Methyltrioxorhenium-catalysed oxidation of pseudocumene in the presence of amphiphiles for the synthesis of vitamin E
Mónica
Carril
a,
Philipp
Altmann
a,
Werner
Bonrath
b,
Thomas
Netscher
b,
Jan
Schütz
b and
Fritz E.
Kühn
*a
aMolecular Catalysis, Catalysis Research Center, Technische Universität München, Ernst-Otto-Fischer-Straße 1, D-85747 Garching bei München, Germany. E-mail: fritz.kuehn@ch.tum.de; Tel: +49 89 289 13081
bDSM Nutritional Products, Research and Development, P. O. Box 2676, 4002 Basel, Switzerland
First published on 21st October 2011
Abstract
Vitamin E is an essential food component and antioxidant of major economic relevance. 2,3,5-Trimethyl-1,4-benzoquinone is a key intermediate in the production of vitamin E, which can now be selectively achieved through MTO-catalysed oxidation of simple and inexpensive pseudocumene (1,2,4-trimethylbenzene), in the presence of hydrogen peroxide and an appropriate amphiphile.
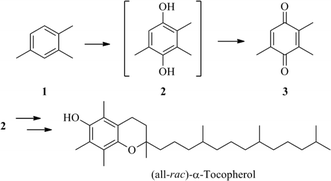
Vitamins are essential organic compounds, which are either not synthesised in the human or animal organism, or not formed in sufficient amounts. Vitamin E and especially its most active component α-tocopherol is of particular biological relevance and industrial interest because of its antioxidant properties.1–4 One of the key intermediates in the synthesis of α-tocopherol is 2,3,5-trimethyl-1,4-benzoquinone (3), which is usually prepared from 2,3,6-trimethylphenol.5–20 Industrially more interesting, but more difficult to achieve, is the oxidation of cheap pseudocumene under mild conditions via hydroquinone 2.21–26 Improving the selective oxidation of pseudocumene, as shown in Scheme 1, compared to existing methods would be an important step towards industrial application. Vitamin E is actually prepared from 2. 3 as the main product of the reaction—due to reaction conditions—can easily be reduced to 2. Previous research by our group on MTO-catalysed oxidation of pseudocumene showed that water is a very influential component, having two major effects. One is the well known but slow decomposition of MTO in the presence of water.8,26–29 The other effect is a reduction of the energy barrier through nitromethane and water as proton transfer agent during the first oxidation step to a phenolic intermediate 3.26 A possible approach to improve the activity of the catalytic system—likely hampered by the presence of two liquid phases—could be the use of amphiphiles with their known beneficial effects in catalysis.30–33 Furthermore, the use of an imidazole-functionalised amphiphilic copolymer linked to MTO has been recently reported as a recyclable catalyst for epoxidation, setting a precedent in its field.34
Hence, given the high importance of benzoquinone 3 as an intermediate in the synthesis of vitamin E, we report herein the MTO-catalysed selective oxidation of pseudocumene in the presence of different amphiphiles as an appealing alternative to the existing methods and to improve product selectivity of 3.
First, a number of commercially available neutral, anionic and cationic amphiphiles, shown in Fig. 1, were probed in the target oxidation of pseudocumene, at 50 °C, using MTO as a catalyst and hydrogen peroxide (30% aqueous solution) as an oxidant.† The results are summarised in Table 1. Side-products of the reaction are usually trimethylphenol, acting as an intermediate on the way to 3,26 or derivatives of 1 with oxidised methyl groups. These by-products were observed with GC/MS techniques. As it can be seen from Table 1, the use of certain amphiphiles can have a positive effect on the conversion of the reaction while maintaining a high selectivity. Indeed, the best results are obtained when using the inexpensive, neutral amphiphile Brij 30. When this additive is used in the presence of aqueous H2O2 solution as only solvent, the product selectivity is 67% (Table 1, entry 3). A reduction of the amount of amphiphile leads to a slightly increased conversion, but is accompanied by a significantly lower selectivity (Table 1, entry 3 vs. 4). Interestingly, the use of an additional solvent, such as nitromethane, affords a selectivity of 80%, which is the highest from all experiments with amphiphiles shown in this work (Table 1, entry 5). A water-miscible solvent such as methanol has no particular effect (Table 1, entry 3 vs. 6).
 | ||
| Fig. 1 Amphiphilic additives used in this work. | ||
| Entry | Reaction conditionsa | Time/h | Temp./°C | Conv. 1 [%] | Sel. 3 [%] |
|---|---|---|---|---|---|
a Reaction conditions: 1 (1.44 mmol; 1 equiv.), MTO (2 mol%), H2O2 (30% in H2O, 4 equiv.), H2O2 (aq. solution)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Brij 30 = 12 (v/v, when indicated in the table), conversion of starting material and formation of 3 were quantified by GC-MS using an internal standard, selectivity of 3 refers to the amount of converted starting material that turned into target product 3, salox = salicylaldoxime.
b H2O2 (30%) Brij 30 = 12 (v/v, when indicated in the table), conversion of starting material and formation of 3 were quantified by GC-MS using an internal standard, selectivity of 3 refers to the amount of converted starting material that turned into target product 3, salox = salicylaldoxime.
b H2O2 (30%)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Brij 30 = 30 (v/v).
c 0.35 mL of solvent per mmol of 1.
d 0.14 mL of solvent per mmol of 1.
e 0.7 mL of solvent per mmol of 1. Brij 30 = 30 (v/v).
c 0.35 mL of solvent per mmol of 1.
d 0.14 mL of solvent per mmol of 1.
e 0.7 mL of solvent per mmol of 1.
|
|||||
| 1 | 20 | 50 | 13 | 100 | |
| 2 | Brij 30/without MTO | 20 | 50 | 0 | 0 |
| 3 | Brij 30 | 20 | 50 | 27 | 67 |
| 4b | Brij 30 | 20 | 50 | 38 | 47 |
| 5c | Brij 30, MeNO2 | 20 | 50 | 28 | 80 |
| 6c | Brij 30, MeOH | 20 | 50 | 31 | 52 |
| 7c | 4 mol% salox, Brij 30, MeNO2 | 22 | 50 | 29 | 72 |
| 8c | 2 mol% salox, Brij 30, MeNO2 | 72 | 50 | 39 | 62 |
| 9c | 2 mol% salox, Brij 30, H2O2 (50%), MeNO2 | 72 | 50 | 50 | 60 |
| 10d | 4 mol% salox, Brij 30, MeNO2 | 72 | 50 | 37 | 57 |
| 11e | 4 mol% salox, Brij 30, MeNO2 | 72 | 50 | 35 | 63 |
| 12 | 4 mol% (Bu4N)Br, Brij 30 | 22 | 50 | 21 | 0 |
| 13 | 4 mol% (Bu4N)PF6, Brij 30 | 19 | 50 | 28 | 46 |
| 14 | 4 mol% (Bu4N)HSO4, Brij 30 | 22 | 50 | 24 | 54 |
| 15e | 4 mol% salox, 10 mol% (Bu4N)HSO4, Brij 30, MeNO2 | 72 | 50 | 21 | 24 |
| 16 | 1 mol% CTAS | 72 | 50 | 36 | 44 |
| 17 | 50 mol% CTAT | 72 | 50 | 55 | 11 |
| 18c | 4 mol% N(Dodec)3, 2 mol% CTAT, CHCl3 | 72 | 40 | 30 | 27 |
| 19 | 2 mol% SDS | 72 | 40 | 31 | 55 |
We have shown that the use of salicylaldoxime as a ligand in combination with MTO improved the yield and selectivity of the oxidation of pseudocumene in comparison with the ligand-free analogue oxidation.26 Hence, salicylaldoxime was tested as a ligand for the target oxidation in the presence of Brij 30, yielding benzoquinone 3 with a selectivity of 72% (Table 1, entry 7). Interestingly, the amount of nitromethane added to the system seems to have two contrary effects reflected by different selectivities (Table 1, entries 7, 10, and 11).
Higher diluted systems (Table 1, entry 11) probably lack amphiphile/salox coordination to MTO, while too concentrated systems (Table 1, entry 10) lose the beneficial effect nitromethane seems to induce during the first oxidation step.26 In both cases, the selectivity is lower than in the medium diluted case (Table 1, entry 7). The addition of ammonium salts as phase transfer catalysts (PTC) to the Brij 30-containing reaction mixture does not improve the conversion or product yield. Nevertheless, the anion of a PTC seems to have an effect on the reaction. For instance, (Bu4N)Br inhibits the reaction, whereas (Bu4N)PF6, and (Bu4N)HSO4 afford selectivities of around 50% (Table 1, entries 12–14). Common cetyltrimethyl derived cationic amphiphiles lead to low selectivities (Table 1, entries 16–18), while anionic sodium dodecyl sulfate (SDS) affords moderate selectivity values (Table 1, entry 19). Generally, the use of ionic additives to the reaction lowers the selectivity significantly. Non-ionic amphiphile Brij 30 alone provides much better selectivities and the probing of similar amphiphiles appears reasonable. Amphiphiles could possibly be improved by additional functionalities like Lewis basic moieties. Such a combination might allow the combination of the positive effects of Lewis bases in MTO catalysed oxidations (higher catalyst stability and improved selectivity) and the positive effect of amphiphiles shown in this work. Amphiphile and Lewis base combined to one molecule could lead to a superior behaviour compared to adding the two separate substances to the reaction mixture (Table 1, entries 7–11 and 15).
Conclusion
In summary, a mild procedure for the MTO-catalysed oxidation of pseudocumene based on the use of amphiphiles to improve the selectivity of the product distribution is presented. The applied strategy leads to a good selectivity of up to 80% and generally improved results when compared to the same reaction conditions in the absence of amphiphiles.26 The use of sustainable hydrogen peroxide and the economic relevance of benzoquinone 3 in the production of vitamin E render the presented procedure very appealing from an industrial point of view. Further, the design of amphiphilic ligands that could also coordinate to MTO and decrease its Lewis acid character is also an object of our ongoing studies.Notes and references
- K. U. Baldenius, L. v. d. Bussche-Hnünefeld, E. Hilgemann, P. Hoppe and R. Stürmer, Ullmann's Encylopedia of Industrial Chemistry, VCH, Weinheim, 1996 Search PubMed.
- T. Netscher, Vitam. Horm., 2007, 76, 155 CrossRef CAS.
- T. Netscher, F. Mazzini and R. Jestin, Eur. J. Org. Chem., 2007, 1176 CrossRef CAS.
- T. Rosenau, E. Kloser, L. Gille, F. Mazzini and T. Netscher, J. Org. Chem., 2007, 72, 3268 CrossRef CAS.
- W. Bonrath, M. Eggersdorfer and T. Netscher, Catal. Today, 2007, 121, 45 CrossRef CAS.
- C. Rein, P. Demel, R. A. Outten, T. Netscher and B. Breit, Angew. Chem., Int. Ed., 2007, 46, 8670 CrossRef CAS.
- A. Wildermann, Y. Foricher, T. Netscher and W. Bonrath, Pure Appl. Chem., 2007, 79, 1839 CrossRef CAS.
- W. Adam, W. A. Herrmann, J. Lin and C. R. Saha-Möller, J. Org. Chem., 1994, 59, 8281 CrossRef CAS.
- W. Adam, W. A. Herrmann, C. R. Saha-Möller and M. Shimizu, J. Mol. Catal. A: Chem., 1995, 97, 15 CrossRef CAS.
- R. Bernini, E. Mincione, M. Barontini, F. Crisante, G. Fabrizi and A. Gambacorta, Tetrahedron, 2007, 63, 6895 CrossRef CAS.
- Y. Çimen and H. Türk, Appl. Catal., A, 2008, 340, 52 CrossRef.
- O. A. Kholdeeva, I. D. Ivanchikova, M. Guidotti and N. Ravasio, Green Chem., 2007, 9, 731 RSC.
- O. A. Kholdeeva, N. N. Trukhan, M. P. Vanina, V. N. Romannikov, V. N. Parmon, J. Mrowiec-Bialon and A. B. Jarzebski, Catal. Today, 2002, 75, 203 CrossRef CAS.
- Y. Li, W. Liu, M. Wu, Z. Yi and J. Zhang, J. Mol. Catal. A: Chem., 2007, 261, 73 CrossRef CAS.
- R. Maassen, S. Krill and K. Huthmacher, EP1132367, 2001 Search PubMed.
- X. Menga, Z. Suna, S. Lin, M. Yang, X. Yang, J. Suna, D. Jiang, F.-S. Xiao and S. Chen, Appl. Catal., A, 2002, 236, 17 CrossRef.
- R. Saladino, V. Neri, E. Mincione and P. Filippone, Tetrahedron, 2002, 58, 8493 CrossRef CAS.
- H. Sun, X. Li and J. Sundermeyer, J. Mol. Catal. A: Chem., 2005, 240, 119 CAS.
- C. Wang, W. Guan, P. Xie, X. Yun, H. Li, X. Hu and Y. Wang, Catal. Commun., 2009, 10, 725 CrossRef CAS.
- O. V. Zalomaeva, I. D. Ivanchikova, O. A. Kholdeeva and A. B. Sorokin, New J. Chem., 2009, 33, 1031 RSC.
- K. Möller, G. Wienhöfer, K. Schröder, B. Join, K. Junge and M. Beller, Chem.–Eur. J., 2010, 16, 10300 CrossRef.
- Y. Asakawa, R. Matsuda, M. Tori and M. Sono, J. Org. Chem., 1988, 53, 5453 CrossRef CAS.
- J. Jacob and J. H. Espenson, Inorg. Chim. Acta, 1998, 270, 55 CrossRef CAS.
- S. Li and J. Fu, Shihua Jishu Yu Yingyong, 2008, 26, 234 CAS.
- S. Yamaguchi, M. Inoue and S. Enomoto, Bull. Chem. Soc. Jpn., 1986, 59, 2881 CrossRef CAS.
- M. Carril, P. Altmann, M. Drees, W. Bonrath, T. Netscher, J. Schütz and F. E. Kühn, J. Catal., 2011, 283, 55 CrossRef CAS.
- W. Adam, W. A. Herrmann, J. Lin, C. R. Saha-Möller, R. W. Fischer and J. D. G. Correia, Angew. Chem., Int. Ed. Engl., 1994, 33, 2476 CrossRef.
- C. C. Romão, F. E. Kühn and W. A. Herrmann, Chem. Rev., 1997, 97, 3197 CrossRef.
- W.-D. Wang and J. H. Espenson, J. Am. Chem. Soc., 1998, 120, 11335 CrossRef CAS.
- T. Dwars, E. Paetzold and G. Oehme, Angew. Chem., Int. Ed., 2005, 44, 7174 CrossRef CAS.
- K. Holmberg, Eur. J. Org. Chem., 2007, 731 CrossRef CAS.
- J. S. Milano-Brusco, H. Nowothnick, M. Schwarze and R. Schomacker, Ind. Eng. Chem. Res., 2010, 49, 1098 CrossRef CAS.
- T. Nishikata and B. H. Lipshutz, J. Am. Chem. Soc., 2009, 131, 12103 CrossRef CAS.
- S.-H. Wei and S.-T. Liu, Catal. Lett., 2009, 127, 143 CrossRef CAS.
Footnote |
† General procedure for the MTO-catalysed oxidation of 1 using amphiphiles: to a solution of MTO (2 mol%), 1 (1.4 mmol, 1 equiv.), the amphiphile of choice (see Table 1) and solvent (when indicated, see Table 1) H2O2 30% (4 equiv.) was added to start the reaction. The reaction mixture was stirred at 40–50 °C temperature for 20–72 h. After the reaction was stopped, the crude mixture was extracted with diethyl ether. Subsequently, a catalytic amount of MnO2 was added to destroy traces of peroxide if necessary, and the resulting solution was dried over anhydrous sodium sulfate, filtered and analysed by GC-MS using 4-methylbiphenyl and n-decane as internal standard. For characterisation, 2,3,5-trimethyl-1,4-benzoquinone (3), was purified through flash chromatography (dichloromethane/pentane 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6) after work-up and obtained as a pale orange solid. Spectroscopic data are in accordance with literature data.10 6) after work-up and obtained as a pale orange solid. Spectroscopic data are in accordance with literature data.10 |
| This journal is © The Royal Society of Chemistry 2012 |