The fluorous effect in biomolecular applications
Massimo
Cametti
a,
Benoit
Crousse
b,
Pierangelo
Metrangolo
*acd,
Roberto
Milani
cd and
Giuseppe
Resnati
*ac
aNFMLab–DCMIC “Giulio Natta”, Politecnico di Milano, via Mancinelli 7, I-20131 Milan, Italy. E-mail: pierangelo.metrangolo@polimi.it; giuseppe.resnati@polimi.it; Fax: +39 02 2399 3180; Tel: +39 02 2399 3041 Tel: +39 02 2399 3032
bLaboratoire BioCIS—CNRS, Faculté de Pharmacie, Univ. Paris-Sud, rue J. B. Clément, F-92296 Châtenay-Malabry, France
cCenter for Nano Science and Technology (CNST) of IIT@POLIMI, via Pascoli 70/3, I-20133 Milan, Italy
dVTT, Technical Research Centre of Finland, Biotechnology, Tietotie 2, FIN-02044 VTT, Finland
First published on 20th June 2011
Abstract
From being a niche area only a few decades ago, fluorous chemistry has gained momentum and is, nowadays, a fervent area of research. It has brought forth, in fact, numerous applicative innovations that stretch among different fields: from catalysis to separation science, from supramolecular to materials and analytical chemistry. Recently, the unique features of perfluorinated compounds have reached the attention of the biochemists' audience. This tutorial review introduces the basic concepts of fluorous chemistry and illustrates its main biomolecular applications. Special attention has been given to fluorous microarrays and their combination with Mass-Spectroscopy (MS) techniques, to protein properties modification by the introduction of local fluorous domains, and to the most recent applications of 19F-Magnetic Resonance Imaging (19F-MRI).
 Massimo Cametti | Massimo Cametti obtained the PhD degree at the University of Rome La Sapienza in early 2005. After post-doctoral research activities in Croatia (Ruđer Bošković Institute, Zagreb), Belgium (Université Libre de Bruxelles, Brussels), Italy (CNR-IGAG, Rome) and Finland (University of Jyväskylä), he has recently become tenure-track assistant professor at the Politecnico di Milano. His research interests include supramolecular and host–guest chemistry, soft-materials and artificial photosynthesis. |
 Benoit Crousse | Benoit Crousse received his PhD from the University Paris VI (Dr G. Linstrumelle) in 1995. Then he joined the group of Prof. P. E. Kündig (University of Geneva, Switzerland). Since 2009 he is Senior Scientist Researcher of the Centre National de la Recherche Scientifique (CNRS) and director of the Laboratory of Fluorinated Molecules at the Faculty of Pharmacy—Paris South (Châtenay-Malabry, France). His research interests include fluorine chemistry, new processes using fluorinated alcohols, and the preparation of fluorinated biomolecules. |
 Pierangelo Metrangolo | Pierangelo Metrangolo is full professor at the Politecnico di Milano since 2011. He has been EU Fellow at the University of Toulouse (France, 2001), visiting Professor at the University of York (UK, 2005) and at the University of Jyväskylä (FI, 2006). Since 2010 he also holds a position of visiting professor of molecular recognition at the VTT—Technical Research Center of Finland. His awards include the 2005 ‘‘G. Ciamician’’ medal of the Division of Organic Chemistry of the Italian Chemical Society and the 2005 Journals Grant Award of the Royal Society of Chemistry. His research interests include crystal engineering, supramolecular and biomimetic materials. |
 Roberto Milani | Roberto Milani obtained a joint PhD degree in Structure and Dynamics of Reactive Systems at the University of Lille I (France) and in Materials Science and Engineering at the University of Padova in 2008. He has been an invited Post-doc at the University of Lille I in 2009. He is currently Senior Post-Doc at the Center for Nanoscience and Technology of the Italian Institute of Technology (CNST-IIT@POLIMI), and visiting scientist at the VTT—Technical Research Centre of Finland. His research interests include supramolecular chemistry, biomimetic and hybrid materials, and surface functionalization. |
 Giuseppe Resnati | Giuseppe Resnati became full professor at the Politecnico di Milano in 2001. He was NATO fellow at the University of Clemson (USA, 1990), visiting scientist at the Université Paris XI (France, 1993) and Nagoya University (Japan, 2001). He is in the Editorial Board of the Journal of Fluorine Chemistry and was awarded the 2008 Research Prize of the Division of Organic Chemistry of the Italian Chemical Society and the European Lectureship in Chemical Science by the Royal Society of Chemistry in 2010. His research interests are fluorine chemistry and self-assembly processes. |
1. Introduction
In general terms, fluorous chemistry deals with the study of the structure, composition, properties, and reactions of highly fluorinated molecules, molecular fragments, materials, and media.1 Nowadays, the term fluorous2 is quite commonly found in the literature, in many different fields of the chemical sciences. It was not so at the time Horváth first coined it as diametrical opposed to the term aqueous. From a historical point of view, the incipiency of fluorous chemistry can be traced back to the PhD defence of M. Vogt, student at the University of Aachen. His thesis described the application of perfluorinated polyethers for the immobilization of homogeneous catalysts.3 Such inconspicuous start was soon followed by the work of Zhu, in 1993, who reported the use of perfluorocarbons (PFCs) as solvents for azeotropic separations.4 However, these works were generally overlooked until 1994, when Horváth and Rábai described, and applied, the concept of “fluorous biphasic catalysis” in a widely advertised article.5 From that time on, a vigorous research in the field has continuously thrived and expanded throughout world laboratories. These efforts have generated many applications in synthesis and separation technologies,6–9 in catalysis (both as fluorous biphasic systems and catalysts)10–13 and supramolecular chemistry,14–17 as well as in novel technological developments (microarrays).18–26The term fluorous has now been adopted into general use and the flow of publications and patents on this topic shows a steady increase in the last few years. The innovative features of fluorous chemistry derive from the unique properties of the fluorine atom exerted when introduced into organic molecular frameworks. The introduction of a single fluorine atom into a molecular fragment already induces significant effects, but as the number of fluorine atoms increases the material starts to behave as a completely novel species. Striking is the comparison between the physico-chemical properties of perfluorocarbons and hydrocarbons, extensively described elsewhere.27–29 Fluorous compounds are usually colourless liquids of high density, inert, nonpolar, and amphiphobic, i.e., at the same time hydrophobic and lipophobic. For these reasons, they are usually immiscible with organic solvents, at room temperature, and water (Fig. 1), as well as with ionic liquids. This behaviour is sometimes referred to as the fluorous effect,30 and can simply be described as the tendency of perfluoroalkyl chains to segregate in order to favour fluorine–fluorine interactions, or, more appropriately, to avoid unfavoured interactions of fluorine atoms with other elements. Indeed, due to the very low polarizability of the fluorine atom, fluorous derivatives have a low tendency to interact with any other molecule and thus to aggregate into hydro- and oleo-phobic phases. These peculiar physico-chemical properties of perfluorocarbons first allowed Curran et al. in 1997 to exploit fluorous tags as analogs of solid phase synthesis,31 and have since resulted particularly useful in several biotechnological applications.32
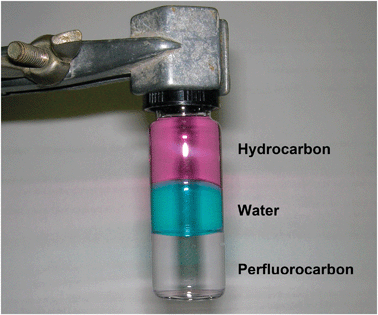 | ||
| Fig. 1 A triphasic system as an example of the immiscibility of PFC solvents with both aqueous and organic phases. | ||
The aim of this tutorial review is to provide the reader with an essential survey of these applications, which all have as a common element the exploitation of the fluorous effect. The most recent and outstanding contributions in the area have been selected and described in detail in order to give an introduction to the field for the non-acquainted. Such contributions encompass fluorous microarrays, sample enrichment/detection for Mass-Spectroscopy (MS) studies, protein properties modification by fluorination, and 19F-Magnetic Resonance Imaging (19F-MRI).
2. Fluorous small molecule microarrays
The evaluation of the affinity which a given host molecule may have towards different, but structurally similar guest species represents a common practice in many research laboratories. In biological systems, such endeavour reaches an acme of complexity and therefore easily implementable methods for multiple affinity assays are highly desirable. Microarray techniques clearly respond to such needs and are nowadays widely applied in molecular biology and DNA studies.These techniques generally rely on robot-assisted ordered spotting of biochemical components onto a solid surface via covalent linkage. This approach is so well developed that incredibly huge arrays, up to tens of thousands of single arrayed spots, can be produced onto a single surface. However, several drawbacks are inherent to such a method. A novel approach to design microarrays, which gets rid of the covalent linkage paradigm, could therefore be extremely valuable. Recently, the particularly strong self-affinity displayed by perfluorocarbons has caught the interest of the people working in the field of microarraying techniques. Indeed, a single perfluoroalkyl tail, typically C8F17-, attached as a tag to a given compound, was demonstrated to ensure its stable immobilization from water solutions onto a perfluorocarbon-coated surface. Hence, the fluorous effect could represent an effective alternative to the covalent immobilization approach.
In 2005 a seminal paper by Pohl et al. introduced the concept of fluorous small molecule microarrays (FSMMAs) applied to the biological screening of carbohydrates.18 A simple synthetic route allowed to append a C8F17-fluorous tag to a series of aldohexose skeletons to form the compounds 1–4 (Scheme 1). Solutions of each of these sugars were then spotted onto a glass microscope slide previously coated via covalent reaction with a perfluoroalkylsilane. After drying, the slide was incubated with fluorescein isothiocyanate-labeled concanavalin A (ConA) and then scanned with a standard fluorescent slide scanner. Where the fluorescent protein found the right sugar partner, association occurred, and a fluorescence signal was detected in the corresponding spot onto the glass surface (Fig. 2).
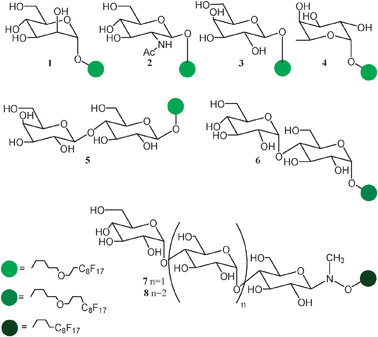 | ||
| Scheme 1 Molecular formulae of fluorous tagged mono-, di-, and poly-saccharides 1–8. | ||
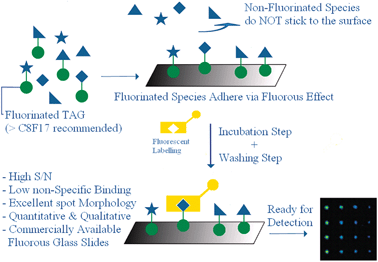 | ||
| Fig. 2 Cartoon illustrating the general concept behind the use of fluorous small molecules microarrays. Positive features are highlighted in blue. | ||
To the best of our knowledge, the study carried out by Pohl et al. was the first proof of the concept that fluorous interactions are strong enough to induce reversible immobilization of biomolecules on microarrays from water solutions. These results were further expanded by Pohl and others. More complex fluorous substrates have been tested on microarrays. For example, disaccharides 5–6,19 and polysaccharides207–8 (Scheme 1) have been successfully spotted onto fluorous-coated glass slides in order to be subjected to affinity tests with lectins.
Future developments in drug design will necessitate fast and reliable screening techniques. The results reported above highlight the FSMMA approach as a very promising method for the fast screening of large synthetic sugars' libraries. Moreover, there is no hamper to quantitative analysis. Studies of the binding of Concanavalin A to L(D)-glycero-D-manno-heptoses in comparison with D-mannose by fluorescent detection on fluorous glass slides were precise enough to demonstrate an unexpectedly small preference of the protein towards the heptose instead of its classically favoured mannose ligand.21
The utility of FSMMAs is not only grounded to the viability of the sugar–lectine interaction in the system under study. The avidin–biotin recognition process has also been investigated to exemplify how FSMMAs have high potential for the discovery of new protein–ligand interactions.22 The same approach can also be applied to the discovery of new enzymes' inhibitors. Indeed, Schreiber and co-workers arrayed a series of candidates, compounds 9a–t, for the inhibition of the histone deacetylase on a fluorous coated surface23 (Scheme 2) and found a good correlation between the data acquired with such a fluorous microarray method and data obtained by conventional biochemical activity assays and by surface plasmon resonance (SPR) methods applied to non-fluorous analogues.
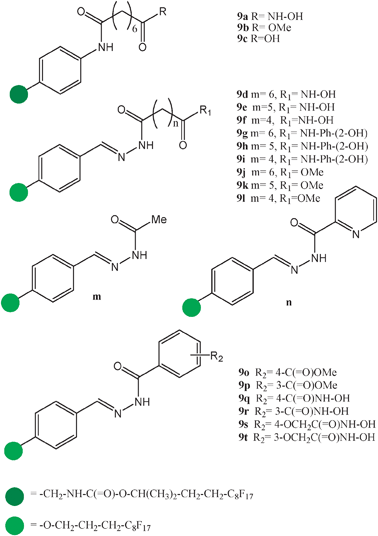 | ||
| Scheme 2 Molecular formulae of a series of fluorous-tagged compounds selected as candidates for the inhibition of the enzyme histone deacetylase and screened for activity onto fluorous microarrays. | ||
Noteworthy are also the studies on enzyme activity conducted on FSMMAs. In a recent work, proteases, i.e., enzymes which catalyse the cleavage of amidic bonds in peptides, have been put at work on a number of peptidic substrates, 10a–f, immobilized onto glass slides via a double C6F13 chain (Scheme 3).24 A fluorescent coumarine unit placed on the linker ensured the fluorescence response, dependent on the cleavage event. The comparison with data produced with more traditional covalent methods, revealed a 10- to 30-fold reduction of the concentration of the peptides necessary to obtain the desired results.
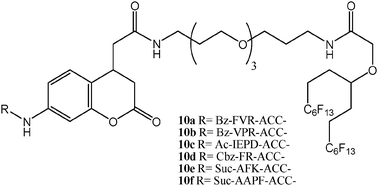 | ||
| Scheme 3 Molecular formulae of peptides decorated with a three section moiety composed of a fluorophore, a linker, and a fluorous tag for microarrays applications (1-letter code identifies amino acids; Bz = benzoyl; Ac = acetyl; Cbz = benzyloxycarbonyl; Suc = 3-carboxy-propionyl). | ||
Remarkably, the non-covalent paradigm underneath the FSMMA approach has an additional distinctive advantage over the traditional covalent method: it allows for a direct comparison of the microarray response with more traditional solution techniques. In fact, the fluorous-tagged molecule can be easily tested in solution as it is, without any modification which could alter the chemical properties of the molecule and render problematic the comparison. This permits us to be confident on the data derived from microarrays, and also to discriminate between contingent effects, if present, derived from the linker identity and surface interactions. Data also show that microarrays responses are valuable for internal comparison (i.e., intensity of spots on the same glass slide), as well as for external comparison (intensity of spots on different glass slides), provided that the same protocols are applied.
All the successful examples of fluorous microarrays applications described above obviously require, to various extents, the synthetic steps which provide the attachment of the fluorous tail to the selected ensemble of target compounds. Therefore, it is important to mention that recent advances in fluorous chemistry techniques, not discussed in detail here, make fluorous-tagged compounds easily accessible. These techniques are, for example, Fluorous Solid Phase Extraction (F-SPE),7 Fluorous Mixture Synthesis,33–36 and others, which constitute essential complements for any effective biomolecular applications of fluorous chemistry. Fluorous microarrays represent a novel technique for biomolecular investigation of complex phenomena. Its applications are likely to increase in number and widen in scope in the near future, as suggested by recent works on proteomics and enzymatic assays studies by mass spectrometry, described in the next section.
3. The fluorous effect in mass spectrometry
A general feature of Mass Spectrometry (MS) techniques is a high chemical sensitivity, with a detection limit which parallels, and sometimes surpasses, that of fluorescence spectroscopy. The combination of MS techniques with fluorous chemistry has been demonstrated to be quite rewarding.As said before, biological samples often display the uttermost complexity. Consider, for example, a cell lysate amid which a given enzyme is present, whose activity assay requires a quantitative analysis. Conventional techniques are nowadays implemented to solve problems of this complexity, however they have many limitations. Again, the non-covalent approach, shown very effective when applied to FSMMAs, can bring in several advantages when combined with MS detection methods. Two examples are following. In the Northen's laboratory, a novel desorption/ionization method (NIMS) extremely suited to perfluorocarbon species was developed.37 This method has found successful application in enzyme activity assays on microbial crude lysates. Recently, thermophilic bacterial communities found in the hot springs of the Yellowstone park were screened for the activity of their galactosidase enzymes in a very simple and effective way.38 A suitable substrate, compound 11, whose structure is depicted in Scheme 4, was synthesized. It can undergo enzymatic transformations leading to a cleavage or an addition of a galactose unit. The substrate has been decorated with a C8F17 tail, a five-carbon linker and an arginine residue. Each of these units has a precise role: the arginine ensures the charge, the linker favours the chemical reactivity and the fluorous tail grants the absorption onto a fluorous surface. After substrate immobilization, cell lysates were added and allowed to react, whereupon they were washed out from the surface. Laser desorption was then employed to vaporize the substrate (whether reacted or not) allowing its detection and quantification. In brief, this protocol (named Nimzyme) makes use of the fluorous effect in order to provide an effective, yet reversible surface immobilization method, suitable for a particular type of MS analysis. The perspective to apply this method to microarrayed systems appears to be particularly appealing. Indeed, the authors seem to be confident on the feasibility of the construction of MS-compatible fluorous microarrays.
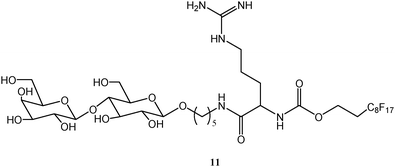 | ||
| Scheme 4 Molecular formula of the substrate for screening the activity of bacterial enzymes by combination of MS/microarrays techniques. | ||
Research on proteomics is based on the large scale analysis of proteins and their building blocks in order to determine their structures and properties. Analytical samples are usually constituted by a complex mixture of chemically treated protein fragments, poly-, oligo-, and single amino acids. Quantitative and qualitative evaluations of the composition of such intricate mixtures are necessary for deductions on protein structure–function relationships. Proteomics is an additional field which can benefit from the integration of MS detection techniques with fluorous chemistry, as documented by the work of Peters and Siuzdak.39 The method, described schematically in Fig. 3, consists on spotting fluorinated samples of various compositions (vide infra) onto a fluorinated silicon surface. The surface is then analysed by an MS technique (DIOS-MS),40 both before and after a washing process. In particular, several different protein samples of increasing complexity were analysed in series. At first, the authors started from a 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of cysteine standards and F-labelled cysteines (perfluorooctyl-iodoacetamide was used as a fluorinating agent to perform the fluorination reaction). Then they applied the method to a sample of fluorous (F-)-labelled cysteines contaminated with significant amounts of trypsin digested bovine serum albumin (BSA). Lastly, BSA was first reacted with the fluorinating agent, then digested into peptides. In all of the three cases, the spectra of the washed samples revealed a high sensitivity enhancement of the fluorous peptides which could be easily identified. Comparison between spectra before and after the washing steps was found to be very informative, and importantly, easily accessible within the proposed protocol. On the other hand, tagging MS analytes with fluorous molecules helps identification also in a somewhat more straightforward way. Unlike most elements, fluorine atoms weigh less than their nominal mass, making it easier for computers in proteomics to identify fluorinated fragments. Applications in metabolomics—involving analysis of complex metabolite profiles—were also both conceptually and experimentally tested with the same method, obtaining promising results.
1 mixture of cysteine standards and F-labelled cysteines (perfluorooctyl-iodoacetamide was used as a fluorinating agent to perform the fluorination reaction). Then they applied the method to a sample of fluorous (F-)-labelled cysteines contaminated with significant amounts of trypsin digested bovine serum albumin (BSA). Lastly, BSA was first reacted with the fluorinating agent, then digested into peptides. In all of the three cases, the spectra of the washed samples revealed a high sensitivity enhancement of the fluorous peptides which could be easily identified. Comparison between spectra before and after the washing steps was found to be very informative, and importantly, easily accessible within the proposed protocol. On the other hand, tagging MS analytes with fluorous molecules helps identification also in a somewhat more straightforward way. Unlike most elements, fluorine atoms weigh less than their nominal mass, making it easier for computers in proteomics to identify fluorinated fragments. Applications in metabolomics—involving analysis of complex metabolite profiles—were also both conceptually and experimentally tested with the same method, obtaining promising results.
 | ||
| Fig. 3 Schematic presentation of the procedural steps for integrated MS-FSMMA analysis of sample mixtures. Adapted with permission from ref. 39. | ||
These few examples suggest that coupling non-covalent fluorous methods with sophisticated analytical techniques results into powerful tools that may facilitate currently problematic biological screening, thus contributing to a better understanding of complex biological processes.
4. Fluorous biomacromolecules
Proteins are efficient biomolecular machines in which the sequence of amino acids that compose their linear polymeric chain translates into a well-defined tridimensional structure responsible for their specific activity. The dynamic progression which molds the primary structure of proteins into their tertiary structure is called folding. This fundamental process is substantially driven by hydrophobic forces and by the exclusion of water molecules from the protein core. Given the peculiar physico-chemical properties of perfluorinated moieties, one might wonder what effect the introduction of perfluorinated units into a protein would produce in terms of its structure, stability, and function. Despite the extensive use of perfluorinated materials in modern technologies, and that the first examples of fluorinated proteins date back to the 1960s,41 detailed studies on the fluorous effect in biomacromolecules have been scarce. By contrast, in the last decade, a renewed interest on fluorinated proteins has emerged, as documented by the examples from leading groups in the field hereafter presented.Helix bundle proteins, composed of coiled-coil arranged helices featuring a sterically close-packed hydrophobic core, are the main protagonists of these studies. Fig. 4a shows the structure of an anti-parallel, four-α-helix bundle protein whose changes in properties upon various degree of fluorination have been investigated in the Marsh's laboratory.42–45 The bundle is a tetramer, composed of four 27 residue long peptides. Its non-fluorinated version is referred to as α4-H. In Fig. 4a, different representations of α4-H are shown, together with its amino acid sequence. α4-H possesses leucine residues at each of the a and d positions on its three heptade units. These hydrophobic residues can be replaced with fluorinated leucine analogues, viz., 5,5,5,-5′,5′,5′-hexafluoroleucine, to yield ensembles of new proteins, fluorinated to various extents (Fig. 4b).
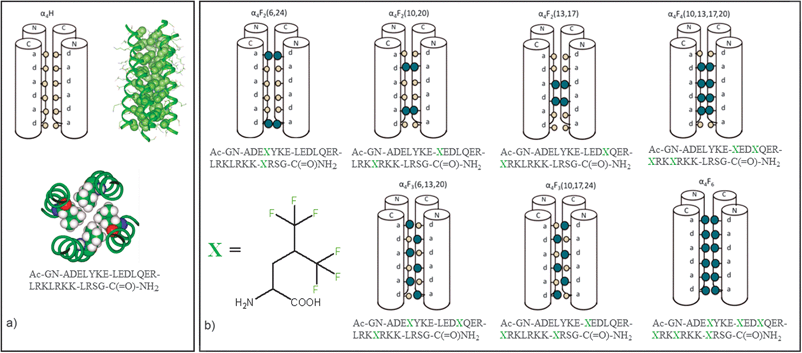 | ||
| Fig. 4 (a) Different representations (schematic and two 3D views) of the non-fluorinated tetrameric bundle α4-H; (b) schematic representation and aminoacidic composition of the fluorinated bundles studied in Marsh's laboratory, α4-Fn (n = 2,3,4,6). 1-letter code identifies amino acids. Adapted from ref. 42 with permission. | ||
Once the quaternary structure is assembled, the leucine (or hexafluoroleucine) residues in the resulting tetrameric bundles are located at the monomer/monomer interfaces, thus constituting a hydrophobic core. Therefore, fluorination was anticipated to strongly influence the aggregation properties of the protein bundles and their behaviour. The fluorinated bundles will be referred to as α4-Fm, with m ranging from 2 to 6 and referring to the number of fluorinated residues present in the monomer peptide.
It must be noted that the fluorine content in the monomers ranges from 12 to 36 fluorine atoms in the studied series, which results in the tetrameric bundles containing from 48 to 144 fluorine atoms. These modifications are quite pronounced, especially in the latter peptide of the series, for which a fluorous effect might be reasonably expected, alongside an estimated increase of the monomer volume by ca. 30%.43
Data on the effect on Gibbs energy variations (ΔΔGofold) related to the position and extent of fluorination obtained by titration experiments with guanidinium chloride showed that the introduction of CF3-groups stabilizes the folded structures in all cases. However, the stabilization is dependent on the position and on the number of the modified residues and it ranges from 0.7 to 14.6 kcal mol−1. Interestingly, such an increase in stability is accompanied by an enhanced chemical robustness. Indeed, tetrameric α4-F6 resists to the digestion by proteases, such as chymotrypsin and trypsin, staggeringly better than the non-fluorinated α4-H bundle.44 In addition, α4-F6 is also more stable to denaturation induced by addition of alcoholic solvents. An increase of the rigidity of the whole system, which follows the extent of fluorination, is also observed. Qualitative lineshape analysis by dynamic 19F-NMR investigations on the series of α4-Fn bundles (n = 2, 4, and 6) indicated a progressive “freezing out” of the system at increasing fluorine content.43
Tuning the protein stability by introduction of fluorous domains may result in interesting applications in many different fields. As far as medical treatments are concerned, fluorination of antimicrobial peptides (AMPs) may be one of the promising options for fighting against antibiotic resistant bacterial strains, whose increasing diffusion in hospital whereabouts has become a serious matter of concern. In this respect, the fluorous analogue of the AMP “MSI-78” synthesized by Marsh et al., a 22 amino acids long peptide bearing four hexafluoroleucine residues, displayed an improved potency against different bacteria strains.46 Its model structure based on the non-fluorinated analogue, together with its amino acid sequence, is shown in Fig. 5. The efficacy of action of AMPs is usually believed to be connected to the ability of these proteins to weaken the defence of bacteria by forming portals in their membranes. Partial fluorination improves the folding process occurring inside the liphophilic membrane. The tendency of fluorous residues to minimize their interactions with the membrane surroundings results in a pronounced self-association within the membrane, which explains the improved biological activity of the above described fluorinated AMP.
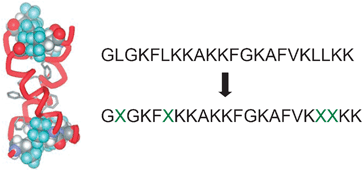 | ||
Fig. 5 Model structure of the fluorinated AMP “MSI-78” synthesized by Marsh et al. (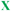 = (L)-5,5,5,-5′,5′,5′-hexafluoroleucine). 1-letter code identifies amino acids. Reproduced with permission from ref. 46. = (L)-5,5,5,-5′,5′,5′-hexafluoroleucine). 1-letter code identifies amino acids. Reproduced with permission from ref. 46. | ||
As demonstrated by the examples above, the fluorous effect can be a powerful tool for tuning the self-association of peptides. However, the introduction of an excessive number of fluorinated residues in a peptide can be counterproductive. The two peptides 12a and 12b shown in Scheme 5 are the fluorinated analogues of a known AMP, magainin-2-amide (12c), and both of them are extensively more resistant to protease activity than 12c.47 Surprisingly, only the first one, 12a, has shown a sensible antimicrobial activity. On the contrary, the more extensively fluorinated species, 12b, which contains five fluorinated residues, is so prone to self-associate in the bulk water that it looses all its efficacy.
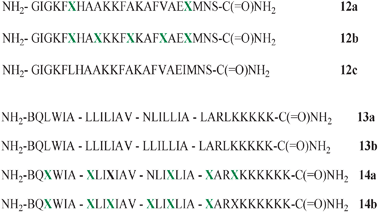 | ||
Scheme 5 Amino acid sequence of AMP 12c and its fluorinated analogues 12a and 12b; amino acid sequence of coiled coil peptides 13a and 13b and their fluorinated analogues 14a and 14b; 1-letter code identifies amino acids (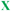 = (L)-5,5,5,5′,5′,5′-hexafluoroleucine). = (L)-5,5,5,5′,5′,5′-hexafluoroleucine). | ||
Fluorous segregation as the driving force for the self-association of peptides was evoked by Niemz and Tirrell48 also in the case of the membrane binding of fluorinated mellitins. These peptides are known venoms able to cause cell lysis by insertion into cell membranes. Substituting up to four of the leucines present in the 26-residues long peptides with trifluoroleucines induced a significant increase in the peptides' self-associative character and membrane affinity. This was inferred by the authors based on the observation of a decreased effective charge (ν) in fluorinated peptides, indicative of aggregation amid the membrane as a consequence of perfluorocarbon–hydrocarbon segregation.
Another prominent example on the same topic, i.e., the aggregation enhancement in membrane environment by the fluorous effect, is given by the work of Kumar et al.,49–51 based on the comparison of the behaviour of different coiled-coil peptides with the primary structures shown in Scheme 5.
The particular design of peptides 13a and 14a, which differentiates them from a similar peptide shown in Fig. 4a, is embodied by an asparagine residue which, from previous studies,50 was demonstrated to ensure effective inter-helical H-bonding, the main driving force to aggregation into bundles. Such behaviour was not observed in 13b, devoid of the essential H-bonding site. Peptide 14a has the six leucines substituted with hexafluoro derivatives; 14b has an additional modification done, the H-bonding donor arginine replaced with a hexafluoroleucine. Importantly, as schematically depicted in Fig. 6, the self-association of the peptides embedded into large unilamellar vesicles (made of glycerophosphocholine lipids) was significantly favoured by the extensive fluorination of the six or seven residues (as in 14a and 14b, respectively). This behaviour, compared to that of the non-fluorinated analogues (13a and 13b), is even more remarkable. Indeed, 14b forms higher order adducts even in the absence of the H-bonding capability, thus showing that the fluorous effect is sufficient for the formation of higher order aggregates within hydrophobic membrane environments.
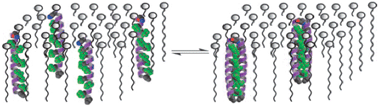 | ||
| Fig. 6 Schematic presentation of the aggregation behaviour of fluorinated peptides in a membrane environment. Reproduced from ref. 51 with permission. Copyright (2004) National Academy of Sciences, USA. | ||
From the examples above, one can notice that leucine is the residue which preferentially undergoes substitution. This is essentially due to the fact that it is common and is the prototype of a hydrophobic residue. For these reasons, it is mostly found in the protein core and/or at protein–protein interfaces, where one might expect the fluorous effect to become more prominent and effective. On the contrary, substitution of a hydrophilic residue with a fluorinated analogue might result in a detriment to the protein stability, folding, and function. That said, other hydrophobic amino acids, such as valine, methionine, alanine, and ethylglycine, although less commonly, have been replaced into proteins with fluorinated analogues.
For the sake of brevity, additional interesting results coming from the work of Kumar, Marsh, Tirrell and their collaborators describing the fluorination of other peptide systems such as β-peptide bundles and GNC4 leucine zippers (a yeast transcription factor with a parallel two-stranded coiled coil) are not discussed here. For more details, the reader is invited to refer to the original articles and other reviews.52–59
All the examples described above forcefully demonstrate that the introduction of fluorinated amino acids has become technically viable by solid phase synthetic methods and, in some cases, by in vivo incorporation.60,61 This ushers in the possibility of an expansion of the use of fluorinated peptides in biomolecular and biomedical applications. Indeed, fluorination may impart novel and advantageous properties to proteins, in terms of enhanced stability, biological activity, and increased robustness towards proteases.
We believe that fluorinated biomolecules, both from de novo design, or derived by modification of wild types, constitute a still widely unexplored research area where the potential of the fluorous effect may be fully exploited in novel and intriguing applications. Support to this is also given by studies on polyfluorinated DNA bases.62
5. Fluorous reporter agents in 19F-MRI
This section focuses on the use of fluorinated compounds for medical imaging purposes. Magnetic Resonance Imaging (MRI) is a powerful technique which is having a tremendous impact in medical research and clinical practice.63 It relies on the absorption of radiofrequency by the 1H nuclei belonging to water molecules, ubiquitous in biomedical samples. A control on the magnetic field (attained by gradient techniques) allows for the acquisition of the spatial distribution of the sample, whereas intensity depends on the nuclei's relaxation rates. Thus, images can be generated.The only natural isotope of fluorine, 19F, has attracted the interest of the MRI community due to its favourable physico-chemical properties (spin = 1/2, γ, 100% abundance), which render 19F Nuclear Magnetic Resonance (NMR) almost as sensitive as 1H-NMR (ca. 83%). Moreover, 19F is essentially absent from any biological tissue, hence any exogenous fluorine would be detected as a “hot spot on a cold background”. Thanks to these favourable properties, fluorinated molecules could basically act as reporter agents. MRI analysis based on the combination of 1H- and 19F-images would thus generate a full picture of the sample.
Cell tracking is definitely one of the research areas which is believed could benefit more from 19F-MRI using next generation reporter agents. The possibility to observe a fundamental biological process at the cellular level in vivo is considered to hold great promises for medical advancement.64 In this review, we make an attempt to explicit the concept underlining the use of perfluorocarbon compounds in cell tracking studies based on 19F-MRI detection. A selection of the most recent examples found in the literature is described. For a more comprehensive picture, please refer to other reviews dedicated to the subject.65–68
As said before, PFCs have the tendency to maximize fluorine–fluorine interactions and form a separate phase, viz., a fluorous phase, being immiscible with both hydrophilic and hydrophobic media. For the same reason, the direct use of PFCs in water solution is impractical. This notwithstanding, it has been demonstrated that the use of nanoemulsions could circumvent such limitation. Nanoemulsions are submicron sized emulsions formed by the mixing of two immiscible liquids (water and PFCs, in this case) by the use of surfactants. Such thermodynamically stable isotropic systems reach dimensions in the 20–200 nm range, and they are currently studied as promising vectors for drug delivery.
Fundamental work on this subject has indeed demonstrated that nanoemulsions containing PFCs can be effectively used for cell loading and detection by 19F-MRI. The first studies by Ahrens et al.69 targeted dendritic cells (DCs) ex vivo. They were incubated with nanoemulsions composed of perfluoro-15-crown-5-ether (PFCE) and lecithin and safflower oil, emulsifier substances, in water. After a comprehensive screening of potential adverse effects of the PFCE loading process on cellular toxicity, metabolism, and proliferativity, which were only minimal, then DCs were administered to mice and MR images were taken. Fig. 7a shows the in vivo images of a muscle tissue where labeled DCs were intramuscularly injected. Instead, Fig. 7b depicts the popliteal lymph node of the animal where the DCs loaded with PFCE had migrated in a 6 h journey started from the animal hind foot pad, where the cells were initially injected. These data fully demonstrate that migration of PFC-loaded cells could be monitored in vivo by 19F-MRI.
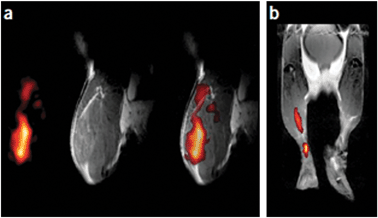 | ||
| Fig. 7 In vivo MRI of PFPE-labelled DCs in mouse (gray scale 1H, hot iron scale 19F). (a) Mouse quadriceps after injection of fluoro-labelled DCs: from left to right are 19F- and 1H-images and a merged19F/1H image. (b) Composite image of DC migration into the popliteal lymph node after injection of DCs into a hind foot pad. Reproduced from ref. 69, with permission. Copyright (2005) Nature Publishing Group's. | ||
Nanoemulsions containing PFCE were also injected into neural stem cells in mice.70 This time, the nanoemulsions were also loaded with rhodamine-based fluorescent derivatives to ensure a dual mode imaging, which can in some cases be extremely useful,71 for example, to allow the histopathology analysis of the sample. Fig. 8 shows images of the injected PFCE loaded cells at different times (after 1 h, after 3, and 7 days) and at different concentrations (left vs. right lobe). Noteworthy, the investigated cells remained functioning and the concentration of PFCE stable. From signal intensity, length of the instrumental data acquisition protocol (5 min), and number of cells injected (4 × 104–5), it might be estimated a lower threshold for detection (at 9.4 T) of 7 pmol of fluorine atoms per cell. Such limit is believed to be possibly improved (lowered) by an improved design of the fluorinated reporter agent and by the optimization of the acquisition protocol.
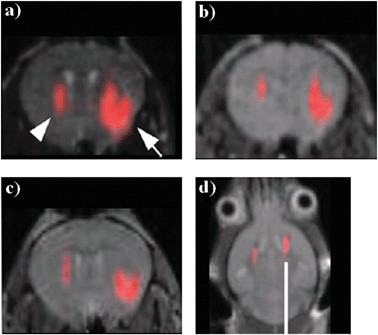 | ||
| Fig. 8 In vivo MR images of transplanted neural stem cells after (a) 1 hour, (b) 3 days and (c) 7 days after injection (19F signal superimposed on the 1H MR images; arrows indicate the left and right lobes where 4 × 104 and 3 × 105 labelled cells, respectively, were injected), (d) MR image after 2 weeks of injection on a different animal of 4 × 105 cells in both hemispheres. Adapted from ref. 70, with permission. | ||
This ex vivo cell loading protocol is not the only possible to be applied. Cells can also be loaded by interaction with nanoemulsions at the time of the introduction into the body sample. This approach has been used, for example, to study local inflammatory processes occurring in the brain, initiated by an ischemic process.72 When injected into the ischemic brain, PFC nanoemulsions were fagocitated by monocyte/macrophage cells, which then transported the fluorine reporters to the damaged zones. Once labeled with the fluorine cargo, the fate of the phagocytes can be monitored over time. Control experiments ensured that the 19F signal observed was due to the PFCE molecules actually entrapped into the cells, and not simply deriving from extracellular deposits of PFCE.
The perfluorinated crown ether used in the works described so far is commercially available, with a trivial chemical structure, yet with a relevant positive feature. All of its 20 fluorine atoms are chemically equivalent, therefore, only one NMR signal is observed in its 19F-NMR spectrum. In fact, chemical shift equivalence avoids the occurrence of signal artifacts when acquiring images,73 and it should be considered an important structural requisite for the design of any fluorous reporter agent, despite many examples in the literature seem to deny so.
Finally, a last example is presented, showing a two-fold use of PFC nanoemulsions in cell tracking. In this work,74 natural killer (NK) non-phagocytic cells were selected as targets for nanoemulsion loading, given their importance in cancer immunotherapy. Perfluorodecaline (PFD) was selected as the PFC and nanoemulsions were obtained through the use of common phospholipid surfactants. In addition to this, and here the novel approach stands, [InGaP/ZnS] quantum dots (QDs) were also incorporated into the nanoemulsions. Such nanometre-sized species possess unique optical properties, and it was envisaged to use them as a tool for dual imaging purposes. The incorporation of QDs was realized by coating them with a layer of perfluoroalkylthiols. Such fluorinated sheath allowed the solubilization of the particles into the PFC phase and hence ensured their presence into the nanoemulsion. Despite the non-optimal selection of the PFC, as far as the chemical shift equivalence is concerned, the system performed well, as demonstrated by the MR images shown in Fig. 9. Such a method relies on the use of a PFC as the 19F-reporter for MRI detection, but, at the same time, the fluorous phase is exploited to selectively entrap the perfluoroalkyl-functionalized QDs into the nanoemulsion, given the strong interactions of the fluorinated sheath with the PFC phase.
![Cartoon illustrating the process for PFD/[InGaP/ZnS QDs] nanoemulsions production. The dispersion of InGaP/ZnS QDs in PFD was facilitated by 1H,1H,2H,2H-perfluorooctanethiol. PFD containing InGaP/ZnS QDs were emulsified in aqueous solutions containing surfactants. Images of the system obtained by TEM, fluorescence spectroscopy and 19F MRI are also shown. Adapted from ref. 74, with permission.](/image/article/2012/CS/c1cs15084g/c1cs15084g-f9.gif) | ||
| Fig. 9 Cartoon illustrating the process for PFD/[InGaP/ZnS QDs] nanoemulsions production. The dispersion of InGaP/ZnS QDs in PFD was facilitated by 1H,1H,2H,2H-perfluorooctanethiol. PFD containing InGaP/ZnS QDs were emulsified in aqueous solutions containing surfactants. Images of the system obtained by TEM, fluorescence spectroscopy and 19F MRI are also shown. Adapted from ref. 74, with permission. | ||
The successful research examples described in this section—selected among the many available—are stimulating many laboratories worldwide to undertake extensive investigations aimed at extending the applicability of such techniques by designing more sophisticated and functional 19F-reporters. Among the many challenges ahead, there are to be taken into serious account both the problems inherent to the use of nanoemulsions, not always providing stable and reproducible systems, and the biological and environmental acceptability of PFCs. Notwithstanding this, 19F-MRI holds the potential to emerge in the near future as a powerful, non-invasive diagnostic tool in clinical monitoring.
6. Other biomedical applications of PFCs
The wide panorama described so far would not be exhaustive if other important biomedical applications involving fluorous chemistry were not mentioned alongside 19F-MRI. Indeed, fluorous emulsions and microbubbles are currently employed as imaging tools for ultrasound methodologies (ultrasonography), as well as in artificial blood and drug delivery applications. For the sake of brevity these topics will be dealt here only briefly, directing the reader towards more exhaustive literature.As far as ultrasonography is concerned, studies dating back to the late 1970s show the testing of PFC emulsions as contrast agents in order to compensate for the often weak difference in tissues echogenicity (i.e., the effectiveness of backscattering of ultrasound pulses).75 Similarly to 19F-MRI, emulsions76 and microbubbles77 are employed for delivering PFC molecules into the target tissue. Recently, emulsion-free systems have also appeared, such as polymeric microcapsules decorated with a phospholipids covering and filled with perfluorooctyl-bromide.78
The use of PFCs as blood substitutes and drug delivery media is also a very well developed area. PFCs have a surprising ability to solubilise gases, and in particular they solubilise oxygen twice as effectively as their hydrocarbon counterparts.79 Therefore, they could function as oxygen carriers to tissues, temporarily replacing human blood under emergency conditions, e.g. as an alternative to blood transfusion during surgery. For readers interested in becoming accustomed to the subject, the extensive review by Riess80 provides a remarkable source of information.
As to drug delivery, PFC nanoparticles formulated as oil-in-water emulsions are attractive candidates for the incorporation and transport of pharmaceutically active compounds. Among recent studies, the investigation on the transport through a model lipid bilayer of Mellitin, a cytolytic peptide, is a very interesting case.81 Thanks to their small dimensions, PFC nanoparticles can be very effective to permeate the Blood Brain Barrier,82 but pulmonary drug delivery83 as well as atherosclerosis treatment84 are also among the most active research fields. Given the wide variety of forms of stable aggregation displayed by perfluorinated amphiphilic compounds, micelles, vesicles and other higher types of aggregates made of (or containing) perfluorocarbon components might provide additional interesting species for drug-delivery and imaging purposes.
This brief account on other possible uses of PFCs clearly shows their importance in modern biomedical research, but one last comment must be devoted here to an important aspect, which actually refers to all the research described in this review. Many PFC compounds can be converted in the human body and/or in the environment into toxic species. This may engender practical problems whenever the use of extensively fluorinated species eventually reached a large scale in technological and biomedical applications. Accumulation of PFC species in humans, animals, and environment may pose serious issues. In this regard, the concentration of perfluoroalkyl compounds such as perfluorooctanoic and perfluorooctane sulfonic acids has been monitored in blood samples on retired workers of the fluoro-chemical industry, and it was demonstrated that the lifetime of these compounds could be of several years. A recent review on the subject deals with all these aspects.85 It is, therefore, unavoidable that the full exploitation of the peculiar properties of PFCs in the field of biomedicine must develop alongside with the study of the effects of these chemicals on the entire ecosystem.
7. Conclusions
Research on fluorous chemistry is presently blooming. The number of publications containing the term fluorous in the title or in the abstract is, year by year, steadily increasing (Fig. 10). Despite that the initial impetus of fluorine chemistry research was focused on coating materials—Teflon® is one of the most illustrious and renown product of those—recently, more attention has been drawn by other, further off research arenas, after the acknowledgement that the unique properties of fluorous compounds might have a broader scope and significance. Whenever included into organic molecular frameworks, fluorine atoms bring in novel properties which result useful, sometimes unexpectedly, in many different fields, such as materials and supramolecular chemistry, separation science and organic synthesis, just to name a few of them.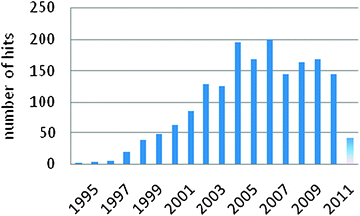 | ||
| Fig. 10 Bar plot of the distribution by year of the publications containing the term “fluorous” in the title (last updated on April 2011, SciFinder®). | ||
This tutorial review, in the authors' intent, shall serve to outline recent research works which may constitute a kernel of experiences, a starting point, to which a non-acquainted reader may rely on, in the case of a growing interest for this exciting field. Several applicative ways to implement the fluorous effect into biomolecular applications have been described in detail. If microarrays techniques have been on the stage already for a while, the non-covalent approach made viable by the fluorous effect, represents a novel tool for development of new applications. Furthermore, its combination with powerful detection techniques, such as MS, is expected to produce significative improvements in the analysis of complex biological samples.
The incorporation of a conspicuous number of fluorine atoms in a small protein was attempted decades ago. Yet, detailed physico-chemical studies have only been recently undertaken by investigators around the world. The results, although still limited to a few systems, some of which described herein, prove that the fluorous effect can indeed increase the stability of proteins, and can modulate their folding and aggregation properties. Despite the absence of significant data on fluorous tagged proteins, one can reasonably expect the direct implementation of some of the techniques described here on proteins to be fruitful in terms of innovative applications.
Finally, the properties of fluorine can be further exploited in MRI applications. 19F-MRI is still at its infancy, despite the enormous interest that it is instigating in the biomedical scientific community. Here, some of the most outstanding contributions were described.
In conclusion, we strongly believe that fluorine chemistry will be increasingly employed by biomolecular chemists to engender novel properties and behaviour into biological molecules, thus extending their field of applications. The selected excellent examples described in this tutorial review demonstrate the huge potential of the biomolecular exploitation of the fluorous effect. The field is only at its infancy, therefore, additional research efforts in fluorous chemistry and technologies are desirable. This would provide biochemists and biologists with new powerful tools for the ready-to-use introduction of fluorous residues into biomolecules in order to control their structures, properties, and applications.
Acknowledgements
Dedicated to Dr Tullio Pilati, an excellent scientist and a friend. The authors gratefully acknowledge financial support from Fondazione Cariplo (project 2150, New Generation Fluorinated Materials as Smart Reporter Agents in19F-MRI, and project 2010-1351, Development of a Technology Platform between the South and the North of Europe: Exchange Research Program between Politecnico di Milano and VTT—Technical Research Centre of Finland (S2N)).Notes and references
- Handbook of Fluorous Chemistry, ed. J. A. Gladysz, D. P. Curran and I. T. Horváth, Wiley-VCH, Weinheim, 2004 Search PubMed.
- J. A. Gladysz and D. P Curran, Tetrahedron, 2002, 58, 3823–3825 CrossRef CAS.
- M. Vogt, PhD thesis, University of Aachen, 1991 Search PubMed.
- D.-W. Zhu, Synthesis, 1993, 10, 953–954 CrossRef.
- I. T. Horváth and R. Rábai, Science, 1994, 266, 72–75 CrossRef CAS.
- W. Zhang, Chem. Rev., 2009, 109, 749–795 CrossRef CAS.
- W. Zhang and D. P. Curran, Tetrahedron, 2006, 62, 11837–11865 CrossRef CAS.
- W. Zhang, Chem. Rev., 2004, 104, 2531–2556 CrossRef CAS.
- W. Zhang, Tetrahedron, 2003, 59, 4475–4489 CrossRef CAS.
- J. A. Gladysz, in Handbook of Green Chemistry, ed. P. Anastas and R. H. Crabtree, Wiley/VCH, Weinheim, 2009, vol. 1, pp. 17–38 Search PubMed.
- C. Cai, W.-B. Yi, W. Zhang, M.-G. Shen, M. Hong and L.-Y. Zeng, Mol. Diversity, 2009, 13, 209–239 CrossRef CAS.
- M. Wende and J. A. Gladysz, J. Am. Chem. Soc., 2003, 125, 5861–5872 CrossRef CAS.
- M. Cavazzini, F. Montanari, G. Pozzi and S. Quici, J. Fluorine Chem., 1999, 94, 183–193 CrossRef CAS.
- G. Cavallo, P. Metrangolo, T. Pilati, G. Resnati, M. Sansotera and G. Terraneo, Chem. Soc. Rev., 2010, 39, 3772–3783 RSC.
- R. Bertani, P. Sgarbossa, A. Venzo, F. Lelj, M. Amati, G. Resnati, T. Pilati, P. Metrangolo and G. Terraneo, Coord. Chem. Rev., 2010, 254, 677–695 CrossRef CAS.
- P. Metrangolo, F. Meyer, T. Pilati, G. Resnati and G. Terraneo, Angew. Chem., Int. Ed., 2008, 47, 6114–6127 CrossRef CAS.
- J.-M. Vincent, J. Fluorine Chem., 2008, 129, 903–909 CrossRef CAS.
- K.-S. Ko, F. A. Jaipuri and N. L. Pohl, J. Am. Chem. Soc., 2005, 127, 13162–13163 CrossRef CAS.
- S. K. Mamidyala, K.-S. Ko, F. A Jaipuri, G. Park and N. L. Pohl, J. Fluorine Chem., 2006, 127, 571–579 CrossRef CAS.
- G.-S. Chen and N. L. Pohl, Org. Lett., 2008, 10, 785–788 CrossRef CAS.
- F. A. Jaipuri, B. Y. M. Collet and N. L. Pohl, Angew. Chem., Int. Ed., 2008, 47, 1707–1710 CrossRef CAS.
- R. L. Nicholson, M. L. Ladlow and D. R. Spring, Chem. Commun., 2007, 3906–3908 RSC.
- A. J. Vegas, J. E. Bradner, W. Tang, O. M. McPherson, E. F. Greenberg, A. N. Koehler and S. L. Schreiber, Angew. Chem., Int. Ed., 2007, 46, 7960–7964 CrossRef CAS.
- B. Y. M. Collet, T. Nagashima, M. S. Yu and N. L. Pohl, J. Fluorine Chem., 2009, 130, 1042–1048 CrossRef CAS.
- N. L. Pohl, Angew. Chem., Int. Ed., 2008, 47, 3868–3870 CrossRef CAS.
- E.-H. Song and N. L. Pohl, Future Med. Chem., 2009, 1, 889–896 Search PubMed.
- M. P. Krafft and J. G. Riess, Chem. Rev., 2009, 109, 1714–1792 CrossRef CAS.
- J. G. Riess, Tetrahedron, 2002, 58, 4113–4131 CrossRef CAS.
- A. V. Grosse and G. H. Cady, Ind. Eng. Chem., 1947, 39, 367–374 Search PubMed.
- C. J. Wilson, D. A. Wilson, A. E. Feiring and V. Percec, J. Polym. Sci., Part A: Polym. Chem., 2010, 48, 2498–2508 Search PubMed and references cited therein.
- A. Studer, S. Hadida, R. Ferritto, S.-Y. Kim, P. Jeger, P. Wipf and D. P. Curran, Science, 1997, 275, 823–826 CrossRef CAS.
- D. P. Curran, Science, 2008, 321, 1645–1646 CrossRef CAS.
- W. Zhang, Z. Luo, C. H.-T. Chen and D. P. Curran, J. Am. Chem. Soc., 2002, 124, 10443–10450 CrossRef CAS.
- W.-H. Jung, S. Guyenne, C. Riesco-Fagundo, J. Mancuso, S. Nakamura and D. P. Curran, Angew. Chem., Int. Ed., 2008, 47, 1130–1133 CrossRef CAS.
- D. P. Curran and T. Furukawa, Org. Lett., 2002, 4, 2233–2235 CrossRef CAS.
- Z. Luo, Q. Zhang, Y. Oderaotoshi and D. P. Curran, Science, 2001, 291, 1766–1769 CrossRef CAS.
- T. R. Northen, O. Yanes, M. T. Northen, D. Marrinucci, W. Uritboonthai, J. Apon, S. L. Golledge, A. Nordström and G. Siuzdak, Nature, 2007, 449, 1033–1036 CrossRef CAS.
- T. R. Northen, J.-C. Lee, L. Hoang, J. Raymond, D.-R. Hwang, S. M. Yannone, C.-H. Wong and G. Siuzdak, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 3678–3683 CrossRef CAS.
- E. P. Go, W. Uritboonthai, J. V. Apon, S. A. Trauger, A. Nordstrom, G. O'Maille, S. M. Brittain, E. C. Peters and G. Siuzdak, J. Proteome Res., 2007, 6, 1492–1499 CrossRef CAS.
- S. A. Trauger, E. P. Go, Z. Shen, J. V. Apon, B. J. Compton, E. S. Bouvier, M. G. Finn and G. Siuzdak, Anal. Chem., 2004, 76, 4484–4489 CrossRef.
- O. M. Rennert and H. S. Anker, Biochemistry, 1963, 2, 471–476 CrossRef CAS.
- B. C. Buer, R. de la Salud-Bea, H. M. Al Hashimi and E. N. G. Marsh, Biochemistry, 2009, 48, 10810–10817 Search PubMed.
- H.-Y. Lee, K.-H. Lee, H. M. Al-Hashimi and E. N. G. Marsh, J. Am. Chem. Soc., 2006, 128, 337–343 CrossRef CAS.
- L. M. Gottler, R. de la Salud-Bea and E. N. G. Marsh, Biochemistry, 2008, 47, 4484–4490 CrossRef CAS.
- K.-H. Lee, H.-Y. Lee, M. M. Slutsky, J. T. Anderson and E. N. G. Marsh, Biochemistry, 2004, 43, 16277–16784 CrossRef CAS.
- L. M. Gottler, H.-Y. Lee, C. E. Shelburne, A. Ramamoorthy and E. N. G. Marsh, ChemBioChem, 2008, 9, 370–373 CrossRef CAS.
- H. Meng and K. Kumar, J. Am. Chem. Soc., 2007, 129, 15615–15622 CrossRef CAS.
- A. Niemz and D. A. Tirrell, J. Am. Chem. Soc., 2001, 123, 7407–7413 CrossRef CAS.
- N. Naarmann, B. Bilgiçer, H. Meng, K. Kumar and C. Steinem, Angew. Chem., Int. Ed., 2006, 45, 2588–2591 CrossRef CAS.
- N. Naarmann, B. Bilgiçer, K. Kumar and C. Steinem, Biochemistry, 2005, 44, 5188–5195 Search PubMed.
- B. Bilgiçer and K. Kumar, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 15324–15329 CrossRef CAS.
- N. C. Yoder and K. Kumar, Chem. Soc. Rev., 2002, 31, 335–341 RSC.
- E. N. G. Marsh, B. C. Buer and A. Ramamoorthy, Mol. BioSyst., 2009, 5, 1143–1147 RSC.
- G. Akçay and K. Kumar, J. Fluorine Chem., 2009, 130, 1178–1182 Search PubMed.
- C. Jäckel and B. Koksch, Eur. J. Org. Chem., 2005, 4483–4503 CrossRef.
- M. A. Molski, J. L. Goodman, C. J. Craig, H. Meng, K. Kumar and A. Schepartz, J. Am. Chem. Soc., 2010, 132, 3658–3659 Search PubMed.
- B. Holzberger, M. Rubini, H. M. Möller and A. Marx, Angew. Chem., Int. Ed., 2010, 49, 1324–1327 CrossRef CAS.
- B. Bilgiçer and K. Kumar, Tetrahedron, 2002, 58, 4105–4112 CrossRef CAS.
- Y. Tang, G. Ghirlanda, N. Vaidehi, J. Kua, D. T. Mainz, W. A. Goddard III, W. F. DeGrado and D. A. Tirrell, Biochemistry, 2001, 40, 2790–2796 CrossRef CAS.
- P. Wang, Y. Tang and D. A. Tirrell, J. Am. Chem. Soc., 2003, 125, 6900–6906 CrossRef CAS.
- J. K. Montclare, S. Son, G. A. Clark, K. Kumar and D. A. Tirrel, ChemBioChem, 2009, 10, 84–86 CrossRef CAS.
- J. S. Lai and E. T. Kool, Chem.–Eur. J., 2005, 11, 2966–2971 CrossRef CAS.
- E. Terreno, D. Delli Castelli, A. Viale and S. Aime, Chem. Rev., 2010, 110, 3019–3042 CrossRef CAS and references therein.
- T. Schroeder, Nature, 2008, 453, 345–351 Search PubMed.
- J. Chen, G. M. Lanza and S. A. Wickline, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2010, 2, 431–440 Search PubMed.
- J. M. Janjic and E. T. Ahrens, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 492–501 Search PubMed.
- G. M. Lanza, P. M. Winter, A. M. Neubauer, S. D. Caruthers, F. D. Hockett and S. A. Wickline, Curr. Top. Dev. Biol., 2005, 70, 57–76 CrossRef CAS.
- J.-X. Yu, V. D. Kodibagkar, W. Cui and R. P. Mason, Curr. Med. Chem., 2005, 12, 819–848 CrossRef CAS.
- E. T. Ahrens, R. Flores, H. Xu and P. A. Morel, Nat. Biotechnol., 2005, 23, 983–987 CrossRef CAS.
- J. Ruiz-Cabello, P. Walczak, D. A. Kedziorek, V. P. Chacko, A. H. Schmieder, S. A. Wickline, G. M. Lanza and J. W. M. Bulte, Magn. Reson. Med., 2008, 60, 1506–1511 CrossRef.
- A. Louie, Chem. Rev., 2010, 110, 3146–3195 CrossRef CAS.
- U. Flögel, Z. Ding, H. Hardung, S. Jander, G. Reichmann, C. Jacoby, R. Schubert and J. Schrader, Circulation, 2008, 118, 140–148 Search PubMed.
- M. Srinivas, L. J. Cruz, F. Bonetto, A. Heerschap, C. G. Figdor and I. J. M. de Vries, Biomaterials, 2010, 31, 7070–7077 CrossRef CAS.
- Y. T. Lim, M. Y. Cho, J.-H. Kang, Y.-W. Noh, J.-H. Cho, K. S. Hong, J. W. Chung and B. H. Chung, Biomaterials, 2010, 31, 4964–4971 CrossRef CAS.
- R. Díaz-López, N. Tsapis and E. Fattal, Pharm. Res., 2010, 27, 1–16 Search PubMed.
- J. M. M. Simons, L. M. Kornmann, K. D. Reesink, A. P. G. Hoeks, M. F. Kemmere, J. Meuldijk and J. T. F. Keurentjes, J. Mater. Chem., 2010, 20, 3918–3923 RSC.
- E. G. Schutt, D. H. Klein, R. M. Mattrey and J. G. Riess, Angew. Chem., Int. Ed., 2003, 42, 3218–3235 CrossRef CAS.
- R. Díaz-López, N. Tsapis, D. Libong, P. Chaminade, C. Connan, M. M. Chehimi, R. Berti, N. Taulier, W. Urbach, V. Nicolas and E. Fattal, Biomaterials, 2009, 30, 1462–1472 CrossRef CAS.
- A. M. A. Dias, R. P. Bonifácio, I. M. Marrucho, A. A. H. Pádua and M. F. Costa Gomes, Phys. Chem. Chem. Phys., 2003, 5, 543–549 RSC.
- J. G. Riess, Chem. Rev., 2001, 101, 2797–2919 CrossRef CAS.
- N. R. Soman, G. M. Lanza, J. M. Heuser, P. H. Schlesinger and S. A. Wickline, Nano Lett., 2008, 8, 1131–1136 CrossRef CAS.
- M. M. Patel, B. R. Goyal, S. V. Bhadada, J. S. Bhatt and A. F. Amin, CNS Drugs, 2009, 23, 35–58 Search PubMed.
- H.-L. Lehmler, Expert Opin. Drug Delivery, 2007, 4, 247–262 Search PubMed.
- S. D. Caruthers, T. Cyrus, P. M. Winter, S. A. Wickline and G. M. Lanza, Wiley Interdiscip. Rev.: Nanomed. Nanobiotechnol., 2009, 1, 311–323 Search PubMed.
- C. Lau, J. L. Butenhoff and J. M. Rogers, Toxicol. Appl. Pharmacol., 2004, 198, 231–241 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
