 2011, 13, 14889–14901 RSC.
- A. G. Stepanov, S. S. Arzumanov, A. A. Gabrienko, V. N. Parmon, I. I. Ivanova and D. Freude, ChemPhysChem, 2008, 9, 2559–2563 CrossRef CAS.
- L. S. Ling and H. Hamdan, J. Non-Cryst. Solids, 2008, 354, 3939–3943 CrossRef CAS.
- J. Ren, Z. Li, S. Liu, Y. Xing and K. Xie, Catal. Lett., 2008, 124, 185–194 CrossRef CAS.
- X. T. Gao and I. E. Wachs, Catal. Today, 1999, 51, 233–254 CrossRef CAS.
- T. Kataoka and J. A. Dumesic, J. Catal., 1988, 112, 66–79 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis of mesoporous TiO2–SiO2; XRD, DRUV, TEM, TPR and acid catalysis experiments. See DOI: 10.1039/c2cy00510g |
| This journal is © The Royal Society of Chemistry 2012 |
