Mesoporous TiO2 photocatalytic films on stainless steel for water decontamination
Jia Hong
Pan
ab,
Zhibin
Lei
a,
Wan In
Lee
c,
Zhigang
Xiong
a,
Qing
Wang
b and
X. S.
Zhao
*ad
aDepartment of Chemical and Biomolecular Engineering, Faculty of Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore 117576, Singapore
bDepartment of Materials Science and Engineering, Faculty of Engineering, NUSNNI-NanoCore, National University of Singapore, 5 Engineering Drive 2, Singapore 117576, Singapore
cDepartment of Chemistry, Inha University, Incheon 402, -75, 1, Korea
dSchool of Chemical Engineering, The University of Queensland, St Lucia, QLD 4072, Australia. E-mail: George.zhao@uq.edu.au; Fax: +61-7-33654199; Tel: +61-7-33469997
First published on 11th October 2011
Abstract
Coating of mesoporous TiO2 nanocrystalline films on stainless steel (Grade 304) was conducted using an evaporation-induced self-assembly (EISA) process in the presence of a PluronicF127 triblock copolymer. The photocatalytic properties of mesoporous TiO2 films were evaluated using orange II in aqueous solution under ultraviolet irradiation. Surface analysis shows that leaching of metals (e.g., Fe, Cr) from the stainless steel occurred during the EISA process. The leached metal species came to the interface between the titanium species and the copolymer, adversely influencing the self-assembly of the mesophase, leading to a poor mesoporous structure and a low photocatalytic activity in the resultant TiO2 films. Pre-treatment of stainless steel by acid washing followed by coating a SiO2–SnO2 interlayer has been demonstrated to effectively avoid the acid leaching and thermal diffusion of metal species from stainless steel. The photocatalytic activities of the subsequently deposited mesoporous TiO2 films were significantly improved, which can be attributed to the enhanced charge carrier separation and excellent optical reflection properties of the interlayer.
1. Introduction
Heterogeneous photocatalysis has been widely explored as an environmentally friendly technology for water purification.1–3Titanium dioxide (TiO2) is amongst the most popular and reliable photocatalysts.4–6 Recent research effort has been focused on the design and preparation of nanostructured TiO2 with a prescribed crystal phase, surface area, and morphology.7,8 Most of the photocatalysts were used as powder and operated in a slurry photoreactor. To recycle TiO2 and get the treated water power-free, separation of the suspended TiO2 is indispensable, which causes additional costs and hinders the commercialization of photocatalytic technology.9–13 Hence, attempts have been made to develop alternative photoreactors based on the immobilized TiO2. Recently, their continuous operations with high energy efficiency and excellent performance in water purification have been demonstrated, which may be beneficial to the current water treatment industry.14–20 Typically in Singapore, short-wavelength UV photolysis has been set as the final treatment process for the ultimate purification of drinking water prior to making it accessible to the public. Immobilized TiO2 on current UV photolysis reactors not only improves the purification efficiency without significantly altering the original process and infrastructure, but also increases the water quality by completely mineralizing the recalcitrant organic pollutants that are resistant to destruction only by UV irradiation.The photocatalytic performance of the immobilized photocatalyst is critically dependent on the specific surface area.17,19,21–23 Increasing effort has been devoted to designing porous TiO2 coating with large surface area, well-crystallized, nanosized anatase, and excellent mechanical stability.6,7 In this context, mesoporous TiO2 films have been investigated to display favourable features, such as proper pore-wall parameters for efficient mass exchange, improved light harvesting,19 as well as superior photocatalytic activities in degrading organic pollutants and inactivating microorganisms.6,24–32 The so-called evaporation-induced self-assembly (EISA) process provides a facile approach to the preparation of highly organized mesoporous TiO2-based films.19,33,34 The preparation is generally derived from a highly acidic (pH <0) sol solution to stabilize the Ti precursor for the subsequent cooperative assembly with the block copolymer template. Various substrates, including Pyrex glass,35–37 transparent conductive oxides,37–39 ceramic membranes,17silicon,40 and stainless steel,41 have been employed for growing mesoporous TiO2 films. It has been observed that the surface roughness, composition, and thermal and chemical stability of the substrate have a direct influence on the purity, crystallinity, mesostructural ordering, and photocatalytic activity of the resultant TiO2 films.19,36,40 Previous studies have mainly focused on glass substrates. Industrially important materials like stainless steel have been investigated only by few groups.41–43 Different from that of glass substrates, the surface of stainless steel is neither smooth nor chemically inert, bringing in many uncertainties into the system of film coating.
In the present work, we investigated the diffusion behaviours of metal species from stainless steel to mesoporous TiO2 films prepared via an EISA process in the presence of a PluronicF127 block copolymer template. The effect of metal diffusion on the morphology, surface property, and photocatalytic activity of the fabricated mesoporous TiO2 films was studied. Attempts to avoid the metal diffusion and thus to improve the photocatalytic activity by introducing a SiO2-based interlayer between the stainless steel and the mesoporous TiO2 film were then described. Ultimately, the underlying mechanism related to the enhanced photocatalytic activity was scrutinized.
2. Experimental section
2.1 Sample preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.005
0.005![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.75
1.75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 25. After being stirred for 3 h, 80 μl of sol solution was taken and deposited on stainless steel substrates by spin-coating. The obtained films were then aged for 3 days in a humid chamber with a constant humidity of 60%. After aging, the films were annealed at 150 °C for 12 h (labelled as as-synthesized samples) and finally calcined at 400 °C in static air for 4 h at a ramping rate of 1 °C min−1. For reference, mesoporous TiO2 films deposited on Pyrex glass were also prepared via the same process. We designated mesoporous TiO2 films deposited on glass, as-received stainless steel, and pre-treated stainless steel as TiO2/Glass, TiO2/as-SS, and TiO2/SS, respectively. All of the calcined films deposited on these three types of solid substrates possessed a similar amount of TiO2 (around 2.7 mg) with a thickness of around 320 nm as we reported before.44,48
25. After being stirred for 3 h, 80 μl of sol solution was taken and deposited on stainless steel substrates by spin-coating. The obtained films were then aged for 3 days in a humid chamber with a constant humidity of 60%. After aging, the films were annealed at 150 °C for 12 h (labelled as as-synthesized samples) and finally calcined at 400 °C in static air for 4 h at a ramping rate of 1 °C min−1. For reference, mesoporous TiO2 films deposited on Pyrex glass were also prepared via the same process. We designated mesoporous TiO2 films deposited on glass, as-received stainless steel, and pre-treated stainless steel as TiO2/Glass, TiO2/as-SS, and TiO2/SS, respectively. All of the calcined films deposited on these three types of solid substrates possessed a similar amount of TiO2 (around 2.7 mg) with a thickness of around 320 nm as we reported before.44,48
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 g mol−1) was then added. The molar ratio of the final composition in the sol was as follows: (Sn or Ce)/Si/NH3/PEG = 1
000 g mol−1) was then added. The molar ratio of the final composition in the sol was as follows: (Sn or Ce)/Si/NH3/PEG = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 × 10−3. The resultant sol was deposited onto stainless steel substrates by spin-coating. The obtained films were calcined at 500 °C for 2 h at a ramp rate of 5 °C min−1. The prepared nonporous SnO2–SiO2 and CeO2–SiO2 passive interlayers with thickness of 250–300 nm were subsequently used for the deposition of mesoporous TiO2 films according to the same procedures described above. Pure SiO2 films were prepared with a similar procedure in a 0.01 mol L−1 solution of TEOS but without using Ti and Ce sources. We designated worm-like mesoporous TiO2 nanocrystalline films deposited on stainless steel with an interlayer of SiO2 (SiO2/SS), SnO2–SiO2 (SnO2/SS), and CeO2–SiO2 (CeO2/SS) as TiO2/SiO2/SS, TiO2/SnO2/SS, and TiO2/CeO2/SS, respectively.
2 × 10−3. The resultant sol was deposited onto stainless steel substrates by spin-coating. The obtained films were calcined at 500 °C for 2 h at a ramp rate of 5 °C min−1. The prepared nonporous SnO2–SiO2 and CeO2–SiO2 passive interlayers with thickness of 250–300 nm were subsequently used for the deposition of mesoporous TiO2 films according to the same procedures described above. Pure SiO2 films were prepared with a similar procedure in a 0.01 mol L−1 solution of TEOS but without using Ti and Ce sources. We designated worm-like mesoporous TiO2 nanocrystalline films deposited on stainless steel with an interlayer of SiO2 (SiO2/SS), SnO2–SiO2 (SnO2/SS), and CeO2–SiO2 (CeO2/SS) as TiO2/SiO2/SS, TiO2/SnO2/SS, and TiO2/CeO2/SS, respectively.
2.2 Characterization
X-ray diffraction (XRD) patterns were obtained by using an X-ray diffractometer (Shimadzu XRD-6000) with a monochromated high-intensity Cu Kα radiation of wavelength λ = 0.15418 nm. X-ray photoelectron spectroscopy (XPS) analyses of thin films were carried out in an ultrahigh vacuum (UHV) chamber with a base pressure below 5 × 10−9 Torr at room temperature. Photoemission spectra were recorded by a Sigma Probe Instrument (Thermo VG, UK) equipped with a standard monochromatic Al Kα excitation source (hν = 1486.6 eV). The binding energy (BE) scale was calibrated by measuring a C 1s peak at 284.5 eV from the surface contamination. The surface structure of TiO2 films was observed by a field-emission scanning electron microscope (JEOL JSM-6700F) operated at 5 kV. For the observation of prepared mesoporous TiO2 films by a transmission electron microscope (TEM, JEOL JEM 2010) operated at 200 kV, the samples were peeled off from the substrate, and the collected flakes were gently dispersed in methanol. The suspension was then dropped on a holey amorphous carbon film deposited on a Ni grid (JEOL Ltd.). N2 adsorption–desorption isotherms were measured at liquid nitrogen temperature (77 K) using a surface area and porosimetry analyzer (Nova 100, Quantachrome Instruments). Before measurement, the mesoporous TiO2 samples were outgassed under vacuum for 5 h at 250 °C. The Brunauer–Emmett–Teller (BET) equation was used to calculate the surface area from adsorption data obtained at P/P0 = 0.01–0.31. The total volume of micro- and mesopores was calculated from the amount of nitrogen adsorbed at P/P0 = 0.975–0.977, assuming that adsorption on the external surface was negligible compared to adsorption in pores. The average pore diameter was estimated using the Barrett–Joyner–Halenda (BJH) method from the desorption branch of the isotherm. The optical reflections of interlayers deposited on stainless steel were recorded by a UV-Vis-NIR scanning spectrophotometer (Shimadzu UV-3101 PC) over a wavelength range of 250–650 nm. Photoluminescence (PL) spectra were collected on a Perkin-Elmer luminescence spectrometer (LS-50B) at room temperature.2.3 Photocatalytic activity measurements
The photocatalytic performances of the synthesized samples were tested in an open reactor under UV irradiation, and orange II was used as a model organic pollutant. The stainless steel coated with mesoporous TiO2 films was immersed vertically in 100 mL orange II aqueous solution with an initial dye concentration of 2 mg L−1. Then the solution was magnetically stirred in the dark for 30 min to reach the adsorption equilibrium of orange II with mesoporous TiO2 film. The film-coated side of the substrate was exposed to a low-pressure germicidal lamp (Philips TUV PL-S 9W) which provides constant short-wave UVC irradiation with a peak wavelength of 253.7 nm. Photocatalytic reaction commenced upon turning on the UV lamps, and UV-Vis absorption spectra were recorded at different time intervals to monitor the reaction. The concentration of orange II left in the aqueous system was measured by detecting the absorption at 485.2 nm on a UV-visible spectrophotometer (UV-1700 Shimadzu).3. Results and discussion
3.1 Diffusion properties of metal species
Our mesoporous TiO2 films were derived from an HCl-stabilized sol solution, which was highly acidic with a pH value much less than 0. Upon deposition, the stainless steel was inevitably subjected to the acid attack, and Cl− ions could accelerate the corrosion of the stainless steel. The treated stainless steel possessing a Cr-rich surface was fairly stable. No observable colour change appeared, whereas for untreated stainless steel, brown colour appeared at the interface between TiO2 coating and stainless steel during the humidity aging process. With elapse of aging time the colour became deeper gradually, suggesting that the metal species leaked from stainless steel gradually. After humidity aging, the hybrid films were calcined at 400 °C to remove the organics and crystallize the TiO2. It was found that both mesoporous TiO2 films showed good adhesions on the stainless steel.XPS measurement was performed to elucidate the surface chemical compositions of the calcined mesoporous TiO2 films. Fig. 1a shows the XPS survey spectra for TiO2/Glass, TiO2/as-SS, and TiO2/SS. Sharp photoelectron peaks located at BE of 458, 531, and 285 eV could be assigned to Ti 2p, O 1s, and C 1s, respectively. The trace amount of carbon originated from the residual carbon in the mesoporous TiO2 films. Additional Fe and Cr species were detected for those films deposited on the stainless steel. Therefore, Fe and Cr were the major diffusion metals from the stainless steel, in agreement with the previous studies.49–51 The molar ratios for Fe/Cr/Ti were estimated to be 0.032/0.007/1 and 0.098/0.02/1 for TiO2/SS and TiO2/as-SS, respectively. It can be concluded that Fe and Cr diffused from the stainless steel substrate to the interface of TiO2/as-SS induced by acid leaching and thermal diffusion, while for TiO2/SS, thermal diffusion was the main pathway. The existence of metal species impurities on the TiO2 surface undoubtedly decreased the number of active sites for photocatalysis.
 | ||
| Fig. 1 XPS survey spectra (a) and high-resolution XPS spectra of Fe 2p (b) and Cr 2p (c) for calcined mesoporous TiO2 films coated on glass and stainless steel substrates with/without pre-treatment. | ||
Fig. 1b and c compare the high-resolution XPS spectra for Fe 2p and Cr 2p for TiO2/SS and TiO2/as-SS. Both samples had similar XPS peaks located at the same BE values. In Fig. 1b, the two main XPS peaks of Fe 2p at the BE of approximately 710.3 and 724.1 eV could be assigned to Fe 2p3/2 and Fe 2p1/2, respectively. These BE values were virtually identical to those for pure Fe2O3.52–54 The two small peaks, indicated with asterisks, were satellite peaks of Fe 2p3/2 and Fe 2p1/2. From Fig. 1c of Cr 2p spectra, two main XPS peaks at the BE of 576.6 and 586.4 eV, assigned to Cr 2p3/2 and Cr 2p1/2, could be seen. However, after pre-treatment of stainless steel, the intensity for the deposited mesoporous TiO2 films was considerably decreased, which was related to relatively less Fe and Cr species present on the film surface. Apparently, the diffused metal species existing on the film surface were fully oxidized after calcination.
3.2 Effect of metal diffusion on the textural properties of films
The effect of substrates on the mesostructural ordering and crystal phase of mesoporous TiO2 films was characterized by XRD measurements as summarized in Fig. 2. Fig. 2a shows the small-angle XRD patterns for the as-synthesized and calcined (inset) mesoporous TiO2 films coated on glass and stainless steel substrates with and without pre-treatments. For the as-synthesized block copolymer-templated TiO2 films, the diffraction pattern for TiO2/Glass showed a main diffraction peak in the 2θ range of 0.9–1.3°, which could be indexed as (200) according to the body-centered-cubic Im3m symmetry. Apparently, the as-synthesized block copolymer–TiO2 hybrid films preserved a highly organized mesostructure. However, sample TiO2/SS presented a broad and less intensive (200) diffraction peak. The notable degradation in mesostructure ordering is largely ascribed to the fact that the cooperative assembly between Ti species and Pluronic F127 was disturbed by the higher roughness of stainless steel and the metal ion diffusion. Ortel et al.42 have demonstrated that metal ions are able to complex with the hydrophilic PEO subunit in Pluronic F127, which significantly interferes with the self-assembly between the triblock copolymer and Ti species and weakens their hydrogen bonding interactions. Eventually, the ordering of the resulting mesostructure is inevitably decreased. Our experiment validated their findings. Indeed, diffraction peaks at the small-angle range were absent for TiO2/as-SS, which further proved that the leached metal species had a negative impact on the self-assembly of the Ti precursor and copolymer. Hence, poor mesostructure ordering was formed on as-received stainless steel. Upon calcination, both TiO2/SS and TiO2/as-SS did not show any diffraction at the low-angle range, as shown in the inset of Fig. 2a, suggesting the loss of long-range ordering in the final mesoporous nanocrystalline structure. | ||
| Fig. 2 (a) Low-angle XRD patterns of the as-synthesized mesostructured F127- TiO2 hybrid films deposited on glass or stainless steel with/without pre-treatment (shown in the inset of Fig. 2a are the low-angle XRD patterns of the samples after calcination), and (b) wide-angle XRD patterns of the samples after calcination. | ||
The crystalline structure and phase composition of the resultant mesoporous TiO2 films were revealed by wide-angle XRD patterns. To eliminate the intervention of substrates, powdered samples were collected by peeling mesoporous TiO2 films off from the substrate. Fig. 2b shows wide-angle XRD patterns for the corresponding samples. The peak positions in all samples matched well with the diffraction patterns of the bulk anatase (JCPDS 21-1272). TiO2/Glass exhibited most intensive and sharpest diffraction peaks, corresponding to the highest crystallinity and largest crystal size. On the other hand, films deposited directly on untreated SS showed the broadest peak width and lowest peak intensity when viewing from the (101) peak at 25.6°. Therefore, part of the leaked metal ions during deposition and aging may be doped into the crystal lattices of TiO2, which retards the phase transformation and crystal growth of anatase.19,20,45
TEM and N2 sorption measurements were carried out to further elucidate the difference in mesoporous ordering of the calcined mesoporous TiO2 films. Representative TEM micrographs are shown in Fig. 3. TiO2/Glass showed a highly ordered 3-D cubic mesoporous structure. The pore size distribution was quite uniform at 6–8 nm (Fig. 3a and b). An obvious degradation in mesoporous structure ordering occurred when the substrate was changed to stainless steel. A worm-like mesoporous structure was formed in TiO2/SS (Fig. 3c), while TiO2/as-SS possessed a similar mesoporous structure but smaller mesopores with some collapses (Fig. 3d).
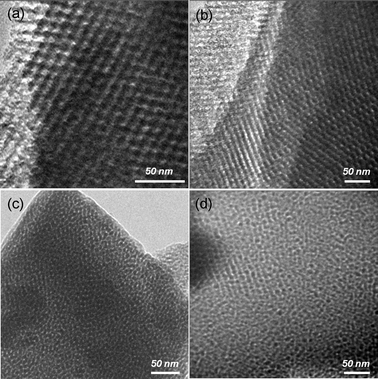 | ||
| Fig. 3 TEM micrographs for mesoporous TiO2 films deposited on glass or stainless steel: TiO2/Glass (a,b); TiO2/SS (c); and TiO2/as-SS (d). | ||
A similar trend was also observed from N2 adsorption analysis. Fig. 4 shows the N2 adsorption–desorption isotherms and pore diameter distribution plots (inset) for mesoporous TiO2 films deposited on different substrates and calcined at 400 °C. All the samples exhibited typical type-IV isotherm plots with sharp capillary condensation steps, indicating the possession of cylinder-shaped mesoporous structures.19,45–47 All the films also possessed similar large surface areas of 96–115 m2 g−1. The pore size for TiO2/Glass and TiO2/SS was around 6.8 nm, whereas TiO2/as-SS possessed a much smaller pore size of 3.9 nm with a wider distribution. This may be due to the smaller crystal size of TiO2 and collapse of mesoporous structure as a result of the poor quality of the self-assembly process due to the metal leakage. Apparently, a small pore size of TiO2/as-SS would decrease its absorption ability and mass exchange efficiency. Table 1 summarizes the textural properties of mesoporous TiO2 films deposited on different substrates.
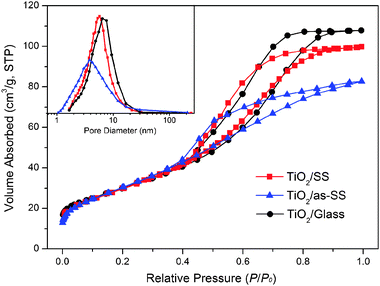 | ||
| Fig. 4 N2 adsorption–desorption isotherms and pore size distribution plots (inset) for mesoporous TiO2 films deposited on glass or stainless steel with/without pre-treatment and finally calcined at 400 °C. | ||
| Sample | L/nm | Crystallinity (%) | S BET/m2 g−1 | D/nm | V p/cm3 g−1 |
|---|---|---|---|---|---|
| L: crystal size; SBET: specific surface area; D: mean pore diameter; and Vp: pore volume. | |||||
| TiO2/Glass | 10.3 | 86.3 | 107.8 | 6.9 | 0.157 |
| TiO2/SS | 10.1 | 85.8 | 98.8 | 6.8 | 0.145 |
| TiO2/as-SS | 8.9 | 72.5 | 112.5 | 3.9 | 0.127 |
3.3 Photocatalytic activities of mesoporous TiO2 films on stainless steel
The photocatalytic activity evaluation was carried out at ambient temperature using orange II as the probe molecule. Fig. 5 shows the photocatalytic degradation results for mesoporous TiO2 films deposited on stainless steel and glass. The initial concentration of orange II was kept at 2 ppm. Control tests under UV irradiation without any photocatalyst revealed that orange II shows excellent resistance to UV irradiation and a negligible amount (∼5%) of orange II was degraded by photolysis. Therefore TiO2 is believed to be the main active site on which photocatalytic reaction takes place. Among the various substrates used, TiO2/Glass showed the highest photocatalytic activity, whereas TiO2/as-SS presented lowest photocatalytic activity. Therefore, stainless steel may not be an appropriate substrate for direct deposition of mesoporous TiO2 films with high performances in photocatalysis. The decrease in photocatalytic activity is mostly due to the metal diffusion from SS. Firstly, appreciable active sites vanish from the TiO2 surface because of the occupation by the diffused Fe and Cr species. Secondly, ion diffusion inhibits the crystallization of TiO2 films and the subsequent crystal growth of anatase nanocrystallites, as can been seen in Table 1. Thirdly, the mass exchange between pollutants and degradation products is partially blocked due to the relatively smaller pore size and lower degree of ordering in mesoporous structure. Finally, Fe and Cr species can act as recombination centers for charge carriers during photocatalysis, which will be demonstrated by photoluminescence analysis and shown in the following section.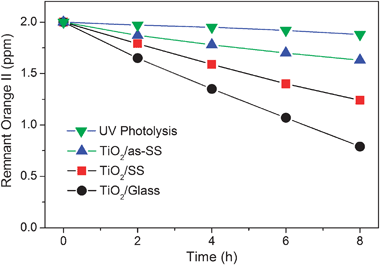 | ||
| Fig. 5 Photocatalytic decoloration of orange II over mesoporous TiO2 films deposited on different substrates under UV irradiation. | ||
3.4 Design of passive interlayers on stainless steel
As discussed above, substrate texture plays an important role in the final microstructures of mesoporous TiO2 films. The mesoporous structure, surface area, final compositions of the surface and pore-wall, and photocatalytic activity of mesoporous TiO2 coatings are negatively affected by high roughness and, more importantly, low chemical resistance to acid and high metal mobility of SS. To inhibit the diffusion of metals and to improve the photocatalytic activity of TiO2 coating on stainless steel, we introduced a passive interlayer between stainless steel and mesoporous TiO2 films. SiO2-based coating was employed due to its high stability, non-toxicity, low cost, easy preparation, as well as the facile formation of an interconnected tetrahedral silica network that shows considerable resistance to the metal diffusion. We further introduced photo-excitable metal oxides, SnO2 and CeO2, into the passive layer to improve the photocatalytic activity of mesoporous TiO2 films. The synthesis was carried out under alkaline conditions by adding NH3·H2O. It was found that NH3·H2O is essential to form the high-quality homogenous coating solution. It not only governs the hydrolysis of TEOS, but also reacts with the precipitated metal hydroxides forming dissolvable metal–ammonia complexing compounds. After aging at 100 °C, the films were calcined at 500 °C, by which nonporous bicomponent thin films with amorphous SiO2 together with crystalline SnO2 or CeO2 were thus obtained.Wide-angle powder XRD patterns confirm that the metal oxides were fully crystallized independently, and no solid solution phase was found in the composite oxides. As shown in Fig. 6, all the diffraction peaks for SnO2–SiO2 and CeO2–SiO2 well matched the tetragonal cassiterite (JCPDS 41-1445) and cubic fluorite (JCPDS 34-394) structures, respectively, and no additional peak for the solid solution phase was found. Obviously, upon calcination in air Sn2+ and Ce3+ were fully oxidized and formed SnO2 and CeO2, respectively.
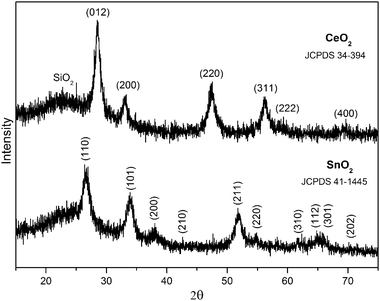 | ||
| Fig. 6 XRD patterns for SnO2–SiO2 and CeO2–SiO2 interlayers. | ||
Fig. 7a shows the XPS survey spectra taken on the surface of SnO2/SS (film thickness: 300 nm). Only Sn, Si, O, C were detected for the XPS spectra of SnO2/SS films. High-resolution XPS analysis was further carried out to clarify the existence of Sn as well as the possibly contained Fe and Cr elements. As shown in Fig. 7b, the single XPS peak located at BE of 716.9 eV could be assigned to Sn 3p with an oxidation state of +4, which agrees with the result from XRD analysis. Interestingly, XPS peaks for Fe 2p that might coexist in the same BE range as Sn 3p did not appear in this case, indicating that the introduced SnO2–SiO2 interlayer sufficiently depresses the thermal diffusion and leaking of the metal species from the stainless steel substrates. To further confirm that the presence of the SnO2–SiO2 layer prohibits the diffusion and leaking of metal from SS, a high-resolution XPS scan was also conducted at a BE range of 565–595 eV for Cr 2p. However, no notable peak was found (Fig. 7c). The acidic and thermal resistances of the SnO2–SiO2 interlayer were further elucidated after subsequent deposition of mesoporous TiO2 films forming TiO2/SnO2/SS. As recorded on their surface, the XPS survey spectrum (Fig. 7a) did not show any peak for Fe and Cr species, while a crack amount (∼0.8%) of SnO2 was detected, which is ascribed to the migration of Sn from the substrate to the surface of mesoporous TiO2 films during calcination.
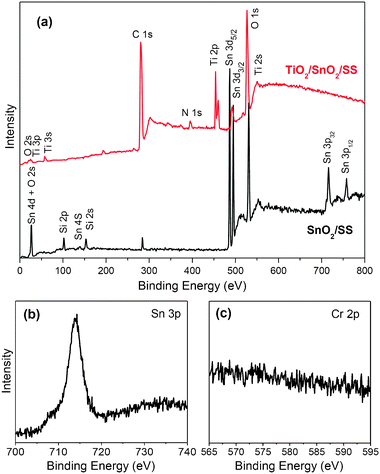 | ||
| Fig. 7 (a) XPS survey spectra of SnO2–SiO2 films deposited on pre-treated stainless steel (SnO2/SS), and the subsequently deposited mesoporous TiO2 films (TiO2/SnO2/SS); high-resolution XPS spectra of Sn 3p (b) and Cr 2p (c) recorded on a SnO2/SS film. | ||
3.5 Enhanced photocatalytic activity with a passive interlayer
The presence of an interlayer sufficiently inhibits the acidic leaking and thermal diffusion of metal from SS substrates, which may be beneficial to the upper photocatalytic TiO2 film. Fig. 8a shows the photocatalytic activity of mesoporous TiO2 films coated with the passive interlayers. The photocatalytic activities of mesoporous TiO2 films were considerably improved with the passive interlayers. For example, the TiO2/SiO2/SS film showed much higher photocatalytic activity than the film directly coated on pre-treated stainless steel. | ||
| Fig. 8 (a) Photocatalytic degradation of orange II over mesoporous TiO2 films with different interlayers on stainless steel; (b) apparent rate constant (k) for mesoporous TiO2 films deposited on various substrates. | ||
According to our previous results,55,56 the degradation of orange II follows the first-order kinetics. Thus, the photocatalytic reaction can simply be described by:
| r = −d[c]/dt = k[c] | (1) |
| −ln[c]/c0 = kt | (2) |
3.6 Role of interlayers in photocatalysis
Photocatalytic activity of TiO2 is dependent on the quantity of separated charge carriers (electrons and holes) participating in photocatalysis. However, a large fraction (60–90%) of electrons and holes can be easily recombined with the emission of photons, which results in the generation of PL signals.57–59 Therefore, PL analysis has been employed recently to indicate the fate of photogenerated electron–hole pairs on the TiO2 surface.60,61Fig. 9a summarizes the PL spectra of mesoporous TiO2 films deposited on different stainless steel-based substrates. Bare stainless steel presented an intensive photoluminescence at a wavelength of around 484 nm, which was derived from the oxygen vacancies in Cr or Fe oxides at the surface of stainless steel.61 With the coating of TiO2 with/without the interlayer, the peak remained due to the strong background from stainless steel and the thin coating with thickness in the range of 300–600 nm.62,63 However, the intensities were significantly decreased, which was ascribed to the relatively lower PL property of TiO2. Thus, the decrease in PL intensity can be used to reflect the charge separation property of TiO2 coating. Lower charge carrier recombination in TiO2 leads to a higher decrease in the intensity of photoluminescence.61 The sequence of decrease in photoluminescence was TiO2/SnO2/SS > TiO2/CeO2/SS > TiO2/SiO2/SS > TiO2/SS > TiO2/as-SS. In our layer-by-layer deposition followed with calcination, a high-quality TiO2–SnO2 heterojunction could be formed at the interface between the mesoporous TiO2 films and the passive SnO2 interlayer, which eases the charge transfer between TiO2 and SnO2 during photocatalysis under UV irradiation. According to the energy-level diagrams of TiO2 and SnO2,64–66 the photo-excited electrons in the TiO2/SnO2/SS system accumulate on the lower-lying conduction band of SnO2, while holes accumulate on the higher-lying valence band of TiO2 consequently (Fig. 9b). Such spatial charge carrier separation would result in high quantum efficiency as well as high photocatalytic activity since holes accumulated at mesoporous TiO2 films are exposed to the contaminants. For narrow-band-gap CeO2 (Eg = 2.7–3.0 eV) with a low lying conduction band, similar electron transfer would occur.67,68
 | ||
| Fig. 9 (a) Photoluminescence spectra of the prepared mesoporous TiO2 films deposited on various substrates or interlayers on stainless steel; (b) an illustration of charge carrier separation on mesoporous TiO2/SnO2/SS films. | ||
The introduction of passive interlayers has been found to tune the optical properties of the substrates. Fig. 10 shows the optical reflections for the SnO2/SS, CeO2/SS and SiO2/SS. The reflection curves for all the films were not smooth, which is due to the rough surface of stainless steel substrates. The only visible edge around 365 nm exhibited in all the samples was related to stainless steel itself. Since the composition in the stainless steel is quite complicated, currently we still do not know which component is related to this characteristic absorption edge. On the other hand, the absorption edges for the semiconducting CeO2 and SnO2 at the wavelength range of 280–350 nm were absent for CeO2/SS and SnO2/SS, which may be due to the strong background from stainless steel. Therefore, only relatively intensive shakes were observed from samples CeO2/SS and SnO2/SS in the UV range. This result also indicated an excellent adhesion between SS and interlayers. Bare stainless steel showed a poor reflection capability. Typically in a UV range of 250–350 nm, the reflection was limited at 1.8–2.7%. In the presence of a passive interlayer, the reflection dramatically increases. For SnO2/SS, the value was 4.8–5.6%. The significant improvement in optical reflection may be mainly attributed to the high reflection nature of SnO2 and CeO2 semiconductors, the decrease in surface roughness with the sol–gel coating, and the probable light interference between the passive interlayers and stainless steel. The enhanced reflection by SnO2/SS or CeO2/SS would greatly improve the reuse of UV light for exciting more charge carriers to participate in the photocatalytic degradation of dye molecules. Further improvement in the photocatalytic activity of mesoporous TiO2 films can be anticipated by optimizing the composition of the semiconductor in the interlayer, nanostructure of the passive interlayer, and band gap engineering in TiO2 and interlayer.
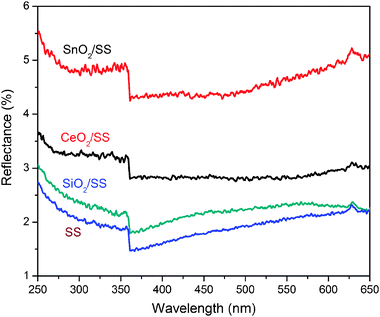 | ||
| Fig. 10 UV-Vis optical reflections over SnO2/SS, CeO2/SS, SiO2/SS and bare stainless steel (SS). | ||
4. Conclusion
In this paper, we have investigated how the metal ion diffusions from a stainless steel substrate negatively influence the surface chemistry, textual property, and photocatalysis of the upper deposited mesoporous TiO2 films. A facile synthesis strategy by integrating substrate pre-treatment and metal oxide-based interlayer deposition has been further developed for the effective suppression of metal ion diffusion. Study on photocatalytic activity in decolouration of orange II serves as an example to establish the composition–structure–property relationship. The presence of interlayers of SnO2 or CeO2 can significantly improve the charge carrier separation and UV light reflection, which leads to enhanced photocatalytic performance. The multi-layer film system may present a promising route to the design and construction of immobilized photocatalysts on stainless steel for efficient water decontamination.Acknowledgements
This work was supported by Environment and Water Industry Development Council (EWI) of Singapore under project number MEWR 651/06/161.Notes and references
- A. Fujishima, T. N. Rao and D. A. Tryk, J. Photochem. Photobiol., C, 2000, 1, 1 CrossRef CAS.
- A. L. Linsebigler, G. Lu and J. T. Yates Jr, Chem. Rev., 1995, 95, 735 CrossRef CAS.
- M. R. Hoffmann, S. T. Martin, W. Choi and D. W. Bahnemann, Chem. Rev., 1995, 95, 69 CrossRef CAS.
- B. Ohtani, Chem. Lett., 2008, 217 CAS.
- J.-M. Herrmann, Appl. Catal., B, 2010, 99, 461 CrossRef CAS.
- A. A. Ismail and D. W. Bahnemann, J. Mater. Chem., 2011, 21, 11686 RSC.
- J. H. Pan, H. Dou, Z. Xiong, C. Xu, J. Ma and X. S. Zhao, J. Mater. Chem., 2010, 20, 4512 RSC.
- X. Wang and R. A. Caruso, J. Mater. Chem., 2011, 21, 20 RSC.
- S. Malato, P. Fernández-Ibáñez, M. I. Maldonado, J. Blanco and W. Gernjak, Catal. Today, 2009, 147, 1 CrossRef CAS.
- X. Zhang, J. H. Pan, A. J. Du, P. F. Lee, D. D. Sun and J. O. Leckie, Chem. Lett., 2008, 424 CrossRef.
- X. Zhang, J. H. Pan, A. J. Du, S. Xu and D. D. Sun, J. Photochem. Photobiol., A, 2009, 204, 154 CrossRef CAS.
- J. H. Pan, X. Zhang, A. J. Du, D. D. Sun and J. O. Leckie, J. Am. Chem. Soc., 2008, 130, 11256 CrossRef CAS.
- X. Zhang, J. H. Pan, A. J. Du, J. Ng, D. D. Sun and J. O. Leckie, Mater. Res. Bull., 2009, 44, 1070 CrossRef CAS.
- D. D. Dionysiou, M. T. Suidan, I. Baudin and J. M. Laiîné, Appl. Catal., B, 2002, 38, 1 CrossRef CAS.
- D. D. Dionysiou, A. A. Burbano, M. T. Suidan, I. Baudin and J. M. Laîné, Environ. Sci. Technol., 2002, 36, 3834 CrossRef CAS.
- G. Balasubramanian, D. D. Dionysiou, M. T. Suidan, I. Baudin and J. M. Laiîné, Appl. Catal., B, 2004, 47, 73 CrossRef CAS.
- A. Julbe, V. Rouessac, J. Durand and A. Ayral, J. Membr. Sci., 2008, 316, 176 CrossRef CAS.
- F. Bosc, A. Ayral and C. Guizard, J. Membr. Sci., 2005, 265, 13 CrossRef CAS.
- J. H. Pan, X. S. Zhao and W. I. Lee, Chem. Eng. J., 2011, 170, 363 CrossRef CAS.
- J. Yu, J. Xiong, B. Cheng and S. Liu, Appl. Catal., B, 2005, 60, 211 CrossRef CAS.
- J. H. Pan, G. Han, R. Zhou and X. S. Zhao, Chem. Commun., 2011, 47, 6942 RSC.
- O. T. Alaoui, Q. T. Nguyen, P. Schaetzel and C. Mbareck, Catal. Sci. Technol., 2011 10.1039/C1CY00179E.
- T. Ochiai, T. Hoshi, H. Slimen, K. Nakata, T. Murakami, H. Tatejima, Y. Koide, A. Houas, T. Horie, Y. Morito and A. Fujishima, Catal. Sci. Technol., 2011 10.1039/C1CY00185J.
- J. H. Pan, S. Y. Chai and W. I. Lee, Mater. Sci. Forum, 2006, 510–511, 58 CrossRef CAS.
- J. C. Yu, X. Wang and X. Fu, Chem. Mater., 2004, 16, 1523 CrossRef CAS.
- M. A. Carreon, S. Y. Choi, M. Mamak, N. Chopra and G. A. Ozin, J. Mater. Chem., 2007, 17, 82 RSC.
- E. Martínez-Ferrero, Y. Sakatani, C. Boissière, D. Grosso, A. Fuertes, J. Fraxedas and C. Sanchez, Adv. Funct. Mater., 2007, 17, 3348 CrossRef.
- H. Li, J. Wang, S. Yin and T. Sato, Res. Chem. Intermed., 2010, 36, 27 CrossRef CAS.
- I. Bannat, K. Wessels, T. Oekermann, J. Rathousky, D. Bahnemann and M. Wark, Chem. Mater., 2009, 21, 1645 CrossRef CAS.
- A. A. Ismail and D. W. Bahnemann, ChemSusChem, 2010, 3, 1057 CrossRef CAS.
- J. Tschirch, D. Bahnemann, M. Wark and J. Rathouský, J. Photochem. Photobiol., A, 2008, 194, 181 CrossRef CAS.
- S. Shamaila, A. K. L. Sajjad, F. Chen and J. Zhang, Appl. Catal., B, 2010, 94, 272 CrossRef CAS.
- C. Sanchez, C. Boissière, D. Grosso, C. Laberty and L. Nicole, Chem. Mater., 2008, 20, 682 CrossRef CAS.
- M. H. Bartl, S. W. Boettcher, K. L. Frindell and G. D. Stucky, Acc. Chem. Res., 2005, 38, 263 CrossRef CAS.
- M. Faustini, L. Nicole, C. Boissière, P. Innocenzi, C. Sanchez and D. Grosso, Chem. Mater., 2010, 22, 4406 CrossRef CAS.
- J. D. Bass, D. Grosso, C. Boissiere and C. Sanchez, J. Am. Chem. Soc., 2008, 130, 7882 CrossRef CAS.
- C. W. Koh, U. H. Lee, J. K. Song, H. R. Lee, M. H. Kim, M. Suh and Y. U. Kwon, Chem.–Asian J., 2008, 3, 862 CrossRef CAS.
- S. Agarwala, M. Kevin, A. S. W. Wong, C. K. N. Peh, V. Thavasi and G. W. Ho, ACS Appl. Mater. Interfaces, 2010, 2, 1844 CAS.
- J. K. Song, U. H. Lee, H. R. Lee, M. Suh and Y. U. Kwon, Thin Solid Films, 2009, 517, 5705 CrossRef CAS.
- Y. Zhang, J. Li and J. Wang, Chem. Mater., 2006, 18, 2917 CrossRef CAS.
- S. Sokolov, E. Ortel and R. Kraehnert, Mater. Res. Bull., 2009, 44, 2222 CrossRef CAS.
- E. Ortel, S. Sokolov and R. Kraehnert, Microporous Mesoporous Mater., 2010, 127, 17 CrossRef CAS.
- S. Sokolov, E. Ortel, J. Radnik and R. Kraehnert, Thin Solid Films, 2009, 518, 27 CrossRef CAS.
- J. H. Pan and W. I. Lee, New J. Chem., 2005, 29, 841 RSC.
- J. H. Pan and W. I. Lee, Chem. Mater., 2006, 18, 847 CrossRef CAS.
- J. H. Pan, D. D. Sun, C. Lee, Y. J. Kim and W. I. Lee, J. Nanosci. Nanotechnol., 2010, 10, 4747 CrossRef CAS.
- J. H. Pan, D. D. Sun and W. I. Lee, Mater. Lett., 2011, 65, 1811 CrossRef.
- Y. J. Kim, Y. H. Lee, M. H. Lee, H. J. Kim, J. H. Pan, G. I. Lim, Y. S. Choi, K. Kim, N.-G. Park, C. Lee and W. I. Lee, Langmuir, 2008, 24, 13225 CrossRef CAS.
- Y. Chen and D. D. Dionysiou, Appl. Catal., B, 2008, 80, 147 CrossRef CAS.
- Y. Chen and D. D. Dionysiou, Appl. Catal., B, 2006, 62, 255 CrossRef CAS.
- Y. Chen and D. D. Dionysiou, Appl. Catal., B, 2006, 69, 24 CrossRef CAS.
- Y. Chen and D. D. Dionysiou, Appl. Catal., A, 2007, 317, 129 CrossRef CAS.
- A. Lv, C. Hu, Y. Nie and J. Qu, Appl. Catal., B, 2010, 100, 62 CrossRef CAS.
- P. Zhang, S. Yin and T. Sato, Appl. Catal., B, 2011, 103, 462 CrossRef CAS.
- G. Li, L. Lv, H. Fan, J. Ma, Y. Li, Y. Wan and X. S. Zhao, J. Colloid Interface Sci., 2010, 348, 342 CrossRef CAS.
- G. Li, X. S. Zhao and M. B. Ray, Sep. Purif. Technol., 2007, 55, 91 CrossRef CAS.
- J. Tang, J. R. Durrant and D. R. Klug, J. Am. Chem. Soc., 2008, 130, 13885 CrossRef CAS.
- H. H. Mohamed, C. B. Mendive, R. Dillert and D. W. Bahnemann, J. Phys. Chem. A, 2011, 115, 2139 CrossRef CAS.
- D. W. Bahnemann, M. Hilgendorff and R. Memming, J. Phys. Chem. B, 1997, 101, 4265 CrossRef CAS.
- Q. Xiang, K. Lv and J. Yu, Appl. Catal., B, 2010, 96, 557 CrossRef CAS.
- J. Zhou, Y. Zhang, X. S. Zhao and A. K. Ray, Ind. Eng. Chem. Res., 2006, 45, 3503 CrossRef CAS.
- B. Liu, X. Zhao, N. Zhang, Q. Zhao, X. He and J. Feng, Surf. Sci., 2005, 595, 203 CrossRef CAS.
- B. Liu, L. Wen and X. Zhao, Mater. Chem. Phys., 2007, 106, 350 CrossRef CAS.
- X. Chen, S. Shen, L. Guo and S. S. Mao, Chem. Rev., 2010, 110, 6503 CrossRef CAS.
- H. Zhang, G. Chen and D. W. Bahnemann, J. Mater. Chem., 2009, 19, 5089 RSC.
- M. Zhou, J. Yu, S. Liu, P. Zhai and L. Jiang, J. Hazard. Mater., 2008, 154, 1141 CrossRef CAS.
- R. Subasri, S. Deshpande, S. Seal and T. Shinohara, Electrochem. Solid-State Lett., 2006, 9, B1 CrossRef CAS.
- P. Patsalas, S. Logothetidis, L. Sygellou and S. Kennou, Phys. Rev. B: Condens. Matter, 2003, 68, 035104 CrossRef.
| This journal is © The Royal Society of Chemistry 2012 |
