Material science for the support design: a powerful challenge for catalysis
Alberto
Villa
,
Marco
Schiavoni
and
Laura
Prati
*
Dipartimento di Chimica Inorganica Metallorganica e Analitica L. Malatesta, Università degli Studi di Milano, via Venezian 21, 20133 Milano, Italy. E-mail: Laura.Prati@unimi.it; Fax: +39 02503 14405; Tel: +39 02503 14357
First published on 13th January 2012
Abstract
The use of heterogeneous catalytic systems for the development of low environmental impact processes has led to an enlargement of studies in this field, especially in order to optimize the selectivity of the reactions. This contribution aims to provide an overview of the role of the supporting materials in determining the activity/selectivity of the catalytic material and to highlight the perspective possibilities of modifying the surface or bulk properties of the support in order to optimize the catalytic performances.
1. Heterogeneous and heterogenized catalytic processes
Catalytic reactions can be historically divided into heterogeneous and homogeneous systems. Although homogeneous systems are normally more selective and easy to study from a mechanistic point of view, heterogeneous catalysts have their main strength in their easier recyclability and therefore higher productivity. Particularly this latter point opens the possibility of a future in which heterogeneous catalysis is used as a green alternative to stoichiometric processes by reducing waste and thus providing a lower environmental impact. However, it clearly appears that, from an applicative point of view, one of the most important aspects is the selectivity. Therefore, a lot of studies were devoted in modifying the catalytic systems in order to optimize the productivity of a single product. Attempts were made to immobilize the homogeneous catalyst and sometimes positive results were achieved. However these studies focused mainly on the stability and activity of the complex, in most cases simply using the support as a device for providing the corresponding heterogeneous system.A different approach was used when an active phase, especially in metal catalysed reactions, is directly deposited on a support. As summarized in Fig. 1, many parameters play some role and this is not always easy to disentangle. In fact the support properties can develop different interactions with the metallic particles dispersed on or in it, thus modifying both their electronic and structural properties. In addition the support can also provide different anchoring sites for the reactant, acting as an active and sometimes reacting surface (bifunctional catalysts).
 | ||
| Fig. 1 Main factors determining activity and selectivity of heterogeneous catalytic systems based on supported metals. | ||
There are a lot of examples where it is clear that the supporting material plays an important role on the catalytic performance. However, the comparison among different data in the literature is very hard, as it is quite usual to find different reaction conditions or other parameters, such as active phase dispersion or nature of the reactant. Thus in the present perspective we focused our attention on general trends that have been recognized through a lot of experimental and theoretical studies in the cases of reactions with H2 or O2 mainly in the liquid phase, even if, due to the presence of different solvents and different reaction conditions, finding general trends is hard work.
Hydrogenation and oxidation/dehydrogenation reactions are typically metal catalyzed reactions. Thus, as summarized in Fig. 1, the activity/selectivity of a catalyst is dominantly ruled by the nature of the metal at constant reaction conditions. However the supporting material has been recognized to have an active role, either for physically stabilizing the active phase (metallic) dispersion (i.e. metal particle dimension and its exposure to the surface) or for modifying its electronic properties. In the following sections we will consider the different role played by the different textural (bulk) or the surface properties of the materials.
2. Bulk properties
There are a lot of examples that have indicated the support effect. Generally it can be seen by varying the supporting material in a single reaction. However, it should be noted that all the other parameters have to remain constant. This fact could be considered trivial, but it is not; in fact all the parameters are interconnected. By varying the support, but using the same procedure, a really different metal dispersion can be obtained. The case of Au as the metal is representative: Au deposited by deposition–precipitation on oxides like Al2O3 or TiO2 can be readily obtained with a mean size of 1–3 nm, whereas on active carbon large aggregates are formed.1,2 Obviously the different dispersion leads to a different activity (the smaller particles are generally expected to be more active for their increased exposed surface3) but no information about the support effect can be derived. In fact, conversely, different methodologies used for depositing Au on the same active carbon produced catalysts that behave very differently in the ethylene glycol selective oxidation. As shown in Table 1, the activity varied as a function of the metal particle size revealed by HRTEM (Table 1).For metals such as Pd or Pt, largely applied in liquid phase catalytic reactions, the metal dispersion appeared not so dependent on the support. Using these metals nanosized NPs of comparable size regardless the support nature are obtained quite easily and therefore the real support effect can be more easily highlighted.
When catalytic processes are carried out in a condensed phase, transport mass limitation can also limit the activity of metal particles less exposed. An example of this phenomenon can be found in the activity of metal nanoparticles on microporous (like active carbon) versus non-porous or mesoporous supports (like oxides), where diffusional problems are of less importance. Indeed, in the liquid phase oxidation of ethylene glycol, AuNPs of medium size centred at 7 nm were more active than those of smaller particle size. In glycerol oxidation similarly sized 2.9 nm AuNPs appeared very poorly active when the surface exposition (%at) decreases on different MgAl2O4 (Table 2).5,6
The negative effect of the microporosity of activated carbon has also been observed by comparing PtNPs supported on active carbon and carbon nanotubes where the enhanced activity of the latter has been addressed to their mesoporous structure.7 In fact the mesoporous nanostructured support makes the mass transfer limitations less significant and gave far better activities than microporous activated carbons in the selective hydrogenation of cinnamaldehyde. By varying the internal diameter of the CNTs it has been reported that selective deposition of PtNPs outside the tubes is possible, thus obtaining even more active catalyst.8 The same conclusion was reached by the study of Pt supported on carbon microspheres of tailored surface area.9
However it has been shown that the activity of MNPs supported on activated carbons did not correlate directly with their exposure as revealed by XPS (%at). Some other parameters can be envisaged to rule up the activity such as the presence of phenol-type groups on activated carbons which prove an active role of the support.6 As outlined in Table 3, considering different activated carbons with similar textural properties but different distribution of functional groups, it was observed that activity of similarly sized Au particles (about 11 nm) increases by increasing the phenol groups despite the decreasing of Au exposure (%Au/C) measured by XPS.
The importance of functional groups in carbonaceous supports will be discussed in more detail in the next section, but it should be stressed that they can deeply influence the activity of the MNPs deposited on them. The surface acidic groups, which have been recently observed to have a detrimental effect on the activity of AuNPs in the glycerol liquid phase oxidation, appeared particularly important.10 Basic oxygen-free supports characterized by a high density of free π-electrons lead to more active catalysts. However, a comparative study between AuNPs supported on active carbon and carbon nitride (C3N4) revealed the importance of oxygenated groups in the O2 activation step.11 PtNPs in the range 1.4–3 nm supported on graphite, a typical scarcely functionalized support, appeared to be less active than when supported on active carbon or fibrils in the selective oxidation of carbohydrates.12 This behaviour has been attributed to the different hydrophilicity, i.e. different density of surface groups, of the support which enhances the adsorption of the substrate and the affinity with the aqueous phase.
Active carbon represented one of the best supports for MNPs for liquid phase applications, both in terms of activity and economy of the process. Indeed, PtNPs were far more active on AC than on oxide and Mordenite in the base-free oxidation of glycerol.13 However, it should be mentioned that when bimetallic AuPt NPs are used, the catalytic activity was inverted and AuPt on Mordenite showed a higher activity than the corresponding catalyst on AC.
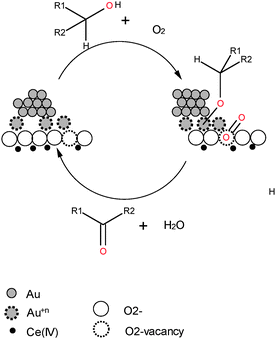
To avoid internal diffusional problems a lot of effort has also been put into the design of oxide supports for which the textural properties can avoid the mass transfer limitation, i.e. materials with controlled porosity or external surface area. In this field the development of synthetic methods for preparing nanoscaled oxides has been successful starting from the first report of Corma on nanoCeO2.14,15 A lot of studies have been performed on Au supported catalysts. With respect to conventional ceria, the nanomaterial of about 5 nm interacts with AuNPs of 2–5 nm promoting the formation of positive oxidation states of gold (Fig. 2).
This interaction leads to a twofold increase of activity in the alcohol oxidation without any solvent or base added. Au on nanoCeO2 showed a high selectivity toward conjugated ketones in the selective oxidation of allylic alcohols.16 Moreover AuNPs supported on nanocube CeO2 appeared more active than when supported on nanorods which apparently stabilized the gold cations which replaced Ce ions on the surface plane of the nanorods. The latter, upon reduction, was very active in gas phase CO oxidation.17 The influence of the support structure on the activity of metallic nanoparticles has been also recognized in the case of Pt on CeO2.18 This latter study identified two types of interactions between Pt and the support: the one which implies electron-transfer from the metal to the support; the second where there is an oxygen transfer from ceria to Pt. The electron transfer is favourable on CeO2 irrespective of its morphology. On the contrary, the oxygen transfer requires the presence of nanostructured ceria in close contact with Pt. This means that this is an inherently nanoscale effect.
A nanoscale effect was also seen in the case of NiO supported AuNPs, where a cooperative effect between Au and the support has been recognized only when the support particles are reduced at the nanoscale (Fig. 3).19
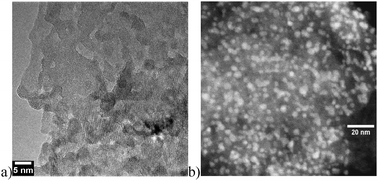 | ||
| Fig. 3 (a) TEM image of nNiO and (b) STEM image of Au/nNiO. | ||
The effect is indeed relevant in the benzyl alcohol oxidation and was not attributed just to the increase of basic site density revealed in the case of nNiO with respect to NiO (Table 4). It has been shown in fact that Au on MgO, more basic than nNiO, is by far less reactive.
Oxide supports can also provide alternative catalytic sites. The γ form of Ga2O3 has been shown to form a bifunctional catalyst when supporting AuNPs are able to catalyse the oxidative transformation of alcohols into the corresponding esters.20 In fact the support is able to catalyze the formation of the hemiacetal form of the intermediate carbonyl compound which can be readily transformed into the corresponding esters through the elimination of water. This reaction takes place in alcoholic medium whereas when a solid solution of γ-Ga2O3 and Al2O3 is used as the support in a solvent-free reaction, the final product is represented by the aldehyde.21
TiO2 has been also largely used as support for noble metals in liquid phase reactions.22–31 However only few studies reported on the support effect for these catalytic applications.
In the case of Au the aerobic alcohol oxidation appeared not very sensitive to the type of oxide support in the presence of base additives32–34 unlike the well-studied CO oxidation carried out in the gas phase.35,36 This finding has been explained by suggesting that only in the case of carbon, the support is directly involved in the rate determining step.37
TiO2 was recognized to establish strong metal–support interactions (SMSI) in the case of Pd only when it is nanosized, whereas SMSI were not detected in the case of micro-sized TiO2.38 SMSI enhanced the specific activity of Pt on TiO2 and also shifted the selectivity of the hydrogenation of unsaturated aldehydes or ketones towards unsaturated compounds.39 Moreover, it has been recognized that SMSI distinguished the catalysts. That is to say that when weak metal–support interactions (WMSI) are present, the catalysts behaviour has not been influenced by the support.40 Ni supported on different Al2O3 (γ or κ) was shown to develop SMSI in the case of γ-Al2O3 whereas only WMSI was formed on κ-Al2O3 due to a different distribution of tetrahedral vacancies.41 From a catalytic point of view, in the selective hydrogenation of isoprene, SMSI provided a more active, more stable and more selective catalyst. The activity of Pd on ZnAl2O4 was analogously improved in the selective hydrogenation of heptyne when SMI are increased.42 In this case the support–metal interactions increased by increasing the crystallite size of the support which corresponded to an increase of defects. The metal–support interactions were invoked to explain the activity of Ni on SiO2 or on Al2O3 (non-reducible oxide) in phenylacetylene hydrogenation.43 Structural metal–support interactions as well as electronic effects help to explain the activity of the catalysts, Ni on SiO2 being the most active.
On TiC it was reported that Au showed an enhanced ability to dissociate H2, but compulsory conditions are the small dimension and the thickness of Au crystallites.44 The active sites involve the Au atoms at the particle edge rather than the ones from the central region; therefore they are in direct contact with the underlying substrate. Small particle size and high dispersion (i.e. low loading) maximized these types of atoms and therefore the catalytic activity.
A tunable inorganic support that has the advantages of a non-porous structure, weak acid–base properties and the possibility to immobilize well-defined monomeric active species is Ca10(PO4)6OH2 hydroxyapatite (HAP). It has been used as support for Pd.45,46 Monomeric PdCl2 species chemisorbed on the HAP surface can be readily transformed into Pd(0) nonoclusters with a narrow particle size distribution in the presence of alcohol. The catalyst showed a remarkably high turn-over number (TON). Ru on HAP was also investigated and attracted particular interest for the high selectivity to aldehyde in the oxidation of alcohol.47,48 This peculiar behaviour was attributed to the coexistence of isolated monomeric Ru3+ species and hydrated RuOx-like phase which shows high redox activity. The catalyst performance can be more than tripled by organically modifying the HAP.49 Efficient modifiers are prolinol, proline, benzoic acid that can form strong H-bonds with the surface OH and phosphate groups of the HAP.
Layered inorganic supports represent another class of well studied materials. Hydrotalcite (HT, Mg6Al2(OH)16CO3·nH2O) is a layered anionic clay consisting of a positively charged two-dimensional brucite layer with anionic species such as hydroxide and carbonate located in the interlayer.50 Its anion exchange ability, its tunability of basic sites and its adsorption capability attracted attention for catalysis. The synergistic effect between the metal and the basic sites of HT can lead to high performance heterogeneous gold catalyst for alcohol dehydrogenation and oxidation which allow avoiding the use of base (Fig. 4).41–58
 | ||
| Fig. 4 Hydrotalcite structure. | ||
Mesostructured silicas such as SBA-15, MCM-41 and MCF with different pore sizes have been used for supporting PdNPs (Fig. 5).59 The allocation of PdNPs in/on the mesostructured silica supports played an important role in the liquid phase hydrogenation of phenylacetylene. The pore diameters of SBA-15 allowed depositing PdNPs inside the pores. This geometrical confinement resulted in a decrease of catalytic activity but in an enhanced selectivity toward styrene. SBA-15 was also modified by the use of dendrimers (organic–inorganic hybrid composite) where AuNPs can be deposited inside the channels.60 Interestingly the size and the location of AuNPs can be tuned by controlling the ratio of Au precursors and the nature of dendrimers. These catalysts are active in the liquid phase oxidation of alcohols under very mild conditions.
 | ||
| Fig. 5 Typical SBA-15 structure (inset MCM-41). | ||
In the Pt catalyzed partial hydrogenation of cinnamaldehyde it was also found that the acidity (Lewis) of the support plays an important role in terms of both activity and (especially) selectivity even if cinnamaldehyde hydrogenation does not require acidic sites to be performed.61 Mesoporous SBA-15, Al-SBA-15 and Al2O3 were used as support for PtNPs of similar dimension (<2 nm). The presence of Lewis acidic sites close to the particles favours the adsorption of the reactant molecules via their polar C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond thus resulting in an enhanced selectivity toward cinnamyl alcohol. Conversely the hydrogenation of C
O bond thus resulting in an enhanced selectivity toward cinnamyl alcohol. Conversely the hydrogenation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond was favoured on SBA-15. The mesoporous structure of SBA-15 was also fruitfully used for avoiding metal (gold) leaching and particle aggregation (sintering) in the benzyl alcohol oxidation.62 The leaching in fact often represents a limiting aspect in the liquid phase reaction. The confinement of the metal particle inside the structure of SBA-15, even reducing the exposure of the metal (thus reducing their intrinsic activity), has a positive effect on the life-time of the catalyst and its recycling.
C bond was favoured on SBA-15. The mesoporous structure of SBA-15 was also fruitfully used for avoiding metal (gold) leaching and particle aggregation (sintering) in the benzyl alcohol oxidation.62 The leaching in fact often represents a limiting aspect in the liquid phase reaction. The confinement of the metal particle inside the structure of SBA-15, even reducing the exposure of the metal (thus reducing their intrinsic activity), has a positive effect on the life-time of the catalyst and its recycling.
Organic polymers have been also employed with the advantage to be suitable to modification during their preparation in terms of both functional groups and porosity (Fig. 6). To this class belong styrene polymers that are able to incarcerate metal nanoparticles (PI).
Polystyrene-based matrix is able to stabilize gold clusters by weak interactions and the cross-linking of the polymer matrix prevents the metal from leaching.63 To circumvent the limitation of the low loading of the metal, the same support was modified by the insertion of carbon black. This new material was able to be loaded at 1 wt% and showed a high stability on recycling.64 It should be noted that a comparative study between Au on nanoCeO2 and Au-PI revealed the superior activity of Au on nanoCeO2 in some reactions namely the aniline oxidation.65 However, as emphasized above this trend should be expected on the basis of metal nanoparticle accessibility.
Pt nanoparticles can be formed inside the nanocavities of hyper-cross-linked polystyrene by impregnation and subsequent reduction.66 This catalyst showed a remarkable stability, being stable over 15 recycles in the L-sorbose oxidation. Polymers could also act as a modifier though different functionalities available on their surface. An example can be provided by triazine-based polymers (CTF) that have been recently shown to be suitable supports for Pd nanoparticles in alcohol oxidation.67 Basic groups are able to stabilize the PdNPs against aggregation and the activity of the metal NPs was improved with respect to differently supported PdNPs like on active carbon.
The last emerging class of materials that should be mentioned is represented by the metal organic framework (MOF). Applications of this wide class of materials have been recently reviewed.68 MOFs are crystalline porous solids composed of a three dimensional network of metal ions held in place by multidentate organic molecules. Thus they can be active by themselves in a lot of reactions. However they have been also used as a host for metal nanoparticles thus providing a sort of mesoporous, functional materials able to physically stabilize the metal nanoparticles but also able to take part in the catalytic process. Examples can be found in alcohol oxidation where Au-containing MOF-5 prepared by solid grinding showed a remarkable activity in the absence of base.69 An electronic interaction between the metal centre and the support was believed to modify the activity of gold clusters in contrast to what happens when Au is supported on active carbon. In fact Au on MOF-5 yields a 69% conversion in the benzyl alcohol oxidation, whereas Au on AC yields only 12%. A favourable electronic effect has been also obtained using MIL-101, a Cr containing MOF characterized by a large surface area (ca. 3000 m2 g−1).70 The electron donation effects of aryl groups to the AuNPs are suggested to be the main reason for the observed high activity of Au/MIL-101 catalyst in the aerobic oxidation of alcohol under base-free conditions. MOF-5 represented a suitable support also for PtNPs which found application in vanillyl and piperonyl alcohols.71
3. Surface properties
The functionalization of catalytic support was of great interest in the last few years. The surface properties of the catalytic materials can be finely tuned by applying appropriate thermal or chemical treatments or by anchoring the desired functional groups. The introduction of functional groups on the surface can change the electronic surface state, modify the acid–base properties and influence the metal dispersion on the surface.72–78 In this paragraph we will focus our attention on the functionalization of two classes of support: carbonaceous materials and silica. Many studies have been done, indeed, on the effect of the functionalization of these materials as support for liquid phase oxidation and hydrogenation.Functionalization of supports, such as activated carbon, carbon nanotubes and carbon nanofibers, with oxygen groups plays a key role in catalyst preparation and in the catalytic performance. Oxidation with nitric acid increases the surface hydrophilicity, which substantially improved the surface wettability and therefore the interaction between the support and the metal precursors normally dispersed in the aqueous phase. Moreover, oxygen groups on the surface remarkably influence the catalytic activity and selectivity remarkably by tuning the preferential reactant adsorption and product desorption. The effect of functionalization (HNO3 65% at 373 K, 2 h) on CNTs and CNFs was reported to be the removal of the residual amorphous carbon from the surface and the introduction of oxygen functionalities such as COOH and OH groups, increasing the hydrophilic properties (Fig. 7).79
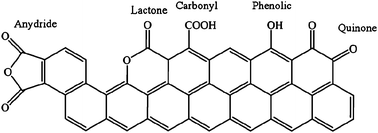 | ||
| Fig. 7 Possible oxygen functionalities introduced by HNO3 treatment. | ||
Importantly, the overall morphology of CNFs and CNTs has not been affected by the pretreatment. During the immobilization of Au preformed sol, oxygen functionalities also improve, with respect to untreated support, the stabilization of Au nanoparticles decreasing the discrepancy between the particle size in the sol and on the support after the immobilization step (Table 5).
| Catalyst | Au/Sol | Au/CNF | Au/CNFox | Au/CNT | Au/CNTox |
|---|---|---|---|---|---|
| Statistical median/nm | 2.45 | 3.80 | 3.65 | 4.61 | 3.83 |
| Standard deviation σ | 0.27 | 0.95 | 0.78 | 1.32 | 0.89 |
The effect of functionalization on catalytic performance in the liquid phase oxidation of glycerol, appeared as a slight increase in the activity (TOF from 648 h−1 to 742 h−1 for CNTs and from 182 h−1 to 186 h−1 for CNF), but, surprisingly, the selectivity to C3 products was boosted from 33% for Au/CNTs to 70% for oxidized Au/CNTs and from 56% for Au/CNFs to 66% for oxidized Au/CNF. The importance of oxygen functionalities was highlighted by Tang et al.80 They showed that Ru supported on functionalized carbon nanofibers by treatment with HNO3 65% and sulfuric acid 95–98% (393 K, 1 h) was very active in the benzyl alcohol oxidation and the partial removal of oxygen species by heat treatment led to a significant reduction of the catalytic conversion. The different Ru/CNF catalysts showed similar Ru particle size (3 nm), suggesting that the different amount of oxygen functionalities does not influence the particle size and distribution. Also in this case the pretreatment did not change the CNF morphology. Therefore, they proposed that a higher amount of carboxylic acid groups are responsible for the promotion of the catalytic activity. The authors attributed this phenomenon to a steric handicap caused by carboxylic groups to the adsorption of benzyl alcohol on the surface due to a reduced π–π interaction. This might lead to a relatively easier interaction of the alcoholic group in benzyl alcohol with carboxylic species through the hydrogen-bond, therefore activating the alcoholic group toward dehydrogenation.81,82 Ovejero et al. also noted that acid functionalization increases the graphitization degree of the CNTs due to the removal of amorphous carbon.83 The treatment of CNTs was performed under stronger conditions than the one previously reported (HNO3 30% at 403 K, 3 h; mixture HNO3/H2SO4, reflux 20 h; HNO3/Na2CO3 under sonication 313 K, 2 h) and changes in morphology were also observed, CNTs resulting in open-ended and cut. In this case, the better performance of acid treated CNTs as supports for Pt, Ru and Cu in aniline wet air oxidation has been attributed to the better metal dispersion as the functional groups act as nucleation centers for metal ions.
The effect of oxygen functionalization on CNTs has been also reported in the liquid phase hydrogenation. Toebes et al. selectively removed oxygen groups from the surface of CNF by heat treatment at different temperatures (573 K to 973 K) in a nitrogen atmosphere and tested these modified CNTs as Pt support in the hydrogenation of cinnamaldehyde.84 The best performance has been obtained with the heat treatment at the highest temperature (973 K), where most of the oxygen groups have been removed. The enhanced activity observed is interestingly mainly due to the increase in the hydrogenation rate of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, with the formation of hydrocinnamaldehyde, whereas only a slight increase in C
C bond, with the formation of hydrocinnamaldehyde, whereas only a slight increase in C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond hydrogenation is observed. Apparently no correlation between number and/or type of oxygen groups and the catalytic activity was revealed as neither electronic nor morphologic modification of the metal has been observed. However in a subsequent study it was found that, as cinnamaldehyde preferentially adsorbs on the non-polar surface,85 an increase of the hydrophilicity of the surface related to the increase of oxygenated functional groups can be related to the hydrogenation rate of cinnamaldehyde. However in this study a decrease in Pt particle size by decreasing the amount of oxygen was also observed. Thus, in accordance with previous studies on the hydrogenation of cinnamaldehyde,86,87 the enhanced activity can be also associated to a particle size effect.
O bond hydrogenation is observed. Apparently no correlation between number and/or type of oxygen groups and the catalytic activity was revealed as neither electronic nor morphologic modification of the metal has been observed. However in a subsequent study it was found that, as cinnamaldehyde preferentially adsorbs on the non-polar surface,85 an increase of the hydrophilicity of the surface related to the increase of oxygenated functional groups can be related to the hydrogenation rate of cinnamaldehyde. However in this study a decrease in Pt particle size by decreasing the amount of oxygen was also observed. Thus, in accordance with previous studies on the hydrogenation of cinnamaldehyde,86,87 the enhanced activity can be also associated to a particle size effect.
It should be noted that other authors reported the opposite effect that by removing oxygen species from the surface, an increase in Pt particle size is observed.88 This discrepancy can arise, however, from the different Pt/CNTs preparation methods employed. Plomp et al. in fact prepared the Pt catalyst by deposition precipitation, whereas Solhy et al. prepared the catalyst via wet impregnation.85,88 The highest catalytic activity corresponded, as before, to the lesser oxygen-rich surface, but this catalyst is the one with the largest Pt particle size. Moreover, they obtained the best selectivity to unsaturated alcohol when fewer oxygen species were present on the surface, in contrast with what was reported before.84 They attributed the best selectivity observed to the biggest particle size tested.
In summary, we could simply conclude that the metal particle size and the amount of oxygen species on the surface are important parameters to tune the catalytic activity, but they are not the only ones that determine the overall performance of a catalytic material. A more recent study showed that the addition and the subsequent selective removal of oxygen species from CNTs have a negligible effect on the Pt particle size and dispersion.89 They observed that the removal of the oxygen species on the surface had a positive effect on activity but also on the selectivity toward cinnamyl alcohol. They attributed this performance to a better adsorption of cinnamaldehyde on the more non-polar surface obtained after the removal of the oxygen species.
Active carbon also represents a widely used, cheap supporting material and its acid functionalization has been intensively studied. The addition of oxygen functionalities to activated carbon enhanced the catalytic activity when AC was used as support for Mn and V in the benzyl alcohol oxidation.90 In this detailed study the pristine AC was treated via different routes: H2O2, 0.5 M or 6 M HNO3, O2. The pretreatment did not affect the carbon morphology and the particle size of the supported Mn and V. However the different functionalization led to different oxygen functionalities. Gas phase oxidation (O2) increases the concentration of hydroxyl groups and carbonyl groups, whereas the liquid phase oxidation with H2O2 or HNO3 forms mainly carboxylic acid. Moreover, the different pretreatments led to different oxygen densities. It has been shown that the abundance of oxygen functionalities is favorable for enhancing the catalytic performance by decreasing surface hydrophilicity, thus facilitating the preferential adsorption of the hydrophobic reactant (benzyl alcohol) and the subsequent desorption of the corresponding aldehyde (benzaldehyde). Moreover the interaction between metal active sites and AC is promoted by the strong anchoring effect of oxygen functionalities. Mn/AC showed the best performance when AC was treated with HNO3, thus in the presence of a higher amount of carboxylic groups, whereas V/AC showed the best performance when O2 was the oxidant, thus in the presence of mainly hydroxyl functional groups. The authors tried to relate the behavior of V and Mn catalysts to a different interaction between the support surface and the metal precursor due to the different acidic/basic properties of the metal solution.91 Indeed, it was reported that the interaction between metal cations and the support surface is favored when the precursor solution is more basic than the aqueous slurry of the support.92 As the precursor solution of Mn(NO3)2 is neutral, it is clear that it was expected to interact in a stronger way with a more acidic AC surface (containing more carboxylic groups). On the other hand, it is not clear why the more basic precursor of NH4VO3 did not take advantage of the greater difference in acidity. Its fruitful interaction with the less acidic OH-functionalized surface is explained.
Gold on carbon catalyzed alcohol oxidations have also been investigated in terms of oxygen-containing functional groups. Zhu et al. compared the activities of Au on AC and C3N4–oxygen free supports in benzyl alcohol oxidation and concluded that the presence of oxygen containing species on the surface of AC improved the catalytic performance, these being responsible for molecular oxygen adsorption and activation.11 Conversely, Rodrigues et al. reported that in glycerol oxidation Au showed improved catalytic activity when oxygen-containing functionalization was removed from the carbon support.10,93 The activation of AC with HNO3 led to a high amount of carboxylic acids and carboxylic anhydrides, lactones, phenols and quinines. The oxygen-containing groups were selectively removed by heat treatment at different temperatures, increasing the basic properties of the support. An oxygen-free activated carbon thus obtained, characterized by a high density of free π-electrons, showed an enhancement of the catalytic performance. The presence of delocalized π-electrons in basic carbon supports leads to a high electronic mobility, whereas oxygen-containing functional groups would decrease the electron density. The mobility of electrons in the basic support could potentially reduce the excess of negative charge forming on gold during the reaction and enhance the possibility of further hydroxide bonding, whereas the transition of these electrons from the support to the metal enhances the regeneration of the hydroxide ions. Therefore, the mobility of the electrons from and to the gold surface could potentially promote both adsorption and regeneration of hydroxide ions and thus improve the catalytic performance.
We could then conclude that an increase of the basic properties of the support might be beneficial in liquid phase oxidation. In this respect, in order to increase the basicity of the support and also in order to stabilize in size the metal nanoparticles and increase the catalytic performance nitrogen groups have been introduced in carbon supports.67,94–109 Jiang et al., for example, observed that the introduction of nitrogen groups on the surface of CNTs drastically increased the dispersion and stability of the gold nanoparticles, due to the strong metal–nitrogen interaction.93 Villa et al. and Prati et al. reported that Au, Pd and Au–Pd nanoparticles immobilized on nitrogen functionalized carbon nanofibers showed better performance than on pristine ones in both the benzyl alcohol and the glycerol oxidation.110,111 Different nitrogen groups on CNFs were obtained from the pre-oxidized CNFs (HNO3 65%) by thermal treatment with NH3 in the temperature range 473 K–873 K for 4 h (Fig. 8).95,112
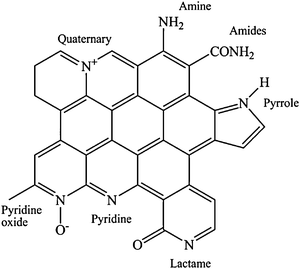 | ||
| Fig. 8 Different nitrogen groups introduced in CNF, by thermal treatment with NH3. | ||
NH3 treatment at different temperatures introduces different nitrogen-containing groups. In particular, increasing the temperature increases the basicity of the support, due to the presence of mainly pyridinic groups. The introduction of nitrogen-containing groups favorably affected the particle dispersion on the support (Fig. 9), as observed in the case of oxygen-containing groups. An enhanced catalytic performance has been always observed consistently when nitrogen-modified supports were employed.
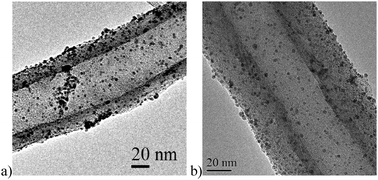 | ||
| Fig. 9 TEM images of (a) Au/CNF, (b) Au/N–CNF 873 K. | ||
Thus, Au nanoparticles supported on CNFs containing pyridine groups in glycerol oxidation showed a TOF of 853 h−1vs. a TOF of only 182 h−1 for Au on pristine CNFs. The basic properties of the support can affect the stability of the reaction products. Indeed, in benzyl alcohol oxidation, Au on basic nitrogen-modified CNFs showed the presence of a consistent amount of benzoic acid and benzyl benzoate deriving from the overoxidation of benzaldehyde and subsequent reaction of alcohol with the acid, respectively. Note that when Au/pristine CNF was used as the catalyst, neither overoxidation nor condensation products have been detected.
Pd on nitrogen-functionalized carbon nanotubes has been studied for the hydrogenation of cinnamaldehyde.113 The support has been synthesized by chemical vapor deposition using a mixture of C2H6/H2 and ammonia at 680 °C over an alumina-supported iron catalyst. The N–CNTs containing pyridine and pyrrole groups increased, as expected, the basicity of the support but also led to a significant improvement of the catalytic activity compared to pristine CNTs. These phenomena were attributed to possible electronic or morphological modifications of the active phase leading to a high TOF. Concerning the selectivity N–CNTs basic supports exhibited a relatively high selectivity towards the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond hydrogenation compared to the one obtained on samples without nitrogen incorporation, regardless of the slight difference in terms of Pd particle size.
C bond hydrogenation compared to the one obtained on samples without nitrogen incorporation, regardless of the slight difference in terms of Pd particle size.
Silica represents the second case that we are concerned with, as it has been extensively studied for many applications, in particular as support for metal nanoparticles for liquid phase reaction.78 Many studies have been performed in order to increase the metal–support interaction. The introduction of different functional groups on the surface that could act as anchoring groups drastically increased the dispersion and the stability of the metal nanoparticles.78,114–121
Aprile et al. reported the synthesis of functionalized mesoporous silica as a support for Au nanoparticles.122 The resulting catalyst showed higher performance than Au supported on non-functionalized silica, in the solventless oxidation of 1-phenylethanol to acetophenone. AuNPs have been anchored to the surface by capping them with quaternary ammonium bonded to a triethoxysilyl group. This methodology of deposition provided a stable dispersion of AuNPs and also limiting their coarsening during the reaction, thus enhancing their catalytic activity. The functionalized silica-supported Au has been obtained through a sol–gel method from tetraethylorthosilicate (TEOS) and colloidal Au nanoparticles stabilized by N-[3-(triethoxysilyl) propyl] O-2(dicetylmethylammonium)ethyl urethane. An interesting finding is the stability on recycling shown by the catalyst. The drawback of this catalytic system is the low stability in aqueous media due to the collapse of the mesoporous structure.
Following a similar strategy, thioether groups have been as well used as anchoring groups for metal nanoparticles as well.123,124 Hu et al. studied the beneficial effect of the introduction of thioether groups into the silica wall via condensation of TEOS with 1,4-bis(triethoxysilyl)propane tetrasulfide on the activity and stability of Au nanoparticles for the benzyl alcohol oxidation.123 Thiother groups were effective in the stabilization of the metal nanoparticles during reaction and the catalyst did not show deactivation phenomena during recycling. Wu et al. synthesized thiother functionalities –(CH2)3–S–S–S–S–(CH2)3 starting from bis(triethoxysilyl)propane tetrasulfide as anchoring groups for Au nanoparticles.124 The catalyst showed good activity and stability in the cyclohexane oxidation due to the high dispersion of the thioether stabilized AuNPs on the surface.124
A slightly different strategy widely used for the functionalization of silica is the grafting of organosilanes on the surface.78,114–121 This synthetic route, that allows the introduction of all the functional groups desired, has been used for anchoring metal nanoparticles for liquid phase applications. For example, the effect of different organosilanes on the stabilization of Pd nanoparticles and on surface properties has been studied. Chen et al. showed that grafting different (3-aminopropyl)triethoxysilane (APS), or [3-(2-aminoethylamino) propyl] trimethoxysilane (ATMS), or hexamethyldisilazane (HMDS) or (mercaptopropyl) trimethoxysilane (MPTMS) drastically affected the catalytic performance of silica-supported Pd catalysts in the benzyl alcohol oxidation (Fig. 10).
 | ||
| Fig. 10 Different functionalities introduced in TUD. | ||
When more hydrophilic amino or thiol groups were used (APS, ATMS, MPTMS), small Pd nanoparticles (1.9, 3.2 and 1.6 nm, respectively, for APS, ATMS, MPTMS) and good Pd dispersion have been obtained, due to the affinity of the ligand groups with the aqueous palladium chloride. On the other hand, hydrophobic trimethyl groups (HMSD) showed a barrier for the adsorption of a Pd precursor, with the formation of large Pd aggregates (17 nm).
APS immobilized on silica displayed the best catalytic performance (TOF of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 571 h−1) due to the match of two favorable conditions: small Pd nanoparticles and the increased basicity of the support surface. In addition, these findings have been verified for bimetallic systems. Au–Pd nanoparticles supported on APS modified silica SBA-16 showed better performance than the Pd based catalyst for a large range of alcohols due to the synergistic effect of the two metals.125
571 h−1) due to the match of two favorable conditions: small Pd nanoparticles and the increased basicity of the support surface. In addition, these findings have been verified for bimetallic systems. Au–Pd nanoparticles supported on APS modified silica SBA-16 showed better performance than the Pd based catalyst for a large range of alcohols due to the synergistic effect of the two metals.125
Oliveira et al. used XAFS analysis to evidence the stronger interaction of amino group-stabilized Au and Au NPs directly adsorbed on the support.126 For this study Fe3O4@SiO2 non-functionalized and functionalized with 3-(aminopropyl) triethoxysilane were chosen. XAFS indicated a stronger interaction of gold with amino-modified silica surfaces than with silanol groups in bare silica. The enhanced interaction leads to a higher catalytic performance of the Au/NH2–Fe3O4@SiO2 than Au/Fe3O4@SiO2 in alcohol oxidation. The advantage of using Fe3O4@SiO2 instead of simple SiO2 facilitated the separation of the spent catalyst by magnetic separation.
4. Remarks and perspectives
Catalyst support is an important factor determining the overall activity of metal nanoparticles. We have first highlighted that a physical constraint i.e. the exposure of MNPs on the support surface could influence the accessibility of reactants to active sites thus in turn affecting the catalytic activity. This can lead to the opinion that supports with a high ratio between external/internal surface represent the best solution for maximizing the contact between reagents. However, it has been shown that the incarceration inside support cavities could be beneficial in terms of resistance to leaching and coarsening of the MNPs. Thus a compromise has to be reached to achieve an acceptable reaction rate and a consistent durability of the catalytic materials. It should be noted that the reaction rate can be also influenced by other parameters such as catalyst/substrate ratio, temperature, concentration, etc. The stability of MNPs has been also achieved through the functionalization of surface support and, except in a few examples, it is normally recognized that surface acid or basic functionalities provide a better dispersion of MNPs which contributes to an increase in the catalytic activity.Secondly, the support may influence the electronic properties of the metal nanoparticles, modification which normally leads to significant differences in terms of activity/selectivity and the durability of the catalyst.
It should be noted that support effects often occur simultaneously with the metal particle size effect. It is almost impossible to vary the metal particle size without changing the physiochemical properties of the support and vice versa. Support effects are often distinguished by concomitant changes of the metal particle diameter or differences in the distribution of metal particles over the support.
Finally it should be remembered that the support can have interaction of its own with the reactant. Hydrophilicity or hydrophobicity of the surface has been shown to possibly tune the catalyst activity through preferential absorption of the reactant. Moreover, the support can actively participate to the reaction providing additional active sites (bifunctional catalyst).
Considering these points it is not difficult to imagine that so few studies give a definite answer on what is the real role of the support. Theoretical studies in the last few years give a substantial help in understanding the mechanism of O2 or H2 activation but it is always experimental data that give a definite confirmation to the theory.127,128
The support preparation, or its modifications, will be the future of catalyst design, making catalyst ever more selective, thereby reducing undesirable by-products, and more resistant to deactivation, thus economically more advantageous. Actually there are not any general trends and a lot of other studies are still needed to unequivocally determine the support role, even for a single reaction.
Notes and references
- L. Prati and G. Martra, Gold Bull., 1999, 32, 96 CrossRef CAS.
- H. Sakurai and M. Haruta, Catal. Today, 1996, 29, 361 CrossRef CAS.
- C. Bianchi, F. Porta, L. Prati and M. Rossi, Top. Catal., 2000, 13, 231 CrossRef CAS.
- G. M. Veith, A. R. Lupini, S. J. Pennycook, A. Villa, L. Prati and N. J. Dudney, Catal. Today, 2007, 122, 248 CrossRef CAS.
- A. Villa, A. Gaiassi, I. Rossetti, C. L. Bianchi, K. van Benthem, G. M. Veith and L. Prati, J. Catal., 2010, 275, 108 CrossRef CAS.
- C. L. Bianchi, S. Biella, A. Gervasini, L. Prati and M. Rossi, Catal. Lett., 2003, 85, 91 CrossRef CAS.
- H. Vu, F. Goncalves, R. Philippe, E. Lamouroux, M. Corrias, Y. Kihn, D. Plee, P. Kalck and P. Serp, J. Catal., 2006, 240, 18 CrossRef CAS.
- H. Ma, L. Wang, L. Chen, C. Dong, W. Yu, T. Huang and Y. Quian, Catal. Commun., 2007, 8, 452 CrossRef CAS.
- Z. Huang, F. Li, B. Chen, F. Xue, Y. Yuan, G. Chen and G. Yuan, Green Chem., 2011, 13, 3414 RSC.
- E. G. Rodrigues, M. F. R. Pereira, X. Chen, J. J. Delgado and J. J. M. Orfao, J. Catal., 2011, 10, 1016 Search PubMed.
- J. Zhu, S. A. C. Carabineiro, D. Shan, J. L. Faria, Y. Zhu and J. L. Figueiredo, J. Catal., 2010, 274, 207 CrossRef CAS.
- J. H. Vleeming, B. F. M. Kuster and G. B. Marin, Catal. Lett., 1997, 46, 187 CrossRef CAS.
- A. Villa, G. M. Veith and L. Prati, Angew. Chem., Int. Ed., 2010, 49, 4499 CrossRef CAS.
- A. Abad, P. Conception, A. Corma and H. Garcia, Angew. Chem., Int. Ed., 2005, 44, 4066 CrossRef CAS.
- A. Corma and M. E. Domine, Chem. Commun., 2005, 4042 RSC.
- A. Abad, C. Almela, A. Corma and H. Garcia, Tetrahedron, 2006, 62, 6666 CrossRef CAS.
- Y. Guan, D. A. J. M. Ligthart, O. Pirgon-Galin, J. A. Z. Pieterse, R. A. van Santen and E. J. M. Hensen, Top. Catal., 2011, 54, 424 CrossRef CAS.
- G. N. Vayssilov, Y. Lykhach, A. Migani, T. Staudt, G. P. Petrova, N. Tsud, T. Skala, A. Bruix, F. Illas, K. C. Prince, V. Matolin, K. M. Neyman and J. Libuda, Nat. Mater., 2011, 10, 310 CrossRef CAS.
- A. Villa, C. E. Chan-Thaw, G. M. Veith, K. L. More, D. Ferri and L. Prati, ChemCatChem, 2011, 3, 1612 CrossRef CAS.
- F.-Z. Su, J. Ni, H. Sun, Y. Cao, H.-Y. He and K.-N. Fan, Chem.–Eur. J., 2008, 14, 7173 CrossRef.
- F.-Z. Su, Y.-M. Liu, L.-C. Wang, Y. Cao, H.-Y. He and K.-N. Fan, Angew. Chem., Int. Ed., 2008, 47, 334 CrossRef CAS.
- N. Dimitratos, A. Villa, C. L. Bianchi, L. Prati and M. Makkee, Appl. Catal., A, 2006, 311, 185 CrossRef CAS.
- D. I. Enache, J. K. Edwards, P. Landon, B. Solsona-Espriu, A. F. Carley, A. A. Herzing, M. Watanabe, C. J. Kiely, D. W. Knight and G. J. Hutchings, Science, 2006, 311, 362 CrossRef CAS.
- D. I. Enache, D. Barker, J. K. Edwards, S. H. Taylor, D. W. Knight, A. F. Carley and G. J. Hutchings, Catal. Today, 2007, 122, 407 CrossRef CAS.
- A. Mirescu, H. Berndt, A. Martin and Ulf Prüße, Appl. Catal., A, 2007, 317, 204 CrossRef CAS.
- W. C. Ketchie, M. Murayama and R. J. Davis, Top. Catal., 2007, 44, 307 CrossRef CAS.
- T. Ishida, N. Knishita, H. Okatsu, T. Akita, T. Takei and M. Haruta, Angew. Chem., Int. Ed., 2008, 47, 9265 CrossRef CAS.
- L. Prati, P. Spontoni and A. Gaiassi, Top. Catal., 2009, 52, 288 CrossRef CAS.
- N. Dimitratos, J. A. Lopez-Sanchez, J. M. Anthonykutty, G. Brett, A. F. Carley, R. C. Tirulavam, A. A. Herzing, C. J. Kiely, D. W. Knight and G. J. Hutchings, Phys. Chem. Chem. Phys., 2009, 11, 4952 RSC.
- N. Dimitratos, J. A. Lopez-Sanchez, D. Morgan, A. F. Carley, R. C. Tirulavam, C. J. Kiely, D. Bhetel and G. J. Hutchings, Phys. Chem. Chem. Phys., 2009, 11, 5142 RSC.
- B. N. Zope, D. D. Hibbitts, M. Neurock and R. J. Davis, Science, 2010, 330, 74 CrossRef CAS.
- P. Haide, B. Kimmerle, F. Krumeich, W. Kleist, J. D. Grunwaldt and A. Baiker, Catal. Lett., 2008, 125, 169 CrossRef.
- T. Mitsudome, A. Noujima, T. Mizugaki, K. Jitsukawa and K. Kaneda, Adv. Synth. Catal., 2009, 351, 1890 CrossRef CAS.
- N. Zheng and G. D. Stucky, Chem. Commun., 2007, 3862 RSC.
- M. Date, M. Okumura, S. Tsubota and M. Haruta, Angew. Chem., Int. Ed., 2004, 43, 2129 CrossRef CAS.
- C. Shang and Z. P. Liu, J. Phys. Chem. C, 2010, 114, 16989 CAS.
- C. Shang and Z.-P. Liu, J. Am. Chem. Soc., 2011, 133, 9938 CrossRef CAS.
- P. Weerachawanasak, P. Praserthdam, M. Arai and J. Panpranot, J. Mol. Catal. A: Chem., 2008, 279, 133 CrossRef CAS.
- M. A. Vannice, Top. Catal., 1997, 4, 241 CrossRef.
- P. Weerachawanasak, O. Mekasuwandumrong, M. Arai, A.-I. Fujita, P. Praserthdam and J. Panpranot, J. Catal., 2009, 262, 199 CrossRef CAS.
- R. Wang, Y. Li, R. Shi and M. Yang, J. Mol. Catal. A: Chem., 2011, 344, 122 CrossRef CAS.
- T. Sirikajorn, O. Mekasuwandumrong, P. Praserthdam, J. G. Goodwin, Jr. and J. Panpranot, Catal. Lett., 2008, 126, 313 CrossRef CAS.
- F. M. Bautista, J. M. Campelo, A. Garcia, D. Luna, J. M. Marinas, R. A. Quiros and A. A. Romero, Catal. Lett., 1998, 52, 205 CrossRef CAS.
- E. Florez, T. Gomez, P. Liu, J. A. Rodriguez and F. Illas, ChemCatChem, 2010, 2, 1219 CrossRef CAS.
- K. Mori, T. Hara, T. Mizugaki, K. Ebitani and K. Kaneda, J. Am. Chem. Soc., 2004, 126, 10657 CrossRef CAS.
- N. Jamwal, M. Gupta and S. Paul, Green Chem., 2008, 10, 999 RSC.
- Z. Opre, D. Ferri, F. Krumeich, T. Mallat and A. Baiker, J. Catal., 2007, 251, 48 CrossRef CAS.
- C. Mondelli, D. Ferri and A. Baiker, J. Catal., 2008, 258, 170 CrossRef CAS.
- Z. Opre, D. Ferri, F. Krumeich, T. Mallat and A. Baiker, J. Catal., 2006, 241, 287 CrossRef CAS.
- F. Cavani, A. Trifirò and A. Vaccari, Catal. Today, 1991, 11, 173 CrossRef CAS.
- T. Mitsudome, Y. Mikami, H. Funai, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., Int. Ed., 2008, 47, 334 CrossRef.
- T. Mitsudome, Y. Mikami, K. Ebata, T. Mizugaki, K. Jitsukawa and K. Kaneda, Chem. Commun., 2008, 4804 RSC.
- T. Mitsudome, A. Noujima, T. Mizugaki, K. Jitsukawa and K. Kaneda, Green Chem., 2009, 11, 793 RSC.
- T. Mitsudome, A. Noujima, T. Mizugaki, K. Jitsukawa and K. Kaneda, Adv. Synth. Catal., 2009, 351, 1890 CrossRef CAS.
- W. Fang, Q. Zhang, J. Chen, W. Deng and Y. Wang, Chem. Commun., 2010, 46, 1547 RSC.
- W. Fang, J. Chen, Q. Zhang, W. Deng and Y. Wang, Chem.–Eur. J., 2011, 17, 1247 CrossRef CAS.
- A. Noujima, T. Mitsudome, T. Mizugaki, K. Jitsukawa and K. Kaneda, Angew. Chem., Int. Ed., 2011, 50, 2986 CrossRef CAS.
- A. Tsuji, K. T. V. Rao, S. Nishimura, A. Takagaki and K. Ebitani, ChemSusChem, 2011, 4, 542 CrossRef CAS.
- N. Tiengchad, O. Mekasuwandumrong, C. Na-Chiangmai, P. Weerachawanasak and J. Panpranot, Catal. Commun., 2011, 12, 910 CrossRef CAS.
- H. Li, Z. Zheng, M. Cao and R. Cao, Microporous Mesoporous Mater., 2010, 136, 42 CrossRef CAS.
- S. Handjani, E. Marceau, J. Blanchard, J.-M. Krafft, M. Che, P. Maki-Arvela, N. Kumar, J. Warna and D. Y. Murzin, J. Catal., 2011, 282, 228 CrossRef CAS.
- C. Y. Ma, B. J. Dou, J. J. Li, J. Cheng, Q. Hu, Z. P. Hao and S. Z. Qiao, Appl. Catal., B, 2009, 92, 202 CrossRef CAS.
- H. Miyamura, R. Matsubara, Y. Miyazaki and S. Kobayashi, Angew. Chem., Int. Ed., 2007, 46, 4151 CrossRef CAS.
- C. Lucchesi, T. Inasaki, H. Miyamura, R. Matsubara and S. Kobayashi, Adv. Synth. Catal., 2008, 350, 1996 CrossRef CAS.
- Y. Perez, C. Aprile, A. Corma and H. Garcia, Catal. Lett., 2010, 134, 134 CrossRef.
- S. N. Sidorov, I. V. Volkov, V. A. Davankov, M. P. Tsyurupa, P. M. Valetsky, L. M. Bronstein, R. Karlinsey, J. W. Zwanzinger, V. G. Matveeva, E. M. Sulman, N. V. Lakina, E. A. Wilder and R. J. Spontak, J. Am. Chem. Soc., 2001, 123, 10502 CrossRef CAS.
- C. E. Chan-Thaw, A. Villa, L. Prati and A. Thomas, Chem.–Eur. J., 2011, 17, 1052 CrossRef CAS.
- A. Corma, H. Garcia and F. X. Llabres i Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS.
- T. Ishida, M. Nagaoka, T. Akita and M. Haruta, Chem.–Eur. J., 2008, 14, 8456 CrossRef CAS.
- H. Liu, Y. Liu, Y. Li, Z. Tang and H. Jiang, J. Phys. Chem. C, 2010, 114, 13362 CAS.
- A. L. Tarasov, L. M. Kustov, V. I. Isaeva, A. N. Kalenchuk, I. V. Mishin, G. I. Kasputin and V. I. Bogdan, Kinet. Catal., 2011, 52, 273 CrossRef CAS.
- L. R. Radovic and F. Rodriguez-Reinoso, in Chemistry and Physics of Carbon 25, ed. P. A. Thrower, Marcel Dekker Inc., 1997 Search PubMed.
- B. Coq and F. Figueras, Coord. Chem. Rev., 1998, 178–180, 1753 CrossRef CAS.
- J. Chen, A. M. Rao, S. Lyuksyutov, M. E. Itkis, M. A. Hamon, H. Hu, R. W. Cohn, P. C. Eklund, D. T. Colbert, R. E. Smalley and R. C. Haddon, J. Phys. Chem. B, 2001, 105, 2525 CrossRef CAS.
- D. Tasis, N. Tagmatarchis, A. Bianco and M. Prato, Chem. Rev., 2006, 106, 1105 CrossRef CAS.
- N. Karouris and N. Tagmatarchis, Chem. Rev., 2010, 110, 5366 CrossRef.
- P. Singh, S. Campidelli, S. Giordani, D. Bonifazi, A. Bianco and M. Prato, Chem. Soc. Rev., 2009, 38, 2214–2230 RSC.
- H. Zou, S. Wu and J. Shen, Chem. Rev., 2008, 108, 3893 CrossRef CAS.
- L. Prati, A. Villa, C. E. Chan-Thaw, R. Arrigo, D. Wang and D. S. Su, Faraday Discuss., 2011, 152, 353 RSC.
- T. Tang, C. Yin, N. Xiao, M. Guo and F.-S. Xiao, Catal. Lett., 2009, 127, 400 CrossRef CAS.
- M. S. P. Shaffer, X. Fan and A. H. Windle, Carbon, 1998, 36, 1603 CrossRef CAS.
- J. Chen, M. A. Hamon, H. Hu, Y. Chen, A. M. Rao, P. C. Eklund and R. C. Haddon, Science, 1998, 282, 95 CrossRef CAS.
- G. Ovejero, J. L. Sotelo, M. D. Romero, A. Rodriguez, M. A. Ocana, G. Rodriguez and J. Garcia, Ind. Eng. Chem. Res., 2006, 45, 2206 CrossRef CAS.
- M. L. Toebes, Y. Zhang, J. Hájek, T. A. Nijhuis, J. H. Bitter, A. Jos van Dillen, D. Y. Murzin, D. C. Koningsberger and K. P. de Jong, J. Catal., 2004, 226, 215 CrossRef CAS.
- J. Plomp, H. Vuori, A O I. Krause, K. P. de Jong and J. H. Bitter, Appl. Catal., A, 2008, 351, 9 CrossRef.
- M. Englisch, A. Jentys and J. A. Lercher, J. Catal., 1997, 166, 25 CrossRef CAS.
- Y. Nitta, K. Ueno and T. Imanaka, Appl. Catal., 1989, 56, 9 CrossRef CAS.
- Solhy, B. F. Machado, J. Beausoleil, Y. Kihn, F. Goncalves, M. F. R. Pereira, J. J. M. Orfao, J. L. Figueiredo, J. L. Faria and P. Serp, Carbon, 2008, 46, 1194 CrossRef CAS.
- B. F. Machado, H. T. Gomes, P. Serp, P. Kalck and J. L. Faria, ChemCatChem, 2010, 2, 190 CrossRef CAS.
- Y. Chen, W. Chen, Q. Tang, Z. Gou, Y. Yang and F. Su, Catal. Lett., 2011, 141, 149 CrossRef CAS.
- M. C. Romanmartinez, D. Cazorlaamoros, A. Linaressolano, C. S. M. Delecea, H. Yamashita and M. Anpo, Carbon, 1995, 33, 3 CrossRef CAS.
- J. M. Solar, C. A. L. Leon, K. Osseoasare and L. R. radovic, Carbon, 1990, 28, 369 CrossRef CAS.
- E. G. Rodrigues, S. A. C. Carabineiro, X. Chen, J. J. Delgado, J. L. Figueiredo, M. F. R. Pereira and J. J. M. Orfao, Catal. Lett., 2011, 141, 420 CrossRef CAS.
- S. van Dommele, k. P. de Jong and J. H. Bitter, Chem. Commun., 2006, 4859 RSC.
- R. Arrigo, M. Haevecker, S. Wrabetz, R. Blume, M. Lerch, J. McGregor, E. P. J. Parrott, J. A. Zeitler, L. F. Gladden, A. Knop-Gericke, R. Schloegl and D. Su, J. Am. Chem. Soc., 2010, 132, 9616 CrossRef CAS.
- J.-P. Tessonnier, A. Villa, O. Majoulet, D. Su and R. Schlögl, Angew. Chem., Int. Ed., 2009, 48, 6543 CrossRef CAS.
- A. Villa, J. P. Tessonnier, O. Majoulet, D. Su and R. Schlögl, Chem. Commun., 2009, 4405 RSC.
- A. Villa, J. P. Tessonnier, O. Majoulet, D. Su and R. Schlögl, ChemSusChem, 2010, 3, 241 CrossRef CAS.
- S. Dommele, K. P. Jong and J. H. Bitter, Top. Catal., 2009, 52(11), 1575 CrossRef CAS.
- S. van Dommele, K. P. de Jong, A. Romero-Izquirdo and J. H. Bitter, Stud. Surf. Sci. Catal., 2006, 162, 29 CrossRef CAS.
- K. N. Naokatsu, S. Okamura, S.-i. Fujita, J.-i. Ozaki and M. Arai, Adv. Synth. Catal., 2010, 352(9), 1476 CrossRef.
- R. Melo Freire, A. Hellen Morais Batista, A. Gomes Souza Filho, J. M. Filho, G. Dantas Saraiva and A. Conceicao Oliveira, Catal. Lett., 2009, 131(1–2), 135 CrossRef.
- K. Jiang, A. Eitan, L. S. Schadler, P. M. Ajayan and R. W. Siegel, Nano Lett., 2003, 3, 275 CrossRef CAS.
- B. Choi, H. Yoon. I. Park, J. Jang and Y. Sung, Carbon, 2007, 45, 2496 CrossRef CAS.
- X. Leprò, E. Terries, Y. Vega-Cantù, F. J. Rodriguez-Macias, H. Muramatsu, Y. Ahm Kim, T. Hayahsi, M. Endo, R. M. Torres and M. Terrones, Chem. Phys. Lett., 2008, 463, 124 CrossRef.
- J. Amadou, K. Chirazi, M. Houllé, I. Janowska, O. Ersen, D. Bégin and C. Pham-Huu, Catal. Today, 2008, 138, 62 CrossRef CAS.
- H. Yoon, S. Ko and J. Jang, Chem. Commun., 2007, 1468 RSC.
- X. Li, Y. Liu, L. Fu, L. Cao, D. Wei, G. Yu and D. Zhu, Carbon, 2006, 44, 3139 CrossRef CAS.
- S. Abate, R. Arrigo, M. E. Schuster, S. Perathoner, G. Centi, A. Villa, D. Su and R. Schloegl, Catal. Today, 2010, 157(1–4), 280 CrossRef CAS.
- A. Villa, D. Wang, P. Spontoni, R. Arrigo, D. Su and L. Prati, Catal. Today, 2010, 157, 89 CrossRef CAS.
- L. Prati, A. Villa, E. Chan-Thaw, R. Arrigo, D. Wang and D. S. Su, Faraday Discuss., 2011, 152, 353 RSC.
- R. Arrigo, M. Hävecker, R. Schlögl and D. S. Su, Chem. Commun., 2008, 4891 RSC.
- J. Amadou, K. Chizari, M. Houlle, I. Janowska, O. Ersen, D. Begin and C. Pham-Huu, Catal. Today, 2008, 138, 62 CrossRef CAS.
- D. V. Leff, L. Brandt and J. R. Helath, Langmuir, 1996, 12, 4723 CrossRef CAS.
- A. Taguchi and F. Schüth, Microporous Mesoporous Mater., 2005, 77, 1 CrossRef CAS.
- F. Hoffman and M. Fröba, Supramolecular chemistry of organic–inorganic hybrid materials, ed. K. Rurat and R. Martinez-Manez, John Wiley & Sons, Hoboken, USA, p. 3 Search PubMed.
- D. Brühwiler, Nanoscale, 2010, 2, 887 RSC.
- J. A. Melero, J. Iglesias and J. Moreno, in Advanced metal containing mesostructured silicas for novel catalytic applications, mesoporous materials, ed. L. T. Burness, Nova publishers, San Diego, USA, 2009, p. 239 Search PubMed.
- P. Kumar and V. V. Guliants, Microporous Mesoporous Mater., 2010, 132, 1 CrossRef CAS.
- N. Linares, E. Serrano, M. Rico, A. M. Balu, E. Losada, R. Luque and J. Garcia-Martinez, Chem. Commun., 2011, 47, 9024 RSC.
- A. Mehdi, C. Reye and R. Corriu, Chem. Soc. Rev., 2011, 40, 563 RSC.
- C. Aprile, A. Abad, H. Garcia and A. Corma, J. Mater. Chem., 2005, 15, 4408 RSC.
- J. Hu, L. Chen, K. P. Loh and X. S. Zhao, Catal. Sci. Technol., 2011, 1, 285 Search PubMed.
- P. Wu, Z. Xiong, K. P. Loh and X. S. Zhao, Catal. Sci. Technol., 2011, 1, 285 CAS.
- Y. Chen, H. Lim, Q. Tang, Y. Gao, T. Sun, Q. Yan and Y. Yang, Appl. Catal., A, 2010, 380, 55 CrossRef CAS.
- R. L. Oliveira, D. Zanchet, P. K. Kiyohara and L. M. Rossi, Chem.–Eur. J., 2011, 17, 4626 CrossRef CAS.
- B. N. Zope, D. D. Hibbits, M. Neurock and R. J. Davis, Science, 2010, 330, 74 CrossRef CAS.
- C. Shang and Z.-P. Liu, J. Am. Chem. Soc., 2011, 133(25), 9938 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |