Role of mixed metal oxides in catalysis science—versatile applications in organic synthesis
Manoj B.
Gawande
*a,
Rajesh K.
Pandey
b and
Radha V.
Jayaram
*c
aREQUIMTE, Departamento de Química, Faculdade de Ciências e Tecnologia, FCT, Universidade Nova de Lisboa, 2829-516 Caparica, Portugal. E-mail: mbgawande@yahoo.co.in; m.gawande@fct.unl.pt; Fax: +351 21 2948550; Tel: +351 21 2948300
bDepartment of Chemistry, Marquette University, Milwaukee, WI-53233, USA
cDepartment of Chemistry, Institute of Chemical Technology, Nathalal Parekh Marg, Matunga, Mumbai, India. E-mail: rvjayaram@udct.org
First published on 3rd February 2012
Abstract
A variety of mixed metal oxides (MMOs), containing alkali, alkaline, rare earth and noble metals, and their applications are presented. In this mini review, we summarize versatile applications of mixed metal oxides in organic synthesis. A variety of reactions such as reduction, oxidation, multicomponent, Mannich, alkylation, condensation, deprotection, cycloaddition, hydroxylation, dehydration, dehydrogenation, transesterification, reactions involving biomimetic oxygen-evolving catalysts and other important C–C bond forming reactions are well presented on the surface of mixed metal oxides under a variety of reaction conditions. The scope of MMOs in important organic reactions, industrial applications, and green chemistry and recent applications of MMOs are well presented in this review.
Introduction
Metal oxides represent one of the most important and widely employed categories of solid catalysts, either as active phases or as supports. Metal oxides are utilized both for their acid–base and redox properties and constitute the largest family of catalysts in heterogeneous catalysis.1 Metals and metal oxides form the bulk of catalysts employed for many synthetic conversions. Transition and noble group metals are frequently used as catalysts and their activity has been attributed to the outer electron configuration.2Single metal oxides can crystallize with different morphologies (isotropic, anisotropic or amorphous) and local co-ordination. All metal oxides can crystallize at room temperature, but many phases may remain amorphous at modest calcination temperatures. The majority of one component metal oxides crystallize with an isotropic morphology (without preferential orientation) and the surface may terminate with M–OH, M–O–M, M![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O or M-( ) functionalities where M-( ) represents an oxygen vacancy.
O or M-( ) functionalities where M-( ) represents an oxygen vacancy.
Among the metal oxide catalysts, those of transition metals occupy a predominant place owing to their low cost of production, easy regeneration and selective action. They are used in widely different types of organic reactions, such as oxidation, dehydration, dehydrogenation and isomerization. Their catalytic activity may be traced to the presence of partially filled d-shells of the metal ion and to the influence of the oxide ligand field on this partially filled d-shell.
In the view of a catalytic chemist, mixed metal oxides are oxygen-containing combinations of two or more metallic ions in proportions that may either vary or be defined by a strict stoichiometry. Solid solutions and mixed metal oxides are classified by physicochemists according to their crystalline systems. Mixed metal oxides are generally obtained in the form of powder or single crystals. They have a wide spectrum of industrial applications in ceramics, electronics, nuclear research and especially in catalysis.3 Since last one decade, there has been focus on synthesis and applications of mixed metal oxides. They were used for selective reduction of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in α,β-unsaturated carbonyls through catalytic hydrogen transfer reaction,4 study of chemical structures and performance of perovskite oxides,5 epoxidation on MoO3/TiO2 oxide,6a study of effect of phosphate ions on the textural and catalytic activity of titania–silica mixed oxide.6b Recently Jackson and Hargreaves published a book on mixed metal oxides.7a
O in α,β-unsaturated carbonyls through catalytic hydrogen transfer reaction,4 study of chemical structures and performance of perovskite oxides,5 epoxidation on MoO3/TiO2 oxide,6a study of effect of phosphate ions on the textural and catalytic activity of titania–silica mixed oxide.6b Recently Jackson and Hargreaves published a book on mixed metal oxides.7a
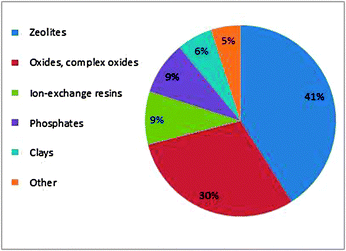
Organic conversions using mixed metal oxides are one of the widely studied classes of reactions in the pharmaceutical industry. Like other heterogeneous catalysts, mixed metal oxides also play very important role in the total catalysts system used3c (Fig. 1). In this review, we focus on application of mixed metal oxides in organic synthesis, synthesis of fine chemicals, industry and green chemistry, which will be addressed briefly (Fig. 2).
 | ||
| Fig. 1 Industrially important heterogeneous catalysts. | ||
 | ||
| Fig. 2 Applications of mixed metal oxides. | ||
Mixed metal oxides
Oxides containing two or more different kinds of metal cations are known as mixed metal oxides. Oxides can be binary, ternary and quaternary and so on with respect to the presence of the number of different metal cations. They can be further classified based on whether they are crystalline or amorphous. If the oxides are crystalline the crystal structure can determine the oxide composition. For instance, perovskites have the general formula ABO3; scheelites, ABO4; spinels, AB2O4; and palmeirites, A3B2O8. The different metal cations (MI and MII) are present as MIn+-Ox and MIIn+-Ox polyhedra, which are connected in various possible ways, such as corner or edge sharing, forming chains MI–O–MII–O, MI–O–MI–O or MII–O–MII–O.The arrangement of cations of a given element differs by the co-ordination and the nature of the neighboring cations and this governs the type of bonding between the cations. Different environment of the cation that constitutes an active center would give rise to different reactivity towards an approaching molecule. It is often not clear, which of the constituent metal cations plays a role of active center in mixed metal oxides. Although the industrial catalysts are usually multiphase systems, the presence of some particular phase appears to be critical to render the activity of the system for a given reaction. One of the most important results of the study of monophasic systems is the demonstration of different catalytic properties of different crystallographic faces in an oxide that provided an experimental proof for structure sensitivity phenomenon in the oxide systems.
There is much interest in understanding the phenomena for the deposition of one oxide on another oxide. There are various methods for the preparation of MMOs such as sol–gel,7b–e wet impregnation,7f,g mechanochemical synthesis,7h,i hydrothermal method,7j,k co-precipitation7l,m and microwave irradiation.19 The mixed metal oxides are characterised by several spectroscopic and analytical techniques such as XRD, FT-IR, TG-DTA, BET, SEM-EDS, TEM, acidity and basicity measurements, XPS and Raman spectroscopy7n which are also additional techniques for catalyst characterization.
Why mixed metal oxides?
Mixed metal oxides play very important role in academic as well as industrial research due to their acid–base and redox properties, and constitute the largest family of catalysts in heterogeneous catalysis. Some of the important applications are depicted here, e.g. CaCoAl-HTlcs are used for NOx capture, decomposition and reduction,8 a mixed oxide from Mo, Nb, Sb and V for the selective oxidation of propane,9 Ni/Mg–Al mixed metal oxide catalyst for steam reforming of ethanol,10 Mg–V–Al mixed metal oxides for oxidative dehydrogenation of propane on ,11 mixed metal oxides La2−xSrxCuO4±λ for hydroxylation of phenol,12 Al2O3 supported mixed metal oxides for destructive oxidation of (CH3)2S2,13 silica/titania mixed oxide-supported zirconocene catalyst for synthesis of linear low-density polyethylene,14 mesoporous Co3O4 and Au/Co3O4 catalysts for low-temperature oxidation of trace ethylene,15 FexCe1−xO2 mixed metal oxides for N2O decomposition,16 LaFe1−xCoxO3 mixed-oxide for combined methane reforming17aetc. Due to their broad scope in the catalysis field,17b they are necessary for organic synthesis, industrial applications and in the pharmaceutical industry.Notably, some of the mixed metal oxides are better in terms of their catalytic activity than component oxides in various reactions. Why is it so? It could be due to increasing active acidic or basic sites, and increasing surface area, which reduces the reaction time, increases the yield of reaction or conversions of reactants.18,31,49a For example, consider liquid phase catalytic transfer hydrogenation of aromatic nitro compounds on La1−xSrxFeO3 perovskites prepared by microwave irradiation. Notably, reduction of aromatic nitro compounds worked well in mixed metal oxides La1−xSrxFeO3 as compared to their component oxides.19 The values of activation energy for LaFeO3, La0.8Sr0.2FeO3 and SrFeO3 are 88.9, 73.9 and 84.4 kJ mol−1, respectively, which are found to follow the order LaFeO3 > SrFeO3 > La0.8Sr0.2FeO3.
Applications of mixed metal oxides in organic synthesis
Application of mixed metal oxides has an important role in organic transformations, due to their simplicity in handling, decreased reactor and plant corrosion problems, cost effectiveness and because most of the MMOs are reusable and recyclable. Some of the important reactions in organic synthesis are depicted below.Antimicrobial drug tinidazole has been synthesized by condensation of 2-methyl-5-nitroimidazole with 2-ethyl-thio-ethanol over a MoO3/SiO2 catalyst to obtain 1-(2-ethyl-thio-ethanol)-2-methyl-5-nitro-imidazole (Scheme 1) which was further oxidized using hydrogen peroxide with the same catalyst to form tinidazole.20
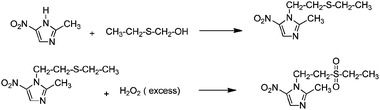 | ||
| Scheme 1 Synthesis of tinidazole over MoO3/SiO2. | ||
Tinidazole, an important pharmaceutical compound, was also synthesized by condensation/oxidation reaction using the MoO3/SiO2 catalyst without any use of acetic acid, tungstic acid or ammonium molybdate used in the conventional process. The MoO3/SiO2 catalyst could be recycled at least five times without any appreciable loss in conversion and selectivity, indicating its potential for use as a bifunctional catalyst.
Song and coworkers have reported a Cu–Mn mixed oxide catalyst supported on carbon which was prepared using the incipient wetness method according to the literature.21 Carbon supported copper–manganese oxide and TEMPO were used for the selective aerobic oxidation of alcohols to corresponding aldehydes or ketones. The synthesized Cu–Mn mixed oxide gave good selectivity, is reusable and separates easily22 (Scheme 2).
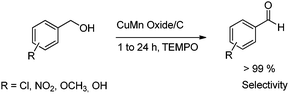 | ||
| Scheme 2 Selective aerobic oxidation of alcohols to corresponding aldehydes or ketones over mixed metal oxides. | ||
Perovskites are a class of mixed oxides, which have interesting catalytic and physicochemical properties. A novel method developed for the synthesis of perovskites of the type LaMO3 (M = Mn, Fe, Co, Cr, Al) involves microwave irradiation for just 15 min using oxalate precursors. The LaMO3 perovskites were used as catalysts for reduction of aromatic nitro compounds with propan-2-ol as hydrogen donor and KOH as promoter.23 Notably, LaFeO3 perovskite is the best catalyst under the same reaction conditions. The corresponding aniline derivatives are obtained in excellent yield (78–98%) in 2–6 hours (Scheme 3). The experimental results obtained for this research work allow us to conclude that perovskites prepared by the microwave irradiation method have activity values similar to those of the ones prepared by conventional methods. The method is simple and does not involve intermittent grindings and calcinations at elevated temperatures. Finally, we have achieved a considerable reduction in the time required for preparation of perovskites.
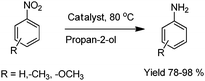 | ||
| Scheme 3 Reduction of aromatic nitro compounds. | ||
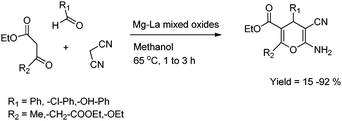
Wadsworth–Emmons reaction was reported on Mg–Al mixed oxides. The Mg–Al mixed oxide was prepared by a simple co-precipitation method by using Mg and Al nitrates and calcining at 923 K. The synthesized Mg–La mixed oxide was found to be an efficient solid base for the selective synthesis of α,β-unsaturated esters and nitriles with greater than 80% yields, avoiding Knoevenagel condensation and aldolisation even with aliphatic aldehydes.24 The catalyst could be recycled once with high activity. The effect of substituents on the phenyl ring proves that the reaction proceeds via a base catalyzed mechanism with a low charge on intermediate species (Scheme 4).
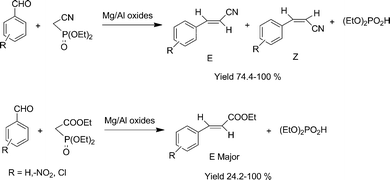 | ||
| Scheme 4 Wadsworth–Emmons reactions of aldehydes with phosphonates. | ||
Cycloaddition of CO2 to various epoxides was carried out on heterogeneous Mg–Al mixed oxides.25 Mg–Al mixed metal oxides obtained by calcination of the hydrotalcites were effective catalysts for the fixation of CO2 to various epoxides to form the corresponding five-membered cyclic carbonate. Particularly, mixed oxide with the Mg–Al ratio of 5 calcined at 400 °C showed the highest catalytic activity. This unique catalytic activity is due to the cooperative action of acid–base sites derived from the formation of Mg–O–Al bonds, i.e. the substitution of Al for Mg in the MgO matrix, on the surface of mixed oxides. The Mg–Al mixed oxide catalyst was reusable and could be proved effective for many organic reactions as environment-friendly acid–base catalysts (Scheme 8).
The possible reaction mechanism for the cyclo-addition reaction is depicted in Fig. 3. The addition reaction is initiated by adsorption of CO2 on the Lewis basic sites to form a carbonate species, and independently, an epoxide is coordinated on the neighboring acid site on the surface. The coordinated epoxide is ring-opened by a nucleophilic attack of the carbonate species, which leads to an oxy anion species yielding the corresponding cyclic carbonate as a product. The Mg–Al mixed oxide has good catalytic activity compared to its physical mixture. Therefore, it is likely that a prominent feature of the active Mg–Al mixed oxide catalysts can be found to originate from the cooperative action of both the basic and acidic sites located in a neighbor on the surface.
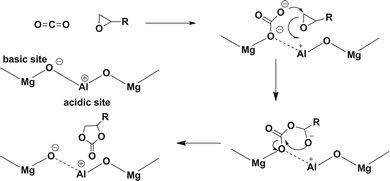 | ||
| Fig. 3 Mechanism of cycloaddition of CO2 catalyzed by Mg–Al mixed metal oxides (ref. 25). | ||
Recently, we have reported Al2O3–OK (K2O supported on alumina) mixed metal oxide for N-alkylation of amines. The Al2O3–OK was prepared by a simple impregnation method and the synthesized Al2O3–OK catalyst was characterized by XRD, SEM–EDS, elemental analysis, particle size analysis, BET surface area, pore size and average pore diameter.26
In order to know about the homogeneity of Al2O3–OK, SEM analysis was carried out. It has been noted that the K2O base was finely and uniformly distributed over the Al2O3 support, which makes the catalyst more active (Fig. 4).
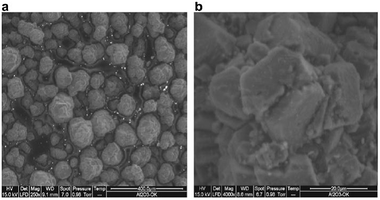 | ||
| Fig. 4 Scanning electron micrograph of Al2O3–OK at 400 μm (left) and scanning electron micrograph of Al2O3–OK at 20 μm (right).26 | ||
N-Alkylation of amines by alkyl halides using Al2O3–OK as a catalyst in acetonitrile at room temperature (30 °C) was described. The corresponding tertiary amines were obtained in good yield (65–95%) (Scheme 5).
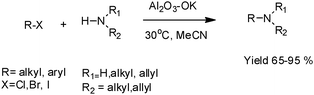 | ||
| Scheme 5 N-Alkylation of primary and secondary amines by alkyl halides. | ||
A series of LaMO3 (M = Cr, Co, Fe, Mn, Ni) perovskites were synthesized by a citrate precursor decomposition method and characterized by a XRD technique. The catalytic activity of the synthesized perovskites was investigated for the oxidative functionalization of alkylaromatics to benzylic ketones using TBHP as an oxidant.27 Of various perovskites screened, LaCrO3 exhibited remarkable catalytic activity, providing the oxidation of alkylarenes selectively at the benzylic position. The LaCrO3/TBHP catalytic system provides excellent yields (84–97%, selectivity 97%) of the desired ketones under solvent-free conditions. The catalyst can be reused successfully up to six consecutive cycles with no significant loss in activity (Scheme 6).
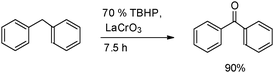 | ||
| Scheme 6 Oxidation of diphenyl methane to benzophenone. | ||
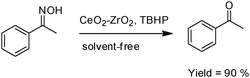
A facile deprotection of oximes over mixed metal oxides (CeO2–ZrO2) under solvent-free conditions was reported. CeO2–ZrO2 was prepared by a co-precipitation method via an inexpensive precursor. The as-synthesized material was characterized by nitrogen adsorption–desorption, surface area, particle size, EDX, XRD and FT-IR techniques. The catalytic activity was tested for deprotection of oximes of ketones and aldehydes efficiently over CeO2–ZrO2 using tert-butyl hydrogen peroxide (TBHP) as an oxidant under solvent-free conditions.28
This method is high yielding, clean, safe, cost effective and therefore very suitable for practical organic synthesis. The corresponding products were obtained in 30–94% yield (Scheme 7).
Potassium iron zirconium phosphate [PIZP-K2FeZrP3O12] was prepared by a sol–gel method.29 The as-synthesized catalyst was characterized by crystal size, elemental analysis, nitrogen adsorption–desorption, pore volume, average pore diameter, XRD, FT-IR and DSC techniques. The SEM images of PIZP are depicted in Fig. 5. The particle size was found to be in the range of 1–3 μm. Benzophenone and its derivatives were synthesized over the PIZP mixed metal oxides catalyst under solvent-free conditions.
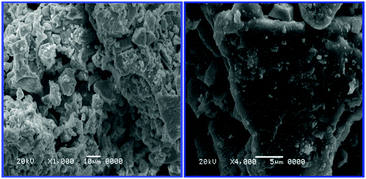 | ||
| Fig. 5 Scanning electron micrograph of PIZP at 10 μm and 5 μm. | ||
The catalytic activity of PIZP was tested for the benzoylation of different arenes using benzoyl chloride as the benzoylating agent. The reaction was carried out at different temperatures and catalyst loadings. The advantage of PIZP is that it can be prepared easily and does not lose its activity even after several runs. The synthesized benzophenone and its derivatives were obtained in excellent yield (87–96%) (Scheme 9).
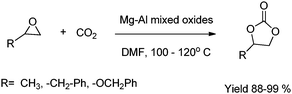 | ||
| Scheme 8 Cycloaddition of carbon dioxide to epoxides in the presence of Mg–Al mixed oxides. | ||
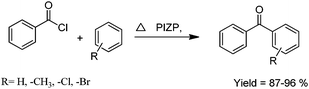 | ||
| Scheme 9 Friedel–Crafts benzoylation of arenes with benzoyl chloride. | ||
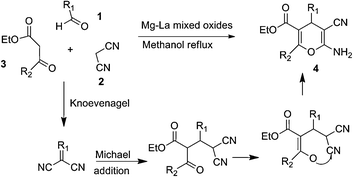
Synthesis of polyfunctionalized 4H-pyrans was carried out in one pot through condensation of an aldehyde, malononitrile, and an active methylenic diketo compound using a heterogeneous strong basic Mg–La mixed oxide catalyst.30 The advantages of this protocol are simple catalyst preparation, mild reaction temperature, easy recovery, and reusability of the catalyst with consistent activity and short reaction times (Scheme 10).
 | ||
| Scheme 10 Mg–La catalyzed synthesis of 4H-pyran derivatives. | ||
The mechanism for the synthesis of 4H-pyrans is depicted in Fig. 6.
The synthesis of 4H pyrans involves a Knoevenagel condensation, catalysed by the as-strong basic sites on the catalyst surface, which initiate the reaction by abstracting a proton from the active methylene group from malononitrile. The aldol type product thus formed suffers a Michael addition from the enolate of the active methylenic diketo compound to yield after cyclization the polyfunctionalized 4H-pyrans
Recently, we reported NaLaTiO4 and HLaTiO4 as heterogeneous and reusable catalysts for O-tert-but-oxycarbonylation of various hydroxyl compounds under mild conditions.31 The observed comparable activity of NaLaTiO4 and HLaTiO4 can be explained on the basis of their structural features as both consist of single layers of TiO6 octahedra that are separated in alternate layers by monovalent cations, which are ion-exchangeable, and La3+, which is non-exchangeable. Thus, the activity may be due to the anionic moiety [LaTiO4]− which is the same for both the catalysts. This was further proved when the influence of the component metal oxides on the reaction was studied. It was observed that both the component metal oxides (Table 1, entries 4 and 5) provided lower yields compared to the perovskites, indicating that the actual catalytic activity resides in the perovskite structure (Scheme 11).
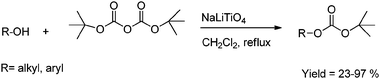 | ||
| Scheme 11 O-tert-butoxycarbonylation of hydroxy compounds. | ||
Hell has reported nickel(II) supported heterogeneous, magnesium–lanthanum mixed oxide for Kumada coupling reaction.32 The catalyst was used successfully in the Kumada coupling of aryl halides, especially aryl bromides. The synthesized compounds were obtained in 11–86% yield (Scheme 12). The Mg–La mixed oxide catalyst is easily separable from reaction mixture, thus the nickel contamination of the product can be avoided.
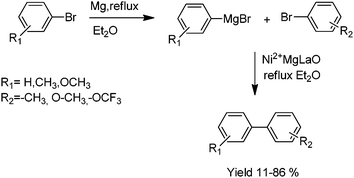 | ||
| Scheme 12 Kumada coupling of Ni2+/Mg–La mixed metal oxides. | ||
Role of mixed metal oxides in industrially important reactions
Several industrially important synthetic conversions have been carried out with mixed metal oxides. MMOs have been used in various important reactions such as reduction of nitroarenes,33 NO reduction,34 with NH3, catalytic wet air oxidation of 2-chlorophenol,35 liquid phase catalytic oxidation of alcohols,36 alcohol dehydration,37 dehydrogenation of 2-octanol,38 transesterification on Na-based mixed metal oxide,39 destructive oxidation of (CH3)2S2,40a solvent-free oxidation of primary alcohols to aldehydes using Au–Pd/TiO2 catalysts,40b ammonia adspecies and catalytic transformation,40c and in some other important applications.40dAlso, some of the mixed metal oxides such as Cu–Mn spinel type oxides are used in methanol steam reforming,40e alumina based oxides for hydrodesulfurization,40f nanostructured oxides for some applications,40g and zinc based oxides for direct conversion of bio-ethanol to isobutene40hetc.
Mannich reaction is a classical method for the preparation of β-amino carbonyl compounds. It is a key step in the synthesis of numerous pharmaceuticals and natural products.41a,b With the increasing interest in developing environmentally benign reactions, the atom-economic catalytic process that employs unmodified carbonyl donor, amine and acceptor aldehyde as an idealized Mannich-type reaction is attracting more and more attention.41c,d Very recently we have reported a one-pot three component Mannich reaction using aldehydes, amines and ketones over a solid MgO/ZrO2 catalyst at 80 °C in acetonitrile.41e Notably, all varieties of aldehydes and amines worked well over the MgO/ZrO2 to afford the corresponding compounds in good yield (Scheme 13).
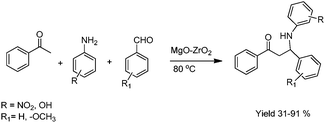 | ||
| Scheme 13 Synthesis of β-amino carbonyl compounds by Mannich reaction using MgO–ZrO2. | ||
 | ||
| Scheme 14 Michael-addition reaction over magnesium–lanthanum mixed oxide. | ||
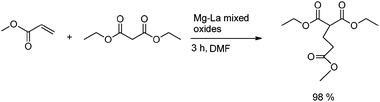
Michael addition reactions were studied by Figueras and co-workers at room temperature with a variety of acceptors and donors, using a magnesium–lanthanum mixed oxide. Notably, all acceptors and donors worked well and obtained a very good yield.42a The reaction of methyl acrylate and diethyl malonate is depicted in Scheme 14.
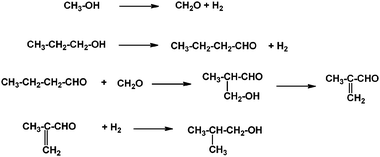
Isobutyl alcohol (iBuOH) has gained an increasing interest in the last decade, owing to its potential use as precursor for the preparation of either gasoline additives, such as methyl-tert-butyl-ether and isooctane, or plasticizers. Synthesis of isobutyl alcohol from methanol and n-propanol through the Guerbet condensation has been studied in batch experiments using bifunctional heterogeneous systems based on a dehydrogenating/hydrogenating metal (Pd, Rh, Ni or Cu) and a basic Mg–Al mixed oxide derived from hydrotalcite-type (HT) precursors42b (Scheme 15).
 | ||
| Scheme 15 Synthesis of isobutyl alcohol over mixed metal oxides. | ||
Mestl has investigated MoVW mixed metal oxides catalysts for acrylic acid production.42c (MoVW)5O14-type oxides were identified as the active and selective components in industrial acrylic acid catalysts. Tungsten is suggested to play an important role as a structural promoter for the formation and stabilization of this oxide. Vanadium is responsible for high catalytic activities but is detrimental for the stability of this oxide at the necessary high concentrations for optimum catalytic performance. The activity of mixed MoVW oxide catalysts for methanol, propene, and acrolein partial oxidation could be considerably improved, when the amount of the (MoVW)5O14-type oxide was increased by thermal annealing. In the model developed by Mestl (Fig. 7a), it can be assumed that the surface oxygen defects generated during catalysis may be replenished either by surface oxygen diffusion, by oxygen diffusion through the near surface layer or by oxygen bulk diffusion. He has also developed a core–shell model of MoVW mixed metal oxide (Fig. 7b).
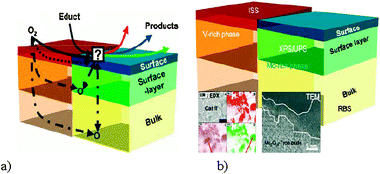 | ||
| Fig. 7 (a) Schematic drawing of different possible oxygen diffusion pathways in mixed oxide partial oxidation catalysts. (b) Schematic drawing of the proposed core–shell model of the MoVW mixed metal oxide catalyst. For detailed information on these models readers are referred to ref. 42c. | ||
Ceria based mixed oxides have attracted much attention due to their versatile applications in various fields such as sensors, fuel cells, biomaterials and especially in catalysis, in automotive catalytic converters for treating emissions and due to some other industrial catalytic applications.43a–k
Kaspar and co-workers have reported ceria-based oxide catalysts in the automotive three-way catalysts (TWCs). In this article they focused on a variety of roles of CeO2 as a TWC promoter and in particular the oxygen storage/release capacities are discussed in detail. Their main focus was on the oxygen storage/release capacities of materials containing ZrO2, which are employed in the last generation of catalytic automotive converters.44a
In 2004, Kaspar and Monte have discussed the role of ceria based catalyst in oxygen storage and release capacity (OSC) in promoting the activity of noble metals in the three-way catalysts (TWCs). In this article they have explained the reduction mechanism for CeO2 using H2 as reducing agent.44b
Kaspar and Monte have published a Feature article on advanced catalytic materials based on nanostructured CeO2–ZrO2 mixed oxides as oxygen storage promoters for the automotive three-way catalysts (TWCs).44c In this article they focused on various factors related to the structures, texture and all other properties of oxide materials. For more detailed information the reader can refer to ref. 44c and d.
The combination of reduction/oxidation treatments yields a variety of different phases and features. Even a modest increase of the re-oxidation temperature easily leads to a disordered type of cation structure.
Applications of MMOs in green chemistry
Scientists need to generate a diverse array of exceptionally complex targets within a short span of time. Because of high molecular complexity in drug discovery accompanied by time constraints, the development of efficient and environmentally benign synthetic protocols becomes the primary driver of chemistry. This can be achieved by the proper choice of starting materials, atom economic methodologies with a minimum number of chemical steps, the appropriate use of greener solvents and reagents, and efficient strategies for isolation and purification.In the current era there is a serious push towards developing processes that are eco-friendly. This process development is generally termed “Green Technology”45 or “Sustainable Technology”. This necessitates a shift from the traditional concepts of process efficiency that focus exclusively on chemical yield to one that assigns economic value to eliminating waste and avoiding the use of toxic and/or hazardous substances and more environmentally acceptable processes.46
The magnitude of the waste problem in the manufacture of chemicals measured as ‘E-Factor’47 in terms of the amount of waste produced per kg of product is depicted in Table 2.
| Industry segment | Annual production/t | E-factor | Waste produced/t |
|---|---|---|---|
| Oil refining | 106–108 | ca. 0.1 | 105–107 |
| Bulk chemicals | 104–106 | <1–5 | 104–5 × 106 |
| Fine chemicals | 102–104 | 5–50 | 5 × 102–5 × 105 |
| Pharmaceuticals | 10–103 | 25–100 | 2.5 × 102–105 |
The waste mainly consists of inorganic salts used or formed during or after the reaction during the workup process. In organic chemistry, the ideal synthesis will be a combination of a number of environmental, health and safety, and economic targets (Fig. 8).
 | ||
| Fig. 8 The ideal synthesis. | ||
To keep the green chemistry concern in mind, many industries are trying to synthesize target compounds by green chemistry routes. We report here, a few examples of mixed metal oxides used in green chemical processes.
Kantam has reported synthesis of β-hydroxy-α-diazo carbonyl compounds and β-amino-α-diazo carbonyl compounds on magnesium/lanthanum mixed oxide at room temperature in water to afford the corresponding products in good yields.48 The catalyst was recovered and reused for several cycles with consistent activity. The corresponding compounds were obtained by condensation of a wide variety of aldehydes and imines with ethyl diazoacetate (Scheme 16).
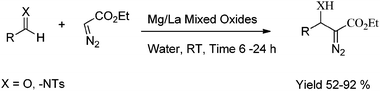 | ||
| Scheme 16 Synthesis of β-hydroxy-α-diazo carbonyl compounds and β-amino-α-diazo carbonyl compounds over mixed metal oxides. | ||
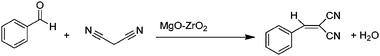
The Knoevenagel condensation of several aldehydes with ethyl cyanoacetate and malononitrile was carried out at 60 °C on a MgO/ZrO2 catalyst under solvent-free conditions.49a The mixed metal oxides (MgO/ZrO2) were prepared by using inexpensive precursors. The synthesized mixed metal oxides were well characterized by several analytical and physicochemical techniques. MgO/ZrO2 worked well for the condensation reaction to obtain the corresponding compounds in good yield (65–98%) (Scheme 17).
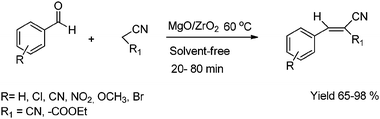 | ||
| Scheme 17 Knoevenagel condensation of aldehydes with ethyl cyanoacetate and malononitrile on MgO/ZrO2. | ||
The atom economy and environmental factor (E) of Knoevenagel reaction are depicted below:
| Atom economy = M.Wt. of the desired product/M.Wt. of all products |
| =242/260 × 100 |
| =93.07% |
| E = Sum of M.Wt. of the by-products/M.Wt. of the desired product |
| =18/242 |
| =0.07 |
The atom efficiency for the condensation reaction of benzaldehyde and malononitrile on several reported catalysts was calculated for comparison (Table 3). It can be seen that in terms of atom efficiency and E-factor the present mixed metal oxide catalyst system is comparable.
Very recently, we have reported MgO–ZrO2 mixed metal oxides for the synthesis of dihydropyrimidinones under solvent-free conditions.49e
The selective oxidation of alcohols is one of the most challenging reactions in green chemistry.50 Cao has reported that mesostructured Ga–Al mixed-oxide solid solutions, which are characterized by unique dehydrogenation properties, may be used as new attractive supports for the fabrication of exceptionally active gold catalysts for the liquid-phase aerobic oxidation of alcohols under mild conditions.51 Aerobic oxidation of benzyl alcohol is depicted in Scheme 18.
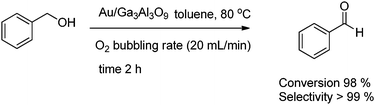 | ||
| Scheme 18 Selective oxidation of benzyl alcohol to benzaldehyde over mixed metal oxides. | ||
Also, other various alcohols viz. 2-octanol, 1-phenyl ethanol etc. yield the corresponding aldehydes with good selectivity >99%.
These catalysts can even efficiently catalyze the aerobic oxidation of several alcohols under ambient conditions in the absence of water or base.
Kobayashi and co-workers recently reported PI/Au nanocatalysts as the most active Au catalysts in the current literature, with a very high turnover frequency (TOF) of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1 for the conversion of 1-phenyl ethanol into acetophenone (at 160 °C).52,53 Under these conditions, an Au/Ga3Al3O9 catalyst gave an exceptionally high TOF of 25
000 h−1 for the conversion of 1-phenyl ethanol into acetophenone (at 160 °C).52,53 Under these conditions, an Au/Ga3Al3O9 catalyst gave an exceptionally high TOF of 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 h−1, thus showing a great potential for practical applications (Scheme 19).
000 h−1, thus showing a great potential for practical applications (Scheme 19).
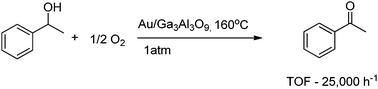 | ||
| Scheme 19 Solvent-free aerobic oxidation of (±)-1-phenylethanol using an Au/Ga3Al3O9 catalyst. Turnover frequency given for the following conditions: t = 30 min, (±)-1-phenylethanol (165.7 mmol), Au (4.0 × 10−4 mol%). | ||
Lande and coworkers have established a series of mixed metal oxides, Ce1MgxZr1−xO2 catalytic material, by a simple co-precipitation method and explored their potential use in the synthesis of 5-arylidine barbituric acid derivatives as a heterogeneous catalyst.54 Catalytic activity results stated that, the Ce1MgxZr1−xO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6
0.6![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4) catalyst exhibits excellent catalytic activity as compared to the Ce1MgxZr1−xO2 (1
0.4) catalyst exhibits excellent catalytic activity as compared to the Ce1MgxZr1−xO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2
0.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8), Ce1MgxZr1−xO2 (1
0.8), Ce1MgxZr1−xO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.4
0.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.6) and Ce1MgxZr1−xO2 (1
0.6) and Ce1MgxZr1−xO2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.8
0.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.2) for the condensation of various aromatic aldehydes and barbituric acid and gave the yield 90 to 94% in short reaction time under microwave irradiation (Scheme 20). The plausible mechanism for the reaction is depicted in Fig. 9.
0.2) for the condensation of various aromatic aldehydes and barbituric acid and gave the yield 90 to 94% in short reaction time under microwave irradiation (Scheme 20). The plausible mechanism for the reaction is depicted in Fig. 9.
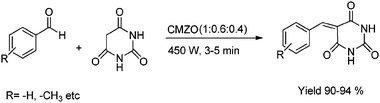 | ||
| Scheme 20 Synthesis of 5-arylidine barbituric acid catalysed by CMZO mixed metal oxides. | ||
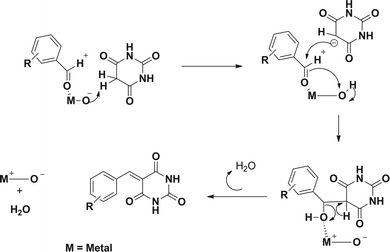 | ||
| Fig. 9 The plausible mechanism for the condensation reaction over CMZO.54 | ||
Recent novel organic reactions on MMOs
The synthesis of mixed metal oxides has attracted much interest from various research groups. The reason for this is that such MMOs have found a wide range of applications in many critical areas of modern technology such as catalysis, solar energy conversion processes, oil recovery and especially in organic synthesis. We herewith present some important organic reactions over mixed metal oxides.Mg–Al mixed-metal oxides were used for the transesterification of soyabean oil with methanol,55 ternary Cu–Ni–Fe catalysts were used in alkyne hydrogenation,56 Ni-based mixed oxide catalyst for carbon dioxide reforming of methane,57 calcined Co–Mn–Al layered double hydroxide (LDH) for catalytic decomposition of N2O, modified with slight amounts of alkali, rare earth, or noble metals,58 nickel-based mixed-metal oxides with catalytic properties,59 Cu–ZnO composite catalysts for selective hydrogenolysis of glycerol to propylene glycol,60 nanosized Ti–V mixed metal oxides with anatase structure for the photo-elimination of toluene,61 nickel-based Ce0.72Zr0.28O2 mixed oxide for methanation of carbon dioxide,62 calcium manganese(III) oxides (CaMn2O4·xH2O) as biomimetic oxygen-evolving catalysts,63 and some other recent applications of mixed metal oxides are depicted below.
Gold supported Cu–Mg–Al-mixed metal oxides of the 10–15 nm range were used for oxidation of a variety of alcohols.64 The mixed-oxide-supported Au catalysts with noble metal loading of 0.6 ± 0.17 wt% were investigated concerning their structural properties and tested in the aerobic liquid-phase oxidation of 1-phenylethanol to phenyl–methyl ketone affording TOFs up to 1300 h−1 (Scheme 21). The catalytic tests showed that the activity of these catalysts depends strongly on the composition of the support, with Cu and Mg being crucial components. Strongly enhanced catalytic activity was observed for gold supported on a ternary mixed oxide containing Cu, Mg, and Al at the molar ratio of 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Extension of the catalytic tests to various structurally different alcohols indicated that the ternary mixed-oxide-supported gold catalyst has excellent catalytic properties in the aerobic oxidation of a broad range of structurally different alcohols, affording selectivities >98%.
2. Extension of the catalytic tests to various structurally different alcohols indicated that the ternary mixed-oxide-supported gold catalyst has excellent catalytic properties in the aerobic oxidation of a broad range of structurally different alcohols, affording selectivities >98%.
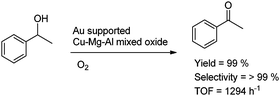 | ||
| Scheme 21 Aerobic liquid-phase oxidation of 1-phenylethanol to phenyl–methyl ketone over mixed metal oxides. | ||
One-step synthesis of methyl isobutyl ketone (MIBK) from acetone and H2 in the gas and liquid phase was carried out on Pd supported on ZnII–CrIII mixed oxide.65 Due to the bifunctional nature of ZnII–CrIII mixed metal oxides, the reaction involves acid-catalyzed condensation of acetone to mesityl oxide, followed by its hydrogenation to MIBK (Scheme 22).
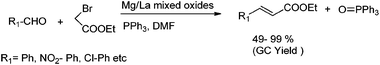
Very recently Kantam and co-workers have reported effective heterogeneous Mg/La mixed oxide (Mg/La MO) for the one pot Wittig reaction involving aldehydes, α-halo esters and triphenylphosphine to afford α,β-unsaturated esters in good yields with high E-stereoselectivity, at room temperature66 (Scheme 23). The corresponding products were obtained in good yields (49–99%).
Hell and coworkers reported copper-free Sonogashira reaction of alkynes and aryl halides by using Pd/MgLa mixed oxide.67 This heterogeneous system allows the full recovery and reuse of the catalyst. Notably, palladium remains on the surface and no leaching was observed. The reaction of alkynes and aryl halides is shown in Scheme 24.
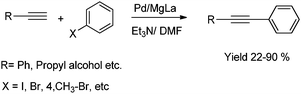 | ||
| Scheme 24 Sonogashira cross-coupling reaction on Pd/MgLa mixed oxide. | ||
The synthesis of benzimidazole structural derivatives has attracted much interest in medicinal chemistry as well as in various pharmacological activities.68 Very recently, we have reported MoO3/SiO2 as a heterogeneous bifunctional catalyst for sequential oxidation of alcohols to their corresponding aldehydes/ketones using H2O2 as a green oxidant and its sequential condensation with O-phenylenediamine (OPDA) to yield benzimidazoles/benzodiazepines with minimum side product formation under mild reaction conditions.69
The prepared catalysts were characterized using FT-IR, Raman spectroscopy, XRD, SEM and NH3-TPD to study their surface properties. A SEM image of SiO2 shows the presence of a smooth surface without any pores, however micrographs for MoO3/SiO2 clearly show marked difference in the morphology from that of SiO2. The differences in SEM images thus illustrate that incorporation of MoO3 changes the morphological structure of the SiO2 surface considerably (Fig. 10).
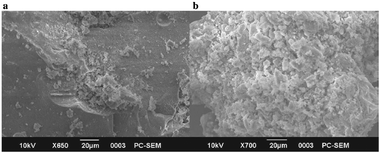 | ||
| Fig. 10 SEM images of (a) SiO2 and (b) MoO3–SiO2. | ||
Acidity measurements were carried out using NH3-TPD analysis (Table 4), and it was found that the acidity of SiO2 (entry 1) was very less compared to the Mo loaded SiO2 (entries 2–7). This showed that the acidity of the catalyst is mainly due to Mo species. It was observed that as the percentage of Mo loading was increased, acidity of the catalyst also increased (entries 2–6).
| Entry | Catalyst | NH3 desorbed/mmol g−1 | Yield (%) |
|---|---|---|---|
| 1 | SiO2 | 0.02 | — |
| 2 | MoO3 | 0.13 | 28 |
| 3 | 5 MoO3/SiO2 | 0.43 | 42 |
| 4 | 10 MoO3/SiO2 | 0.62 | 63 |
| 5 | 15 MoO3/SiO2 | 0.77 | 77 |
| 6 | 20 MoO3/SiO2 | 0.82 | 73 |
| 7 | 25 MoO3/SiO2 | 0.80 | 72 |
However, the best results were obtained with 15 mol% Mo loadings (entry 4) although 20% Mo loading had higher acidity. Similar results were obtained by Wang and his co-workers.70a
The decreased activity of the MoO3/SiO2 catalyst at higher MoO3 loading was attributed to the aggregation of MoO3 particles on the SiO2 surface. MoO3/SiO2 shows good catalytic activity and reusability for both the reaction systems giving high yields (52–85%) of the desired products (Scheme 25).
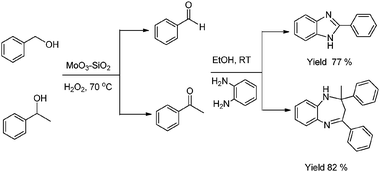 | ||
| Scheme 25 Sequential oxidation and condensation for synthesis of benzimidazole and benzodiazepine over MoO3–SiO2. | ||
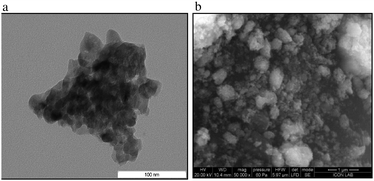
Very recently, we have prepared mixed metal oxides (MgO–ZrO2) nanoparticles by an ultradilution–coprecipitation method and gradient heating.70b The as-synthesized catalyst was characterized by several techniques such as XRD, TG-DTA, FT-IR, SEM and TEM. The SEM and TEM images are depicted in Fig. 11. TEM clearly indicates that the size of MgO–ZrO2 particles are in the 20–35 nm range. The MgO–ZrO2 catalyst is then used for various reactions such as cross-aldol condensation, Cbz protection of amines, reduction of nitroarenes and in synthesis of 1,5-bezodiazepines.
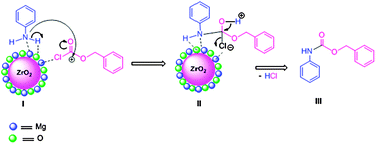
All reactions worked well under mild conditions and gave excellent yields of the corresponding products. The MgO–ZrO2 catalyst was reused and recycled for all reactions without any loss of yield of the products (Scheme 26). The mechanism of Cbz protection of aniline over nano MgO–ZrO2 is depicted in Fig. 12. In the first step, the –NH2 group of aniline and Cbz–Cl adsorbed on the catalyst as shown in I. It is well known that functional groups containing electronegative atoms such as N, O, and S are adsorbed on metal or Lewis acid site and hydrogen adsorbed on the oxygen of the catalyst. Then in the second step electrophilicity of carbonyl carbon of Cbz–Cl is increased and it generates an intermediate state II, which, finally yields final product III along with liberation of HCl. The MgO–ZrO2 catalyst participates in all these steps, weakening the chemical bonds of reactants and subsequently lowering the activation energy.
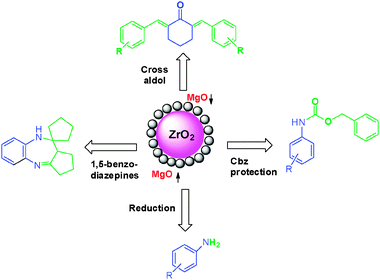 | ||
| Scheme 26 Nano MgO–ZrO2 catalyzed reactions. | ||
The mechanism of reduction of aromatic nitrobenzene to aniline is shown in Fig. 13. During adsorption of propan-2-ol the hydrogen from the –OH group adsorbed as a proton and the hydrogen of C–H migrated via hydride transfer to the substrate. Hence the rate of reaction can show dependence on the strength of adsorption of both propan-2-ol and substrate. First nitrobenzene and isopropyl alcohol adsorbed on nano-MgO–ZrO2I, then reduction of nitrobenzene with IPA as hydrogen source generated an intermediate II with elimination of an acetone molecule, then intermediate III with elimination of a water molecule and finally aniline Vvia intermediate IV.
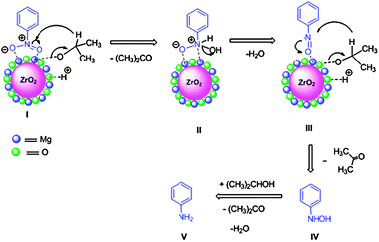 | ||
| Fig. 13 Mechanism of reduction of nitrobenzene to aniline over nano MgO–ZrO2 mixed metal oxide. | ||
Very recently, Baiker has reported metal-support for the hydrogenation of cinchonidine (CD) over acid- and base-doped flame-made Pt/alumina.71 They have studied the effect of support acidity/basicity on the adsorption geometry of CD at the surface of supported Pt nanoparticles. The model catalysts were prepared by the reported procedure.72 The chemo- and diastereoselective hydrogenation of CDH2 are represented in Scheme 27.
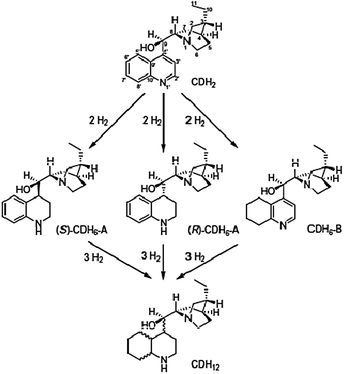 | ||
| Scheme 27 Chemo- and diastereoselective hydrogenation of CDH2.71 | ||
The support effect of Cs2O and SiO2 over alumina on diastereoselective hydrogenation of CDH2 to CDH2-A is shown in Fig. 14. The support effect on the orientation of the alkaloid on Pt during hydrogenation of its heteroaromatic ring is depicted in Fig. 15.
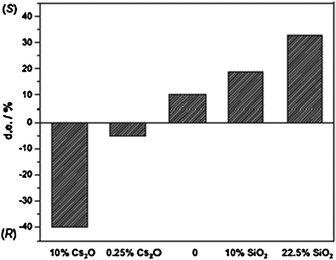 | ||
| Fig. 14 Diastereomeric excess (d.e.) in the hydrogenation of CDH2 to CDH6-A as a function of the promoter content in the alumina support.71 | ||
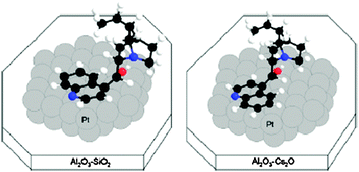 | ||
| Fig. 15 Schematic drawing of the probable adsorption geometry of CDH2 on a Pt (111) model surface located on acidic and basic supports. H—white, C—black, N—blue (gray), O—red (gray).71 | ||
The past few years have seen a development in the synthesis and characterization of mixed metal oxides and their versatile applications, making them very challenging and demanding in field catalysis science and technology.73–84
Future scope of mixed metal oxides
Mixed metal oxides are the heart of heterogeneous catalysis and are being used in chemical industries, pharmaceutical industries as well as in academic research. They are modified day by day and are applied in several fields. Various combinations of noble metals with basic oxides can be used for Sonogashira reaction, Suzuki coupling, other important C–C, C–O, C–S and C–N bond forming reactions and some industrially important multicomponent reactions. Ferrites containing mixed metal oxides as well as pervoskites85 (ABO3) can be used for various name reactions and other important organic conversions. These ferrites containing mixed metal oxides can be separated magnetically and reused for next cycles.In the field of mixed oxides, there are many kinds of fundamental structures (of double oxides and salts of oxoacids) and numerous compositions for each structure are possible. Scientists are reporting novel mixed metal oxides in various international journals of repute and those mixed metal oxides exhibit various valuable functions derived from a variety of electronic, magnetic and chemical properties. The mixed metal oxides shall be used in various fields, especially in catalysis and organic synthesis.
Conclusions
The aim of this review is to illustrate the significance of a variety of mixed metal oxides as catalysts and catalyst supports as employed for a wide variety of catalytic applications both in the liquid and gaseous phases. As discussed, these mixed metal oxides represent an interesting class of heterogeneous catalysts and catalyst supports for alkali, rare earth and noble metals which have been widely employed in reductions, oxidation, one-pot synthesis reactions, one-pot Mannich reactions, alkylations, condensations, deprotection, cycloadditions, hydroxylations, dehydration, dehydrogenations, transesterification, reactions involving biomimetic oxygen-evolving catalysts and other important C–C bond forming reactions. These mixed metal oxides are going to be modified in future and find numerous applications in the coming years both as catalysts and catalyst carriers.Acknowledgements
We are grateful to CSIR, New Delhi, India, for providing financial support for the research work. Manoj Gawande is thankful to Faculty of Science and Technology, Lisbon, Portugal, for the award of Research grants (SFRH/BPD/64934/2009).Notes and references
- (a) H. H. Kung, Transition Metal Oxides: Surface Chemistry and Catalysis; Stud. Surf. Sci. Catal., Elsevier, Amsterdam, 1989, vol. 45, pp. 1–277 Search PubMed; (b) V. E. Henrich and P. A. Cox, The Surface Science of Metal Oxides, Cambridge University Press, Cambridge, UK, 1994 Search PubMed; (c) C. Noguera, Physics and Chemistry at Oxide Surface, Cambridge University Press, Cambridge, UK, 1996 Search PubMed; (d) S. U. Sonavane, M. B. Gawande, S. S. Deshpande and R. V. Jayaram, Catal. Commun., 2007, 8, 1803 CrossRef CAS.
- G. C. Bond, Catalysis by Metals, Academic Press, New York, 1962 Search PubMed.
- (a) C. Mercier, P. Chabardes, Heterog. Catal. Fine Chem., III, Stud. Surf. Sci. Catal., 1993, vol. 78, p. 677 Search PubMed; (b) Y. Izumi, N. Natsume, H. Takamine, I. Tamaoki and K. Urabe, Bull. Chem. Soc. Jpn., 1989, 62, 2159 CrossRef CAS; (c) K. Tanabe and W. F. Holderich, Appl. Catal., A, 1999, 181, 399 CrossRef CAS; (d) B. M. Reddy and A. Khan, Catal. Rev. Sci. Eng., 2005, 47, 257 CrossRef CAS.
- S. U. Sonavane and R. V. Jayaram, Synlett, 2004, 146 CAS.
- M. A. Pena and J. L. G. Fierro, Chem. Rev., 2001, 101, 1981 CrossRef CAS.
- (a) H. Kanai, Y. Ikeda and S. Imamura, Appl. Catal., A, 2003, 247, 185 CrossRef CAS; (b) S. K. Samantaray and K. Parida, Appl. Catal., A, 2001, 220, 9 CrossRef CAS.
- (a) S. D. Jackson and J. S. J. Hargreaves, Metal Oxide Catalysis, Wiley-VCH, 2008, ISBN-10: 3527318151 Search PubMed; (b) H. Cui, M. Zayat and D. Levy, J. Sol-Gel Sci. Technol., 2005, 35, 175 CrossRef CAS; (c) A. Elia, P. Martin Aispuro, N. Quaranta, J. M. Martın-Martınez and P. Vazquez, Macromol. Symp., 2011, 301, 136 CrossRef CAS; (d) Y. J. Kim, S. B. Rawal, S. D. Sung and W. I. Lee, Bull. Korean Chem. Soc., 2011, 32, 141 CrossRef CAS; (e) P. F. Fulvio, S. Pikus and M. Jaroniec, ACS Appl. Mater. Interfaces, 2010, 2, 134 CrossRef CAS; (f) B. M. Reddy, B. Chowdhury and P. G. Smirniotis, Appl. Catal., A, 2001, 211, 19 CrossRef CAS; (g) G. Sankar, C. N. R. Rao and T. Rayment, J. Mater. Chem., 1991, 1, 299 RSC; (h) A. Tang, H. Yang and X. Zhang, Int. J. Phys. Sci., 2006, 1, 101 Search PubMed; (i) V. V. Zyryanov, Inorg. Mater., 2003, 39, 1163 CrossRef CAS; (j) S. Ajaikumar and A. Pandurangan, Appl. Catal., A, 2009, 357, 184 CrossRef CAS; (k) W. C. Sheets, E. S. Stampler, H. Kabbour, M. I. Bertoni, L. Cario, T. O. Mason, T. J. Marks and K. R. Poeppelmeier, Inorg. Chem., 2007, 46, 10741 CrossRef CAS; (l) D. Jiang, L. Su, L. Ma, N. Yao, X. Xu, H. Tang and X. Li, Appl. Surf. Sci., 2010, 256, 3216 CrossRef CAS; (m) B. M. Reddy and I. Ganesh, J. Mol. Catal. A: Chem., 2001, 169, 207 CrossRef CAS; (n) I. E. Wachs and C. A. Roberts, Chem. Soc. Rev., 2010, 39, 5002 RSC.
- J. J. Yu, X. P. Wang, L. D. Li, Z. P. Hao, Z. P. Xu and G. Q. (Max) Lue, Adv. Funct. Mater., 2007, 17, 3598 CrossRef CAS.
- C. Lucarelli, P. Moggi, F. Cavani and M. Devillers, Appl. Catal., A, 2007, 325, 244 CrossRef CAS.
- L. J. I. Coleman, W. Epling, R. R. Hudgins and E. Croiset, Appl. Catal., A, 2009, 363, 52 CrossRef CAS.
- S. Blanco, S. R. G. Carrazan and V. Rives, Appl. Catal., A, 2008, 342, 93 CrossRef CAS.
- C. Liu, Z. Zhao, X. yang, X. Ye and Y. Wu, Chem. Commun., 1996, 1019 RSC.
- C.-H. Wang and H.-S. Weng, Ind. Eng. Chem. Res., 1997, 36, 2537 CrossRef CAS.
- B. Jongsomjit, S. Ngamposri and P. Praserthdam, Ind. Eng. Chem. Res., 2005, 44, 9059 CrossRef CAS.
- C. Y. Ma, Z. Mu, J. Jun Li, Y. G. Jin, J. Cheng, G. Q. Lu, Z. P. Hao and S. Z. Qiao, J. Am. Chem. Soc., 2010, 132, 2608 CrossRef CAS.
- F. J. Perez-Alonso, I. Melián-Cabrera, M. L. Granados, F. Kapteijn and J. L. G. Fierro, J. Catal., 2006, 239, 340 CrossRef CAS.
- (a) M. R. Goldwasser, M. E. Rivas, M. L. Lugo, E. Pietri, J. Perez-Zurita, M. L. Cubeiro, A. Griboval-Constant and G. Leclercq, Catal. Today, 2005, 107, 106 CrossRef; (b) J. A. Rodriguez and D. Stacchiola, Phys. Chem. Chem. Phys., 2010, 12, 9557 RSC.
- (a) A. V. Biradar, S. B. Umbarkar and M. K. Dongare, Appl. Catal., A, 2005, 285, 190 CrossRef CAS; (b) A. P. Amrute, A. Bordoloi, N. Lucas, K. Palraj and S. B. Halligudi, Catal. Lett., 2008, 126, 286 CrossRef CAS.
- A. S. Kulkarni and R. V. Jayaram, J. Mol. Catal. A: Chem., 2004, 223, 107 CrossRef CAS.
- J. G. Chandorkar, S. B. Umbarkar, C. V. Rode, V. B. Kotwal and M. K. Dongare, Catal. Commun., 2007, 8, 1550 CrossRef CAS.
- (a) F. Wang, G. Yang, W. Zhang, W. Wu and J. Xu, Chem. Commun., 2003, 1172–1173 RSC; (b) F. Wang, G. Yang, W. Zhang, W. Wu and J. Xu, Adv. Synth. Catal., 2004, 346, 633 CrossRef CAS.
- G. Yang, W. Zhu, P. Zhang, H. Xue, W. Wang, J. Tian and M. Song, Adv. Synth. Catal., 2008, 350, 542 CrossRef CAS.
- A. S. Kulkarni and R. V. Jayaram, Appl. Catal., A, 2003, 252, 225 CrossRef CAS.
- M. L. Kantam, H. Kochkar, J.-M. Clacens, B. Veldurthy, A. Garcia-Ruiz and F. Figueras, Appl. Catal., B, 2005, 55, 177 CrossRef CAS.
- K. Yamaguchi, K. Ebitani, T. Yoshida, H. Yoshida and K. Kaneda, J. Am. Chem. Soc., 1999, 121, 4526 CrossRef CAS.
- M. B. Gawande, S. S. Deshpande, J. R. Satam and R. V. Jayaram, Catal. Commun., 2007, 8, 576 CrossRef CAS.
- S. J. Singh and R. V. Jayaram, Catal. Commun., 2009, 10, 2004 CrossRef CAS.
- S. S. Deshpande, S. U. Sonavane and R. V. Jayaram, Catal. Commun., 2008, 9, 639 CrossRef CAS.
- M. B. Gawande, S. S. Deshpande, S. U. Sonavane and R. V. Jayaram, J. Mol. Catal. A: Chem., 2005, 241, 151 CrossRef CAS.
- N. S. Babu, N. Pasha, K. T. Venkateswara Rao, P. S. Sai Prasad and N. Lingaiah, Tetrahedron Lett., 2008, 49, 2730 CrossRef CAS.
- S. J. Singh and R. V. Jayaram, Tetrahedron Lett., 2008, 49, 4249 CrossRef CAS.
- A. Kiss, Z. Hell and M. Balint, Org. Biomol. Chem., 2010, 8, 331 CAS.
- Q. Shi, R. Lu, L. Lu, X. Fu and D. Zhao, Adv. Synth. Catal., 2007, 349, 1877 CrossRef CAS.
- M. Kang, E. D. Park, J. M. Kim and J. E. Yie, Catal. Today, 2006, 111, 236 CrossRef CAS.
- N. Li, C. Descorme and M. Besson, Appl. Catal., B, 2007, 76, 92 CrossRef CAS.
- S. S. Deshpande and R. V. Jayaram, Catal. Commun., 2008, 9, 186 CrossRef CAS.
- Q. Zhuang and J. M. Miller, Appl. Catal., A, 2001, 209, L1 CrossRef CAS.
- M. Crivello, C. Pérez, E. Herrero, G. Ghione, S. Casuscelli and E. Rodrıguez-Castellon, Catal. Today, 2005, 107–108, 215 CrossRef CAS.
- A. K. Singh and S. D. Fernando, Energy Fuels, 2009, 23, 5160 CrossRef CAS.
- (a) C. Wang and H. Weng, Ind. Eng. Chem. Res., 1997, 36, 2537 CrossRef CAS; (b) D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362 CrossRef CAS; (c) G. Centi and S. Perathoner, Catal. Rev. Sci. Eng., 1998, 40, 175 CrossRef CAS; (d) M. A. Carreon and V. V. Guliants, Eur. J. Inorg. Chem., 2005, 27 CrossRef CAS; (e) Q. Liu, L. Wang, M. Chen, Y. Liu, Y. Ca, H. He and K. Fan, Catal. Lett., 2008, 121, 144 CrossRef CAS; (f) G. M. Dhar, B. N. Srinivas, M. S. Rana, M. Kumar and S. K. Maity, Catal. Today, 2003, 86, 45 CrossRef CAS; (g) M. Fernandez-Garcia, A. Martınez-Arias, J. C. Hanson and J. A. Rodriguez, Chem. Rev., 2004, 104, 4063 CrossRef CAS; (h) J. Sun, K. Zhu, F. Gao, C. Wang, J. Liu, C. H. F. Peden and Y. Wang, J. Am. Chem. Soc., 2011, 132, 356 Search PubMed.
- (a) A. Cordova, Acc. Chem. Res., 2004, 37, 102 CrossRef CAS; (b) A. Cordova, Chem.–Eur. J., 2004, 10, 1987 CrossRef CAS; (c) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1045 CrossRef CAS; (d) S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069 CrossRef CAS; (e) D. M. Nagrik, D. M. Ambhore and M. B. Gawande, Int. J. Chem., 2010, 2, 98 CAS.
- (a) B. Veldurthy, J. M. Clacens and F. Figueras, Adv. Synth. Catal., 2005, 347, 767 CrossRef CAS; (b) C. Carlini, M. Marchionna, M. Noviello, A. M. R. Gallettia, G. Sbrana, F. Basile and A. Vaccari, J. Mol. Catal. A: Chem., 2005, 232, 13 CrossRef CAS; (c) G. Mestl, Top. Catal., 2006, 38, 69 CrossRef CAS.
- (a) S. Gallardo, T. Aida and H. Niiyama, Korean J. Chem. Eng., 1998, 15, 480 CrossRef CAS; (b) G. K. Reddy, G. Thrimurthulu and B. M. Reddy, Catal. Surv. Asia, 2009, 13, 237 CrossRef CAS; (c) G. Zhou, P. R. Shah and R. J. Gorte, Catal. Lett., 2008, 120, 191 CrossRef CAS; (d) A. E. Nelson and K. H. Schulz, Appl. Surf. Sci., 2003, 210, 206 CrossRef CAS; (e) A. Trovarelli, Catal. Rev. Sci. Eng., 1996, 38, 439 CrossRef CAS; (f) A. Trovarelli, Catalysis by Ceria and Related Materials, Catalytic Science Series, ed. G. J. Hutchings, Imperial College Press, London, 2002, vol. 2 Search PubMed; (g) J. Kaspar and P. Fornasiero, J. Solid State Chem., 2003, 171, 19 CrossRef CAS; (h) J. Kaspar, P. Fornasiero, G. Balducci, R. Di Monte, N. Hickey and V. Sergo, Inorg. Chim. Acta, 2003, 349, 217 CrossRef CAS; (i) H. Vidal, S. Bernal, J. Kaspar, M. Pijolat, V. Perrichon, G. Blanco, J. M. Pintado, R. T. Baker, G. Colon and F. Fally, Catal. Today, 1999, 54, 93 CrossRef CAS; (j) P. Fornasiero, G. Balducci, J. Kaspar, S. Meriani, R. Di Monte and M. Graziani, Catal. Today, 1996, 29, 47 CrossRef CAS; (k) R. Di Monte and J. Kaspar, Catal. Today, 2005, 100, 27 CrossRef CAS.
- (a) J. Kaspar, P. Fornasiero and M. Graziani, Catal. Today, 1999, 50, 285 CrossRef CAS; (b) R. Di Monte and J. Kaspar, Top. Catal., 2004, 28, 47 CrossRef CAS; (c) R. Di Monte and J. Kaspar, J. Mater. Chem., 2005, 15, 633 RSC and references cited therein; (d) S. Otsuka-Yao, H. Morikawa, N. Izu and K. Okuda, Nippon Kinzoku Gakkaishi, 1995, 59, 1237 CAS.
- Production-Integrated Environmental Protection and Waste Management in the Chemical Industry, ed. C. Christ, Wiley-VCH, Weinheim, 1999 Search PubMed.
- (a) Green Chemistry Frontiers in Chemical Synthesis and Processes, ed. P. T. Anatas and T. C. Williamson, Oxford Univ. Press, Oxford, 1998 Search PubMed; (b) R. K. Pandey and R. Kumar, Catal. Commun., 2007, 8, 379 CrossRef CAS; (c) M. B. Gawande and P. S. Branco, Green Chem., 2011, 13, 3355 RSC.
- R. A. Sheldon, Chem. Ind. (London), 1992, 903 CAS and 1997, 12.
- M. L. Kantam, V. Balasubrahmanyam, K. B. Shiva Kumar, G. T. Venkanna and F. Figueras, Adv. Synth. Catal., 2007, 349, 1887 CrossRef CAS.
- (a) M. B. Gawande and R. V. Jayaram, Catal. Commun., 2006, 7, 931 CrossRef CAS; (b) P. D. L. Cruz, E. Diez-Barra, A. Loupy and F. Lunga, Tetrahedron Lett., 1996, 37, 1113 CrossRef; (c) P. S. Rao and R. V. Venkatratnam, Tetrahedron Lett., 1991, 32, 5821 CrossRef CAS; (d) T. I. Reddy and R. S. Varma, Tetrahedron Lett., 1997, 38, 1721 CrossRef CAS; (e) M. B. Gawande, D. M. Nagrik and D. M. Ambhore, Lett. Org. Chem., 2012, 9, 12 CrossRef CAS.
- R. A. Sheldon and J. K. Kochi, Metal-Catalyzed Oxidation of Organic Compounds, Academic Press, New York, 1981 Search PubMed.
- F.-Z. Su, Y.-M. Liu, L.-C. Wang, Y. Cao, H.-Y. He and K.-N. Fan, Angew. Chem., 2008, 120, 340 CrossRef.
- H. Miyamura, R. Matsubara, Y. Miyazaki and S. Kobayashi, Angew. Chem., 2007, 119, 4229 CrossRef.
- H. Miyamura, R. Matsubara, Y. Miyazaki and S. Kobayashi, Angew. Chem., Int. Ed., 2007, 46, 4151 CrossRef CAS.
- S. B. Rathod, A. B. Gambhire, B. R. Arbad and M. K. Lande, Bull. Korean Chem. Soc., 2010, 31(2), 339 CrossRef CAS.
- J. Tantirungrotechai, P. Chotmongkolsap and M. Pohmakotr, Microporous Mesoporous Mater., 2010, 128, 41 CrossRef CAS.
- B. Bridier and J. Perez-Ramırez, J. Am. Chem. Soc., 2010, 132, 4321 CrossRef CAS.
- P. Kumar, Y. Sun and R. O. Idem, Energy Fuels, 2008, 22, 3575 CrossRef CAS.
- K. Karásková, L. Obalová, K. Jirátová and F. Kovanda, Chem. Eng. J., 2010, 160, 480 CrossRef.
- X. Xiang, H. I. Hima, H. Wang and F. Li, Chem. Mater., 2008, 20, 1173 CrossRef CAS.
- S. Wang, Y. Zhang and H. Liu, Chem.–Asian J., 2010, 5, 1100 CrossRef CAS.
- A. Kubacka, A. Fuerte, A. Martınez-Arias and M. Fernandez-Garcıa, Appl. Catal., B, 2007, 74, 26 CrossRef CAS.
- F. Ocampo, B. Louis and A. Roger, Appl. Catal., A, 2009, 369, 90 CrossRef CAS.
- M. M. Najafpour, T. Ehrenberg, M. Wiechen and P. Kurz, Angew. Chem., Int. Ed., 2010, 49, 2233 CrossRef CAS.
- P. Haider and A. Baiker, J. Catal., 2007, 248, 175 CrossRef CAS.
- F. A. Wadaani, E. F. Kozhevnikova and I. V. Kozhevnikov, J. Catal., 2008, 257, 199 CrossRef.
- M. L. Kantam, K. B. Shiva Kumar, V. Balasubramanyam, G. T. Venkanna and F. Figueras, J. Mol. Catal. A: Chem., 2010, 321, 10 CrossRef CAS.
- A. Cwik, Z. Hell and F. Figueras, Tetrahedron Lett., 2006, 47, 3023 CrossRef CAS.
- (a) A. R. Porcari, R. V. Devivar, L. S. Kucera, J. C. Drach and L. B. Townsend, J. Med. Chem., 1998, 41, 1252 CrossRef CAS; (b) A. W. White, N. J. Curtin, B. W. Eastman, B. T. Golding, Z. Hostomsky, S. Kyle, K. A. Maegley, D. J. Skalitzki, S. E. Webber, X.-H. Yu and R. J. Griffin, Bioorg. Med. Chem. Lett., 2004, 14, 2433 CrossRef CAS.
- K. D. Parghi and R. V. Jayaram, Catal. Commun., 2010, 11, 1205 CrossRef CAS.
- (a) J. Chang, A. Wang, J. Liu, X. Li and Y. Hu, Catal. Today, 2010, 149, 122 CrossRef CAS; (b) M. B. Gawande, P. S. Branco, K. Parghi, J. J. Shrikhande, R. K. Pandey, C. A. A. Ghumman, N. Bundaleski, O. M. N. D. Teodoro and R. V. Jayaram, Catal. Sci. Technol., 2011, 1, 1653–1664 RSC.
- E. Schmidt, F. Hoxha, T. Mallat and A. Baiker, J. Catal., 2010, 274, 117 CrossRef CAS.
- B. Schimmoeller, F. Hoxha, T. Mallat, F. Krumeich, S. E. Pratsinis and A. Baiker, Appl. Catal., A, 2010, 374, 48 CrossRef CAS.
- I. E. Wachs, Catal. Today, 2005, 100, 79 CrossRef CAS.
- S. Tanasoi, G. Mitran, N. Tanchoux, T. Cacciaguerra, F. Fajula, I. Sandulescu, D. Tichit and I.-C. Marcu, Appl. Catal., A, 2011, 395, 78 CrossRef CAS.
- R. Di Monte and J. Kaspar, Catal. Today, 2005, 100, 27 CrossRef CAS.
- S. M. Saqer, D. I. Kondarides and X. E. Verykios, Appl. Catal., B, 2011, 103, 275 CrossRef CAS.
- V. I. Sobolev and K. Y. Koltunov, ChemCatChem, 2011, 3, 1143 CrossRef CAS.
- P. Bandyopadhyay, M. Sathe, G. K. Prasad, P. Sharma and M. P. Kaushik, J. Mol. Catal. A: Chem., 2011, 341, 77 CrossRef CAS.
- J. Geserick, T. Froschl, N. Husing, G. Kucerova, M. Makosch, T. Diemant, S. Eckle and R. J. Behm, Dalton Trans., 2011, 40, 3269 RSC.
- K. Taejin and I. E. Wachs, Metal Oxide Catalysis, ed. J. Hargreaves and S. D. Jackson, Wiley, New York, 2009 Search PubMed.
- S. V. Merzlikin, N. N. Tolkachev, L. E. Briand, T. Strunskus, C. Woll, I. E. Wachs and W. Grunert, Angew. Chem., Int. Ed., 2010, 49, 8037 CrossRef CAS.
- J. B. Park, J. Graciani, J. Evans, D. Stacchiola, S. Ma, P. Liu, A. Nambu, J. F. Sanz, J. Hrbek and J. A. Rodriguez, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 4975 CrossRef CAS.
- H. Tian, C. A. Roberts and I. E. Wachs, J. Phys. Chem. C, 2010, 114, 14110 CAS.
- W. Zhou, E. I. Rossa-Medgaarden, W. V. Knowles, M. S. Wong, I. E. Wachs and C. J. Kiely, Nat. Chem., 2009, 1, 722 CrossRef CAS.
- J. E. Tasca, A. Ponzinibbio, G. Diaz, R. D. Bravo, A. Lavat and M. G. González, Top. Catal., 2010, 53, 1087 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |