Macro–meso-porous TiO2, ZnO and ZnO–TiO2-composite thick films. Properties and application to photocatalysis†
Jayasankar
Mani
,
Hazem
Sakeek‡
,
Salah
Habouti
,
Matthias
Dietze
and
Mohammed
Es-Souni
*
Institute for Materials & Surface Technology, University of Applied Sciences Kiel, Grenzstrasse 3, 24149 Kiel, Germany. E-mail: me@fh-kiel.de
First published on 14th November 2011
Abstract
We report on a new and versatile method for preparing homogenous, crack-free and macro–meso-porous thick films of TiO2, ZnO and ZnO–TiO2 composites using a single step coating procedure and fairly low annealing temperatures of a maximum of 400 °C. The method relies on an oxide nanopowder filler in an optimized precursor solution. The films are well adherent to the substrate, show homogeneously distributed open porosity, and are hydrophilic. The photocatalytic activity of these films was characterized using methyl orange as a model dye. We show that the TiO2 films have better photocatalytic activity than ZnO and ZnO–TiO2 composite films. The advantages of these composite films over particulate photocatalysts lie in their robustness and ease of application as no filtration is needed. Furthermore it is possible to apply them on suitable large area membranes.
1. Introduction
TiO2 and ZnO are the most versatile semi-conductor oxides with applications spanning a wide range from cosmetics to air purification.1,2 Most of the applications rely on the generation of electron–hole pairs upon excitation with electromagnetic waves that have energies higher than their band gaps. TiO2 and ZnO are also reported as good photocatalysts for the degradation of environmental contaminants.3,4 When illuminated with appropriate light sources, the photocatalyst generates electron–hole-pairs, which initiate a series of chemical reactions and produce the hydroxyl radical (OH˙) and superoxide anions (O2−˙), when the semiconductor is in contact with water and oxygen.5Most of the applications require thick films with tailored porosity, and until now the method of choice for their processing is screen-printing that requires a number of steps, including paste formulation and processing, transfer to the substrate, burning and sintering at high temperatures. Sol–gel may constitute an alternative to screen-printing, though it is more appropriate for thin film processing. Thick films may be fabricated using layer-by-layer coating, but this is tedious, cost-intensive, can generate cracks, and the thickness achieved rarely exceeds 1 μm. We have shown in a number of studies that thick film fabrication using sol–gel can be made possible by loading sols with nanopowders at high loadings (hybrid sol–gel–powder method).6 We demonstrated that this method worked well for lead–zirconate–titanate (PZT) thick films with which high functional properties, e.g. ferroelectric polarization, piezoelectricity and pyroelectricity, could be obtained. Advantages of the method encompass the possibility of combining different materials, and thus making nanocomposite films, and tailoring porosity.
In the present paper we extend the hybrid sol–gel–powder method to the processing of thick, macro–meso-porous TiO2, ZnO and ZnO–TiO2 composite films, and show that these films can be advantageously applied for the photocatalytic degradation of dye molecules, as one of possible applications. Degradation of toxic organic compounds by using UV irradiated TiO2 and ZnO in the form of powders (e.g. Evonik P25 30 nm particles of TiO2), thin film coatings and membranes has been studied and practiced both at the laboratory and industrial scales.7–16 The application of powder is generally accompanied by complications arising from the need for filtration and separation of the powder from the treated solution, which is considered a challenge to large scale applications.17,18 From a practical point of view, it is therefore advantageous to use semiconductor coatings on suitable substrates to immobilize the catalyst.19,20 In this respect sol–gel thick films with micro- and nanoporous structure were proposed as potential candidates,21,22 and some attempts have been undertaken to process thick films containing TiO2 nanoparticles fillers,21,22 though also in these reports numerous dip-coating sequences were necessary to fabricate thick films that resulted in microstructures with rather small active surface and fairly clumped nanoparticles (see Fig. 3 in ref. 22).
The results presented hereafter show a procedure for mixing TiO2 and ZnO nanopowders with optimized TiO2 and ZnO precursor solutions, respectively, to process thick films with homogeneously distributed nanoparticles and porosity. ZnO–TiO2 composite thick films were also investigated. The films were processed viaspin coating and annealed at a fairly low temperature of 400 °C to yield approximately 4 μm thick films in one coating sequence. The films are then applied for the photocatalytic degradation of methyl orange.
2. Materials and methods
The following chemicals were used: titanium (IV)-isopropoxide (TTIP, 97%, Aldrich, Germany), diethanolamine (DEA, 99%, Aldrich, Germany) as a stabilizing agent, polyethylene glycol with molecular weight 400 (PEG 400, ABCR, Germany), acetylacetone (AcAc, 99%, Aldrich, Germany), absolute ethanol (Merck, Germany), nanocrystalline TiO2 (Evonik P25 powder (50 m2 g−1; 85–70% anatase and 15–30% rutile; mean particle diameter of 30 nm)), zinc 2-ethyl hexanoate (ABCR, Germany), nanocrystalline ZnO (Evonik powder; mean particle diameter of 25 nm).2.1 Preparation of TiO2–P25 films
Thick TiO2–P25 composite films were deposited on 2.5 × 2.5 cm2 glass substrates by a spin-coating technique. Sol A contained 1.253 g of titanium (IV) isopropoxide and 0.525 g of DEA as stabilizer. Sol B was made of 1.5 g (22 wt%) of hydrophobic Evonik P25, 4.6 g of ethanol and 0.2 g of AcAc. Sol A and B were mixed and sonicated for 1 hour in a cooled water bath to maintain the temperature of the sol around room temperature. The ultrasonication step was necessary for a homogeneous dispersion of the P25 nanoparticles. Addition of 0.659 g (10 vol%) of PEG was found to be optimum to produce crack-free films.2.2 Preparation of ZnO films
Thick ZnO films were also prepared on 2.5 × 2.5 cm2 glass substrates by a spin-coating technique. Sol A contained 0.879 g of zinc 2-ethyl hexanoate and 0.439 g of DEA as stabilizer. Sol B was made of 1.125 g (22 wt%) of hydrophobic Evonik ZnO, 3.6 g of ethanol and 0.2 g of AcAc. Sol A and B were mixed and sonicated for 1 hour in a cooled water bath to maintain the temperature of the sol around room temperature. Addition of 0.05 g (1 vol%) of PEG was found to be optimum to produce crack-free films.2.3 Preparation of ZnO–TiO2–P25 composite films
Thick films of ZnO–TiO2–P25 composites were prepared on glass substrates by a spin coating technique. The composite sol was prepared by dispersing hydrophobic TiO2–P25 powder (22 wt%) in the ZnO sol which was prepared as described above. The PEG addition was optimum at 1 vol%. The glass substrates were cleaned with ethanol in an ultrasonic bath for 20 min and kept in a drying oven. The films were spin coated at 3000 rpm for 20 s, dried and heat treated at 400 °C for 10 min in air atmosphere.2.4 Characterization
Structure and phase composition were determined by X-ray diffraction (XRD, PanAnalytical X'Pert, Holland) and Cu Kα radiation (λ = 1.5418 Å) at 40 kV and 40 mA. The diffraction patterns were taken over a 2θ range from 20°–60°. Film morphology was characterized by a high resolution scanning electron microscope SEM (Zeiss Ultra plus 40) and an atomic force microscope (AFM, SIS Nanostation, Germany). UV-Vis measurements were conducted on a spectrophotometer (Lambda 35, Perkin-Elmer). The films for transmission measurement were processed on quartz glass substrates and the thickness of the film was 4 μm. These films were not completely opaque, hence we used transmission mode for the measurement. The water contact angle measurements were performed using a commercial set-up (dataphysics OCA instrument, Germany).2.5 Photocatalytic activity
The photocatalytic activity of the films was evaluated by the photodecolorization of methyl orange (MO) aqueous solution. 25 mL aqueous solution of MO (Aldrich, Milwaukee, WI, USA) with initial concentration of 2 mM and a pH of 5.6 was placed in a 50 mL pyrex glass vessel with magnetic stirring, cooled by a circulating water bath to maintain the temperature constant and prevent any evaporation during measurements. The temperature inside the reactor was monitored by a thermocouple and maintained at 25.5 °C.The photocatalyst films were placed in solution. The solution was kept in the dark under stirring for 30 min to reach the adsorption–desorption equilibrium. The UV source consisted of filtered low-pressure mercury UV tubes with 150 watt emitting radiation in the wavelength range between 250 and 450 nm with a peak maximum at 365 nm. A metal cover with an open slit was placed on top of the vessel to limit the illumination area. 0.5 mL of the sample were taken from the MO solution every 30 min for 6 hours, and the absorbance was monitored using a UV-Vis spectrophotometer (Lambda 35, Perkin Elmer).
3. Results and discussion
3.1 Characterization of the TiO2–P25, ZnO and ZnO–TiO2–P25 composite films
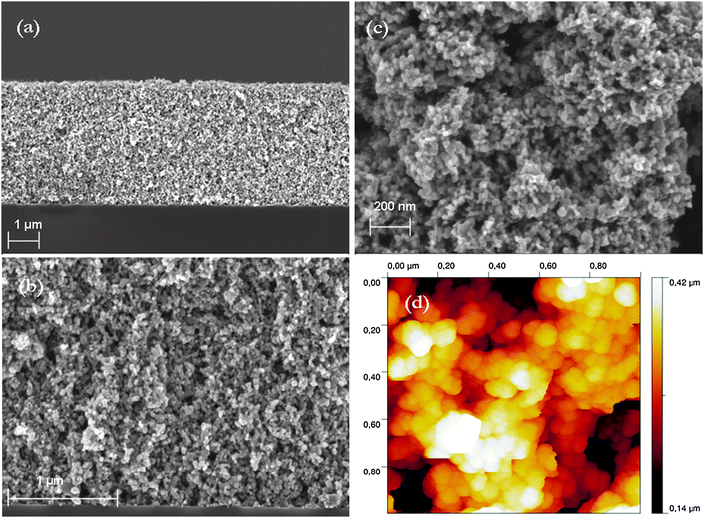 | ||
| Fig. 2 Cross section SEM micrographs of a TiO2–P25 film on the silicon substrate at low magnification (a); it shows uniformity of the film obtained after one spin-coating sequence and annealing at 400 °C; (b) higher magnification image to illustrate the porous structure of the films and the interconnectivity of the pores throughout film thickness. (c) Top-view SEM micrograph of the TiO2–P25 film surface showing a distribution of pore size, (d) AFM micrograph of TiO2–P25 film surface morphology also showing a distribution of pore size. The color bar indicates the height difference between the highest (white) and lowest features (dark). | ||
 | ||
| Fig. 3 Energy dispersive X-ray (EDX) spectrum of ZnO–TiO2–P25 composite showing the presence of ZnO in the TiO2–P25 matrix. Si and Ca are from the glass substrate. | ||
The hydrophilicity and hydrophobicity of the surface are usually analyzed by water contact angle (WCA) measurement. It is well known that WCA depends strongly on the chemical composition, as well as the morphology, of the outermost surface. The TiO2–P25 film was found to be superhydrophilic with a water contact angle of nearly 0° (not measurable). Usually TiO2 films are rather hydrophobic unless they are treated either with UV light or specific molecules.28 However, it was demonstrated that heat treatment at temperatures around 300 °C leaves hydroxyl Ti-OH terminal groups exposed at the surface, thus promoting hydrophilicity.29 In our case we may suppose that the high film surface area could offer a high density of Ti-OH terminal sites that in turn should promote hydrophilicity. A similar result was obtained by Balaur et al. who demonstrated that tubular TiO2 films were hydrophilic in the as-processed state.30 The ZnO films are also hydrophilic, although their WCA of 25.3° was larger than that of TiO2–P25, whereas superhydrophilic ZnO–TiO2–P25 films with WCA of 0° were also obtained.
The formation of the particular microstructures described above greatly benefits from the addition of PEG polymer and introduction of TiO2 powder in the sol–gel solution. Particularly, crack-free films could only be obtained after adding an optimum concentration of PEG. The influence of PEG on the microstructure formation is explained taking TiO2 as a model system. PEG has a two-fold effect on film formation. It is known as plasticizer that promotes film formation, and as dispersing agent (surfactant) in particle synthesis.31 The first effect arises from the fact that PEG could be hybridized with the titania precursor at the molecular scale through strong hydrogen bonding between the –CH2OH groups of the PEG and the –OH groups of the titania precursor, which efficiently prevents crack formation through the retardation of the condensation reaction and the promotion of film structural relaxation. The second effect is related to the ability of PEG as (non-ionic) dispersing agent. It has been reported that PEG could form a nearly spherical micelle around the particle via strong hydrogen bonding and avoids in this way particle agglomeration (micelles repel each other through homopolar surface charges). We surmise that PEG interacts with the TiO2 nanoparticles introduced in the precursor solution and prevents their agglomeration in the way described above. Both effects are schematically summarized in Fig. 4. The addition of PEG and its further elimination during the heat treatment leads to an increase in the surface area and porosity of the samples, thus presumably reducing the internal mass transfer limitation of the pollutant to the active sites.32
 | ||
| Fig. 4 A schematic description of the effects of PEG on Ti–O-network in the precursor solution (1) and as a dispersing agent for TiO2-particles (2). See also the main text for discussion. | ||
 | ||
| Fig. 5 UV-Vis transmission spectra of the TiO2–P25, ZnO and ZnO–TiO2–P25 composite films deposited on glass and annealed at 400 °C. | ||
The photocatalytic activity of TiO2–P25, ZnO and ZnO–TiO2–P25 composite films was studied by using MO degradation experiments as explained above in the experimental section. The illumination of the dye in the absence of the photocatalyst films for 3 hours showed no degradation. Fig. 6(a–c) shows the absorbance of MO as a function of time in the presence of different thick composite films in the spectral range from 350 to 600 nm. The absorbance of MO is taken as a measure for its degradation. The results show that the TiO2–P25 film reduces the absorbance of the dye to 50% of its initial value within 150 min and to 90% within 360 min. In comparison, the ZnO thick film has a lower photocatalytic activity with an absorbance reduction of only 50% of the initial value after 360 min, a result that is also shown by the ZnO–TiO2–P25 composite film.
 | ||
Fig. 6
Absorption spectra of the MO dye solution in contact with (a) TiO2–P25, (b) ZnO and (c) ZnO–TiO2–P25 composite films as a function of UV-irradiation time. (d) Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (Ao/A) vs. UV irradiation time of MO dye solution in contact with TiO2–P25, ZnO and ZnO–TiO2–P25 composite films. (Ao/A) vs. UV irradiation time of MO dye solution in contact with TiO2–P25, ZnO and ZnO–TiO2–P25 composite films. | ||
The photodegradation kinetics of methyl orange can be analyzed using the Langmuir–Hinshelwood model.35 When the pollutant amount is in the millimolar concentration range, the reaction rate R is proportional to the surface coverage θ.
 | (1) |
 | (2) |
As shown in Fig. 6d, Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (A/A0) is linear vs. time which is in accordance with eqn (2). The apparent reaction rate constants (kapp) of MO decomposition with different photocatalyst films are summarized in Table 1. Among the thick films TiO2–P25 showed the best photocatalytic activity with a rate constant value of 4.4 × 10−3 min−1.
(A/A0) is linear vs. time which is in accordance with eqn (2). The apparent reaction rate constants (kapp) of MO decomposition with different photocatalyst films are summarized in Table 1. Among the thick films TiO2–P25 showed the best photocatalytic activity with a rate constant value of 4.4 × 10−3 min−1.
| Samples | Rate constant × 10−3 min−1, kapp | R 2 |
|---|---|---|
| TiO2–P25 | 4.40 | 0.9806 |
| ZnO | 2.50 | 0.9852 |
| ZnO–TiO2–P25 | 1.64 | 0.9845 |
Degradation of MO and other organic pollutants arises as a result of electron–hole-pair formation upon irradiation of the semi-conducting oxide with appropriate light. In case recombination does not occur the electron can move to the surface of the titania nanoparticles and combine with absorbed oxygen to form the O2− radicals. Combination of a radical and a hole can reduce water present in the reaction medium to produce OH˙ radicals. Both these radicals are highly reactive species, resulting in the decomposition of the dyes.36 It has been well-established that, upon photoexcitation, the hole that is generated in the valence band has sufficient oxidation potential to initiate aerobic oxidation of many organic compounds.
The photoexcitation depends on different parameters such as pH, pollutant and catalyst concentrations, light intensity and temperature which makes a direct comparison with the results already published on the topic quite difficult.37 However, we may state that our results compare advantageously to those published for layer-by-layer coatings reported by Sharma et al. and others.38–41 Arconada et al. reported porous thin TiO2-anatase films (2-layer films), obtained by dip-coating glass slides and silicon wafers, where methyl orange degradation was completed within 15 hours.42 The photocatalytic activity of titania films is closely related to the specific surface area of the film. Macro–meso-porous structures have in this respect a large influence on the photocatalytic activity. The macroporous channels could enhance light harvesting efficiency by increasing light-transfer paths for the distribution of photon energy onto the mesoporous network. Such structure-in-structure arrangements also enhance molecular transport control and avoid photocatalyst poisoning by inert deposits. Thus the macro–meso-porous structure provides a readily accessible pore-wall for molecules and also minimizes the pressure drop over monolithic material.43,44 All these favorable conditions are present in our films. The higher film thickness and the formation of the specific microstructure shown in Fig. 2a–c with its small crystallite and large open pores as well as its superhydrophilic character may impart improved photocatalytic activity to our films as large open pores in an otherwise nanostructured film should be beneficial for contaminant transport to the active sites.
With respect to ZnO films similar reactions take place upon irradiation with photons that have higher energy than the band gap of 3.37 eV,45 and principally similar dye degradation reactions occur.
The reaction rate constant kapp obtained for our ZnO thick film is 2.5 × 10−3 min−1 (Table 1). This value is rather conservative as the peak wave length of our UV lamp is far beyond what is required for ZnO. However, the result obtained is better than that reported by Kansal et al.46 They tested the photocatalytic degradation of the MO dye (25 mg L−1), irradiating the aqueous solutions of a dye containing ZnO photocatalyst with UV and solar light, and changing several process parameters (amount of catalyst, concentration of dye, and pH). They found a maximum decolouration at basic pH (8–10) and reported a rate constant of about 1.3 × 10−3 min−1. The high photoactivity in our case may be discussed in similar terms as for TiO2 above.
The vertical transfer of electrons and holes in heterojunctions of semiconductor particulates is a significant process in the photo-oxidative degradation of organic pollutants as charge separation should be improved and hole–electron recombination rather decreased. For ZnO–TiO2 particle junctions charge transfer should involve electron transfer from the conduction band of ZnO to the conduction band of TiO2 and hole transfer from the valance band of TiO2 to that of ZnO.47,48 It was reported that this efficient charge separation should promote charge transfer to the adsorbed molecules due to the longer lifetime of the charge carriers.48
However in the present case the rate constant kapp for the ZnO–TiO2–P25 composite film of 1.64 × 10−3 min−1 (Table 1) was lower than that measured for both monolithic films. These results are consistent with the finding of Mallick and Scurrell who reported that the catalytic activity of TiO2 was hampered by ZnO-shells.49 A similar observation was reported in an Fe2O3–TiO2 composite; on UV exposure iron oxide was reported to quench the photocatalytic activity of TiO2 by a factor of 3–4.48 Explanation may be sought if we consider that the TiO2 particles are surrounded by a ZnO-film. Upon excitation the electrons accumulate on the ZnO-film while the holes are confined to the particles which should reduce the number of sites available for photodegradation. A strategy for enhancing the photocatalytic activity of TiO2–ZnO composites could be viananoparticle junctions instead of core–shell structures, though also in this case an optimal ZnO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) TiO2 ratio is required.50
TiO2 ratio is required.50
4. Conclusion
In summary, homogenous, crack-free and macro–meso-porous and hydrophilic thick films of TiO2–P25, ZnO and ZnO–TiO2–P25 composites were successfully fabricated by a hybrid sol–gel–powder method, using PEG as dispersant and film formation agent. The main role of PEG was to enlarge the inter-particular distance between the metal oxide particles. An annealing temperature of 400 °C was sufficient for film consolidation and high adherence to substrate. Using a similar processing method, thick ZnO-films were also processed with similar microstructural characteristics. To demonstrate the suitability of the method for preparing composite materials, TiO2–ZnO composite films were processed by dispersing the TiO2 nanoparticles in a ZnO precursor solution and further processing. The photocatalytic activity of the various films was demonstrated using methyl orange. The TiO2–P25 film had a higher photocatalytic activity than ZnO that in turn had a higher activity than the ZnO–TiO2–P25 composite film. The main advantages of the proposed thick film are reproducibility and reusability for better catalysis applications.Acknowledgements
A part of this work was conducted as a part of a DAAD scholarship awarded to Dr Sakeek June–September 2010.References
- S. Matsuda and A. Kato, Appl. Catal., 1983, 8, 149 CrossRef CAS.
- A. Sharma, P. Rao, R. P. Mathur and S. C. Ametha, J. Photochem. Photobiol., A, 1995, 86, 197 CrossRef CAS.
- S. Sakthivel, B. Neppolian, M. V. Shankar, B. Arabindoo, M. Palanichamy and V. Murugesan, Sol. Energy Mater. Sol. Cells, 2003, 77, 65 CrossRef CAS.
- Y. Shaogui, Q. Xie, L. Xinyong, L. Yazi, C. Shuo and C. Guohua, Phys. Chem. Chem. Phys., 2004, 6, 659 RSC.
- J. Yu and B. Wang, Appl. Catal., B, 2010, 94, 295 CrossRef CAS.
- M. Es-Souni, S. Maximov, A. Piorra, J. Krause and C.-H. Solterbeck, J. Eur. Ceram. Soc., 2007, 27, 4139 CrossRef CAS.
- I. K. Konstantinou, T. M. Sakellarides, V. A. Sakkas and T. A. Albanis, Environ. Sci. Technol., 2001, 35, 398 CrossRef CAS.
- M. S. Dieckmann and K. A. Gray, Water Res., 1996, 30, 1169 CrossRef CAS.
- C. C. Hsu and N. L. Wu, J. Photochem. Photobiol., A, 2005, 175, 269 CrossRef.
- M. Lindner, J. Theurich and D. W. Bahnemann, Water Sci. Technol., 1997, 35, 79 CrossRef CAS.
- D. S. Muggli, J. T. McCue and J. L. Falconer, J. Catal., 1998, 173, 470 CrossRef CAS.
- Y. Ohko, D. A. Tryk, K. Hashimoto and A. Fujishima, J. Phys. Chem. B, 1998, 102, 2699 CrossRef CAS.
- M. Zhang, T. An, X. Hu, C. Wang, G. Sheng and J. Fu, Appl. Catal., A, 2004, 260, 215 CrossRef CAS.
- S. N. Frank and A. J. Bard, J. Phys. Chem., 1977, 81, 1484 CrossRef CAS.
- B. Kraeutler and A. J. Bard, J. Am. Chem. Soc., 1978, 100, 5985 CrossRef CAS.
- C. A. Martin, M. A. Baltanas and A. E. Cassano, Catal. Today, 1996, 27, 221 CrossRef CAS.
- S. Parra, S. E. Stanca, I. Guasaquillo and K. R. Thampi, Appl. Catal., B, 2004, 51, 107 CrossRef CAS.
- R. Yuan, R. Guan, W. Shen and J. Zheng, J. Colloid Interface Sci., 2005, 282, 87 CrossRef CAS.
- H. T. Chang, N. M. Wu and F. Zhu, Water Res., 2000, 34, 407 CrossRef CAS.
- M. G. Chiovetta, R. L. Romero and A. E. Cassano, Chem. Eng. Sci., 2000, 56, 1631 CrossRef.
- D. T. On, D. D. Giscard, C. Danumah and S. Kaliaguine, Appl. Catal., A, 2001, 222, 299 CrossRef.
- Y. Chen and D. D. Dionysiou, Appl. Catal., B, 2008, 80, 147 CrossRef CAS.
- F. Peng, H. Wang, H. Yu and S. Chen, Mater. Res. Bull., 2006, 41, 2123 CrossRef CAS.
- A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1 CrossRef CAS.
- Q. Zhang, L. Gao and J. Guo, Appl. Catal., B, 2000, 26, 207 CrossRef CAS.
- H. Zhang and J. Banfield, J. Phys. Chem. B, 2000, 104, 3481 CrossRef CAS.
- M. Bizarro, M. A. Tapia-Rodriquez, M. L. Ojeda, J. C. Alonso and A. Ortiz, Appl. Surf. Sci., 2009, 255, 6274 CrossRef CAS.
- R. Wang, K. Hashimoto, A. Fujishima, M. Chikuni, E. Kojima, A. Kitamura, M. Shimohigoshi and T. Watanabe, Nature, 1997, 388, 431 CrossRef CAS.
- A. Kanta, R. Sedev and J. Ralston, Langmuir, 2005, 21, 2400 CrossRef CAS.
- E. Balaur, J. M. Macak, H. Tsuchiya and P. Schmuki, J. Mater. Chem., 2005, 15, 4488 RSC.
- D. Hotza and P. Greil, Mater. Sci. Eng., A, 1995, 202, 206 CrossRef.
- S. Suarez, M. Yates, P. Avila and J. Blanco, Catal. Today, 2005, 105, 499 CrossRef CAS.
- J. C. Yu, J. G. Yu and J. C. Zhao, Appl. Catal., B, 2002, 36, 31 CrossRef CAS.
- J. G. Yu, J. F. Xiong, B. Cheng and S. W. Liu, Appl. Catal., B, 2005, 60, 211 CrossRef CAS.
- D. S. Tsoukleris, A. I. Kontos, P. Aloupogiannis and P. Falaras, Catal. Today, 2007, 124, 110 CrossRef CAS.
- Y. J. Chen and D. D. Dionysiou, Appl. Catal., B, 2006, 62, 255 CrossRef CAS.
- D. W. Chen and A. K. Ray, Water Res., 1998, 32, 3223 CrossRef CAS.
- S. D. Sharma, D. Singh, K. K. Saini, C. Kant, V. Sharma, S. C. Jain and C. P. Sharma, Appl. Catal., A, 2006, 314, 40 CrossRef.
- C. H. Lu, C. Y. Hu and C. H. Wu, J. Hazard. Mater., 2008, 159, 636 CrossRef CAS.
- M. S. Wong, S. W. Hsu, K. K. Rao and C. P. Kumar, J. Mol. Catal. A: Chem., 2008, 279, 20 CrossRef CAS.
- A. Alem and H. Sarpoolaky, Solid State Sci., 2010, 12, 1469 CrossRef CAS.
- N. Arconada, Y. Castro and A. Duran, Appl. Catal., A, 2010, 385, 101 CrossRef CAS.
- D. R. Rolison, Science, 2003, 299, 1698 CrossRef CAS.
- J. Yu, L. Zhang, B. Cheng and Y. Su, J. Phys. Chem. C, 2007, 111, 10582 CAS.
- P. L. Washington, H. C. Ong, J. Y. Dai and R. P. H. Chang, Appl. Phys. Lett., 1998, 72, 3261 CrossRef CAS.
- S. K. Kansal, M. Singh and D. Sud, J. Hazard. Mater., 2007, 141, 581 CrossRef CAS.
- V. Sukharev and R. Kershaw, J. Photochem. Photobiol., A, 1996, 98, 165 CrossRef CAS.
- N. Serpone, P. Maruthamuthu, P. Pichat, E. Pelizzetti and H. J. Hidaka, J. Photochem. Photobiol., A, 1995, 85, 247 CrossRef CAS.
- K. Mallick and M. S. Scurrell, Appl. Catal., A, 2003, 253, 527 CrossRef CAS.
- D. L. Liao, C. A. Badour and B. Q. Liao, J. Photochem. Photobiol., A, 2008, 194, 11 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1cy00302j |
| ‡ Al-Azhar University-Gaza, Department of Physics, Gaza, Palestine. |
| This journal is © The Royal Society of Chemistry 2012 |

