High catalytic activity of CuO nanorods for oxidation of cyclohexene to 2-cyclohexene-1-one†
Maiyong
Zhu
and
Guowang
Diao
*
College of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, P. R. China. E-mail: gwdiao@yzu.edu.cn; Fax: +86-514-87975244; Tel: +86-514-87975436
First published on 20th September 2011
Abstract
Copper oxide (CuO) nanorods were synthesized via a facile hydrothermal process, which exhibit excellent catalytic oxidation of cyclohexene to 2-cyclohexene-1-one by tert-butyl hydrogen peroxide (TBHP) in acetonitrile. This would provide a novel method for directly synthesizing α,β-unsaturated ketones from olefins.
The α,β-unsaturated ketones are of great importance because they are widely used in many fields for example as chemosensors for metal ions,1,2 intermediates of many natural products and bioactive compounds,3–12 building blocks and products of pharmaceutical interest,13,14 in perfumery,15 as probes for papain-family cysteine proteases,16 scaffolds in carbohydrate chemistry,17 zwitterionic intermediates,18 and in surface modification of nanomaterials.19 Traditionally, they are prepared by either elimination reactions of the precursors such as steroidal ketones,20 α-halide ketones,21,22 and 3-oxo steroids;23 or aldol condensation of aldehydes and ketones.24,25 However, there are at least two drawbacks for these reactions. For one thing, obtaining the precursors is a challenge for many researchers because their synthesis often contains many steps from simple organic compounds. For another thing, these reactions tend to give some abnormal products, which makes the purification of the products troublesome.
To date, some new methods have been reported for synthesis of α,β-unsaturated ketones. Yu et al. have developed an efficient strategy for synthesis of (Z)-α,β-unsaturated ketones with high selectivity using phosphonate derivatives and aldehydes as precursors.26 Kim et al. have reported a general way to get α,β-unsaturated ketones through C–C bond forming reaction between primary alcohols and ketones in the presence of gold nanoparticles.27 Huang and Yang have prepared a variety of substituted α,β-unsaturated ketones by the reaction of alkylidenecyclopropanes with various acyl chlorides in the presence of aluminium chloride.28 Yadav et al. have provided a one-pot process for synthesis of α,β-unsaturated ketones through the cation exchange resin-promoted coupling reaction of alkynes with aldehydes.29 Recently, the direct oxidation of olefins to the corresponding α,β-unsaturated ketones has been reported.30,31 For example, Zhang et al. employed cobalt oxide as the catalyst for the oxidation of olefins with hydrogen peroxide to the corresponding α,β-unsaturated ketones. Unfortunately, the yield is very low.30 Tong et al. have systematically investigated the cyclohexene oxidation by molecular oxygen in the presence of 1,4-diamino-2,3-dichloro-anthraquinone (DADCAQ) and N-hydroxyphthalimide (NHPI). They found that an 89% conversion and 71% selectivity for 2-cyclohexene-1-one was obtained under the optimal conditions.32 More recently, Ghiaci and his co-workers developed a trimetallic hybrid nano-mixed oxide (RuO2/Co3O4/CeO2) catalyst for oxidation of cyclohexene. They reported the selective oxidation of cyclohexene to 2-cyclohexene-1-one (97.7, conversion and 95% selectivity). Unfortunately, the cost of rare earth and noble metals is too high for them to be widely used.33
The methods above suffer from either lower yield or higher cost. There is an urgent need to develop methods for obtaining α,β-unsaturated ketones directly from olefins with preferable conversion and selectivity, lower cost as well. In the present work, a facile hydrothermal method had been developed to synthesize CuO nanorods, which was demonstrated to be effective in catalytic oxidation of cyclohexene to 2-cyclohexene-1-one, in the presence of sodium citrate using CuCl2 and NaAc as a copper resource and mineralizer, respectively. Representative TEM images of the CuO nanorods are shown in Fig. 1. A low-magnification TEM image (Fig. 1a) shows that the panoramic morphology of the as-obtained CuO is in large quantity and mainly composed of rod-like architectures. However, the size of these structures was not uniform. From the higher magnification image (Fig. 1b), it is clear that the sizes of the CuO nanorods range from 30 nm to 90 nm in width and from 120 nm to 300 nm in length.
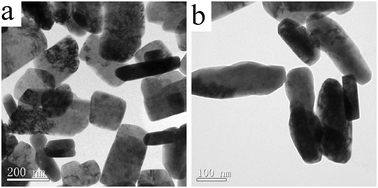 | ||
| Fig. 1 TEM images of the as-prepared CuO nanorods at different magnifications: (a) a lower magnification and (b) a higher magnification. | ||
The morphology and structure of the as-prepared CuO nanorods were further characterized by FE-SEM accompanied by EDS. As shown in Fig. 2a, the morphology of the individual CuO was not clear at low magnification. It seems that there are rod-like and plate-like morphologies of CuO in the as-prepared sample. From Fig. 2b it can be observed that these CuO nanorods are not uniform. Most of them are about 200 nm in length and 50 nm in width. Fig. 2c shows the enlarged FE-SEM image of the upper left corner of rod-like CuO in Fig. 2b. Fig. 2d shows the EDS spectra of the as-synthesized CuO nanorods. The signal of Al came from the substrate of Al foil. The atomic ratio of Cu/O is 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 for the product, which is smaller than the pure CuO. The reason may be attributed to the adsorption of sodium citrate onto the CuO nanorods to avoid the aggregation of CuO nanorods. So the percentage of O in the prepared sample is larger than pure CuO.
30 for the product, which is smaller than the pure CuO. The reason may be attributed to the adsorption of sodium citrate onto the CuO nanorods to avoid the aggregation of CuO nanorods. So the percentage of O in the prepared sample is larger than pure CuO.
 | ||
| Fig. 2 Typical FE-SEM images (a–c) at different magnifications and EDS spectra (d) of the as-prepared CuO nanorods. | ||
An X-ray diffraction technique can yield much information on structures, and phases of materials under investigation. A typical XRD pattern of the as-prepared sample is shown in Fig. 3. All the peaks in the diffraction diagram can be clearly indexed to the monoclinic phase of CuO, which is in good accordance with JCPDS card No. 48-1548. The obvious main peaks correspond to (110), (002), (111), (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 02), (020), (202), (
02), (020), (202), (![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 13), (022), (220), (311), and (
13), (022), (220), (311), and (![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 22) Bragg reflection of CuO. No impurities were detected by XRD, which suggested that the nanostructures obtained by reaction of CuCl2 and NaOH in the presence of sodium citrate under the hydrothermal conditions are pure monoclinic CuO with single crystalline structure. The peaks are strong and sharp, which reveals that the crystallization is very good.
22) Bragg reflection of CuO. No impurities were detected by XRD, which suggested that the nanostructures obtained by reaction of CuCl2 and NaOH in the presence of sodium citrate under the hydrothermal conditions are pure monoclinic CuO with single crystalline structure. The peaks are strong and sharp, which reveals that the crystallization is very good.
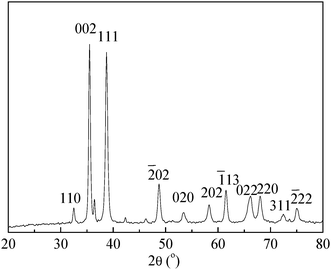 | ||
| Fig. 3 Typical XRD pattern of the as-prepared CuO sample. | ||
Nanostructured CuO materials have been reported to exhibit excellent catalytic performance in many reactions.34–40 The oxidation of cyclohexene by tert-butyl hydrogen peroxide (TBHP) was employed to evaluate the catalytic activity of the as-prepared CuO nanorods (see Scheme 1). Fig. S1 (ESI†) shows the GC graph of the oxidation system of cyclohexene. The structures and contents of each component are listed in Table S1 (ESI†). It was found that the oxidation preferentially occurred in the α-C–H bond, which yielded 2-cyclohexene-1-one (1). Only a very small amount of cyclohexene epoxide (2) was formed. It can be derived that almost a 100% conversion of cyclohexene and 95% selectivity for 1 were obtained. This result is compared to the best one reported previously by others using the three-mixed RuO2/Co3O4/CeO2 oxide catalyst.33 The prospect of CuO nanorod catalyst in this work lies in two aspects. One is the low cost compared to noble and/or rare earth metal-based oxide. The other is the simple preparation and convenient quality-controlling, which is difficult for multicomponent materials. In order to further show the excellence of CuO nanorod catalysts prepared in this work, Table 1 compares the conversion and selectivity for oxidation of cyclohexene to 2-cyclohexene-1-one (1) using different catalysts.30,32,33,41–43
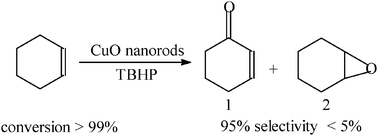 | ||
| Scheme 1 Oxidation of cyclohexene by TBHP in the presence of CuO nanorods. | ||
| Entry | Catalyst | Solvent | Conversion (%) | Selectivity (%) | Ref. |
|---|---|---|---|---|---|
| a DCE = 1,2-dichloroethane. b CN = 1-chloronaphthalene. c DMAC = N,N′-dimethylacetamide. d Co-HAP = Co nanoparticles dispersed on the surface of hydroxyapatite. | |||||
| 1 | CuO | CH3CN | >99 | 95 | This work |
| 2 | RuO2/Co3O4/CeO2 | DCE a | 97.7 | 95 | 33 |
| 3 | CrO3 | CNb | 42 | 75 | 41 |
| 4 | CrMCM | — | 41.5 | 74.2 | 42 |
| 5 | NHPA + DADCAQ | DMAC c | 84 | 69 | 32 |
| 6 | Co-HAP d | CH3CN | 45.6 | 57.4 | 30 |
| 7 | Co3O4 | CH3CN | 48.1 | 51.3 | 43 |
To conclude, a facile hydrothermal route to single-crystalline CuO nanostructures with a rod-like morphology is developed. The as-synthesized sample exhibits excellent catalytic oxidation of cyclohexene to 2-cyclohexene-1-one. A nearly 100% cyclohexene conversion with 95% selectivity of 2-cyclohexene-1-one has been obtained using TBHP as the oxidant in CH3CN with the as-prepared CuO nanorod catalysts. These results may provide a novel method for synthesis of α,β-unsaturated ketones directly from olefins.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 20973151), Specialized Research Fund for the Doctoral Program of Higher Education (SRFDP, 20093250110001), the Foundation of Jiangsu Provincial Key Program of Physical Chemistry in Yangzhou University and the Foundation of Jiangsu Key Laboratory of Fine Petrochemical Technology. The authors also acknowledge the Foundation of the Educational Committee of Jiangsu Provincial General Universities Graduate Student Scientific Research Invention Plan.Notes and references
- I. T. Ho, J. H. Chu and W. S. Chung, Eur. J. Org. Chem., 2011, 1472 CrossRef CAS.
- N. Zhao, Y. H. Wu, L. X. Shi, Q. P. Lin and Z. N. Chen, Dalton Trans., 2010, 39, 8288 RSC.
- A. Nakhai and J. Bergman, Tetrahedron, 2009, 65, 2298 CrossRef CAS.
- M. Oba, T. Saegusa, N. Nishiyama and K. Nishiyama, Tetrahedron, 2009, 65, 128 CrossRef CAS.
- E. Alvarez-Manzaneda, R. Chahboun, E. Cabrera, E. Alvarez, A. Haidour, J. M. Ramos, R. Alvarez-Manzaneda, R. Tapia, H. Es-Samti, A. Fernández and I. Barranco, Eur. J. Org. Chem., 2009, 1139 CrossRef CAS.
- T. Kajiwara, T. Terabayashi, M. Yamashita and K. Nozak, Angew. Chem., Int. Ed., 2008, 47, 6606 CrossRef CAS.
- A. Deshmukh, A. Kinage, R. Kumar and R. Meijboom, Polyhedron, 2010, 29, 3262 CrossRef CAS.
- Y. Cai, X. Liu, J. Jiang, W. Chen, L. Lin and X. Feng, J. Am. Chem. Soc., 2011, 133, 5636 CrossRef CAS.
- M. Sada and S. Matsubara, J. Am. Chem. Soc., 2010, 132, 432 CrossRef CAS.
- A. Böhme, D. Thaens, F. Schramm, A. Paschke and G. Schüürmann, Chem. Res. Toxicol., 2010, 23, 1905 CrossRef.
- A. Böhme, D. Thaens, F. Schramm, A. Paschke and G. Schüürmann, Chem. Res. Toxicol., 2009, 22, 742 CrossRef.
- X. Huang, Y. H. Wen, F. T. Zhou, C. Chen, D. C. Xu and J. W. Xie, Tetrahedron Lett., 2010, 51, 6637 CrossRef CAS.
- A. W. Zijl, A. J. Minnaard and B. L. Feringa, J. Org. Chem., 2008, 73, 5651 CrossRef.
- L. Alessandra, Curr. Org. Synth., 2008, 5, 117 CrossRef.
- M. Hubert and G. K. Wolfgang, US Patent, 2001, US 6177400 Search PubMed.
- Z. Yang, M. Fonovic, S. H. L. Verhelst, G. Bluma and M. Bogyo, Bioorg. Med. Chem., 2009, 17, 1071 CrossRef CAS.
- N. M. Xavier and A. P. Rauter, Carbohydr. Res., 2008, 343, 1523 CrossRef CAS.
- J. Jia, M. G. Steinmetz, R. Shukla and R. Rathore, Tetrahedron Lett., 2008, 49, 4621 CrossRef CAS.
- A. Imanishi, S. Yamane and Y. Nakato, Langmuir, 2008, 24, 10755 CrossRef CAS.
- D. N. Kirk and M. P. Hartshorn, Steroid reaction mechanisms, Elsevier, Amsterdam, 1969 Search PubMed.
- E. W. Warnhoff, J. Org. Chem., 1962, 27, 4587 CrossRef CAS.
- S. J. Ji, E. Takahashi, T. T. Takahashi and C. A. Horiuchi, Tetrahedron Lett., 1999, 40, 9263 CrossRef CAS.
- R. A. Jerussi and D. Speyer, J. Org. Chem., 1966, 31, 3199 CrossRef CAS.
- Y. W. Zhu, W. B. Yi and C. Cai, J. Fluorine Chem., 2011, 132, 71 CrossRef CAS.
- J. M. Concellón, H. Rodríguez-Solla and C. Méjica, Tetrahedron, 2006, 62, 3292 CrossRef.
- W. Yu, M. Su and Z. Jin, Tetrahedron Lett., 1999, 40, 6725 CrossRef CAS.
- S. Kim, S. W. Bae, J. S. Lee and J. Park, Tetrahedron, 2009, 65, 1461 CrossRef CAS.
- X. Huang and Y. Yang, Org. Lett., 2007, 9, 1667 CrossRef CAS.
- J. S. Yadav, B. V. S. Reddy and P. Vishnumurthy, Tetrahedron Lett., 2008, 49, 4498 CrossRef CAS.
- Y. Zhang, Z. Li, W. Sun and C. Xia, Catal. Commun., 2008, 10, 237 CrossRef CAS.
- H. E. B. Lempers and R. A. Sheldon, Appl. Catal., A, 1996, 143, 137 CrossRef CAS.
- X. Tong, J. Xu, H. Miao, G. Yang, H. Ma and Q. Zhang, Tetrahedron, 2007, 63, 7634 CrossRef CAS.
- M. Ghiacia, B. Aghabararia, A. M. B. Regob, A. M. Ferrariab and S. Habibollahic, Appl. Catal., A, 2011, 393, 225 CrossRef.
- L. Xu, S. Sithambaram, Y. Zhang, C. H. Chen, L. Jin, R. Joesten and S. L. Suib, Chem. Mater., 2009, 21, 1253 CrossRef CAS.
- L. Rout, S. Jammi and T. Punniyamurthy, Org. Lett., 2007, 9, 3397 CrossRef CAS.
- V. P. Reddy, A. V. Kumar, K. Swapna and K. R. Rao, Org. Lett., 2009, 11, 951 CrossRef CAS.
- D. Alves, C. G. Santos, M. W. Paixäo, L. C. Soares, D. d Souza, O. E. D. Rodrigues and A. L. Braga, Tetrahedron Lett., 2009, 50, 6635 CrossRef CAS.
- S. Jammi, S. Sakthivel, L. Rout, T. Mukherjee, S. Mandal, R. Mitra, P. Saha and T. Punniyamurthy, J. Org. Chem., 2009, 74, 1971 CrossRef CAS.
- R. M. Liou and S. H. Chen, J. Hazard. Mater., 2009, 172, 498 CrossRef CAS.
- Y. Feng and X. Zheng, Nano Lett., 2010, 10, 4762 CrossRef CAS.
- G. Rothenberg, H. Wiener and Y. Sasson, J. Mol. Catal. A: Chem., 1998, 136, 253 CrossRef CAS.
- S. E. Dapurkar, H. Kawanami, K. Komura, T. Yokoyama and Y. Ikushima, Appl. Catal., A, 2008, 346, 112 CrossRef CAS.
- N. R. E. Radwana and H. G. El-Shobaky, Thermochim. Acta, 2000, 360, 147 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details and the GC-MS diagrams of all the products. See DOI: 10.1039/c1cy00274k |
| This journal is © The Royal Society of Chemistry 2012 |
