The first example of rhombic dodecahedral CuBr clusters in a novel mixed-valence Cu(I,II)–benzimidazole complex†
Sisi
Feng
a,
Haigang
Lv
a,
Zhongping
Li
b,
Guoqin
Feng‡
a,
Liping
Lu
*a and
Miaoli
Zhu
*a
aInstitute of Molecular Science, Key Laboratory of Chemical Biology and Molecular Engineering of the Education Ministry, Shanxi University, Taiyuan, Shanxi 030006, People's Republic of China. E-mail: miaoli@sxu.edu.cn; luliping@sxu.edu.cn
bResearch Center of Environmental Science and Engineering, Shanxi University, Taiyuan, 030006, People's Republic of China
First published on 15th November 2011
Abstract
Hydrothermal reaction of CuBr2 and 1,2-di(1H-benzoimidazol-2-yl)ethane-1,2-diol leads to the formation of a new mixed-valence copper(I,II) complex [(C7H5N2)(CuICuIIBr2)], which exhibits a two-dimensional layer structure, containing the first example of a rhombic dodecahedral CuBr [4,3] polyhedral column. The synthesis, crystal structure, electronic property, thermal stability and electrochemical characterization of this complex are investigated.
Intriguing physical properties and tremendous structural variety have made the design and synthesis of new hybrid inorganic–organic materials become a prolific domain in the field of coordination chemistry.1–6 In this field, metal halides have been of increasing interest for their intriguing topologies and rich photoluminescent properties.7–10 Further, the copper halides are the dominant species because of their variable coordination modes11–15 and rich photoelectricity,16,17 as copper(I) possesses the coordination numbers of two, three and four, and copper(II) coordination configuration ranges from four-connected planar mode to five-connected pyramid and six-connected octahedron, etc., which complicates the coordination chemistry of copper18 and makes the copper halides family attractive.
To date, the basic motifs in copper(I) halide polymeric species are the cyclic Cu2X2 dimer and the ring-open form of the dimer.19 Most reported copper(I) halide polymeric species are based on 0D clusters, like cube20 and hexagon prism cluster,21 and one dimensional chains, including typical motifs of single chains,22 double chains,23 looped chains,24,25 helical chains,26 ribbons and columns,11etc., and 2D and 3D copper(I) halide aggregates are sporadic.10,26–29 To the best of our knowledge, an example of a dodecahedral CuX cluster has not been reported. Fortunately, we observed the CuBr cluster as a rhombic dodecahedron in complex 1 firstly.
The hydrothermal method, as one of good choice in modern synthesis, has been widely applied in in situ synthesis,30 producing various polymeric solids with new coordination modes, beautiful architectures and interesting topologies.31–34 By means of this synthesis method we have obtained a series of fascinating metal–organic coordination compounds from ligand 1,2-di(1H-benzoimidazol-2-yl)ethane-1,2-diol (TDB).35–37 In the continued process, we obtained accidentally a mixed-valence Cu(I,II)–bromine complex with in situbenzimidazole as ligands. According to the literature recently reported,38 it is well accepted that Cu(II) ions can be reduced to Cu(I) by N-containing ligands by the hydrothermal method. Interesting structures and reactions can be achieved when Cu(II) is reduced during the hydrothermal synthesis. However, a predictable synthesis result is still a challenge when we hope to control the final products, especially for those mixed-valence Cu(I,II) complexes which are of great biological importance and electronic properties.39–41
In this paper, we establish that Cu(II) ions could catalyse the decomposition of ligand TDB to single benzimidazole and be deoxidized to mixed valence ions in a certain condition, then the title Cu(I,II)–bromine complex was obtained, which is quite difficult to synthesise under routine synthetic conditions. The 2D copper–bromine coordination polymer formed through the linkage of columns of novel rhombic dodecahedral CuBr clusters [CuI2CuII2Br4]∞ by benzimidazole ligands as the bridges. We present a new configuration of the CuX oligomer which is different from those reported earlier, and report the synthesis, crystal structure, electronic property, thermal stability and electrochemical characterization of this complex.
Complex 1[(C7H5N2)(CuICuIIBr2)]n was synthesised as follows: a mixture of CuBr2, 1,2-di(1H-benzoimidazol-2-yl)ethane-1,2-diol and distilled water in a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 was mixed in a 25 ml stainless steel reactor with a Teflon liner and heated from room temperature (RT) to 448 K in 0.5 h. The temperature was kept constant at 448 K for 92 h, then cooled down naturally to RT. Light yellow gladiate crystals were collected in 15% yield.
500 was mixed in a 25 ml stainless steel reactor with a Teflon liner and heated from room temperature (RT) to 448 K in 0.5 h. The temperature was kept constant at 448 K for 92 h, then cooled down naturally to RT. Light yellow gladiate crystals were collected in 15% yield.
We have reported that Ni5L6 structural units can be formed by in situ ligand I from ligand TDB in certain hydrothermal conditions, no matter which NiX2 (X = Cl, Br, I, NO3) salt was used. It means that anions did not influence the main structure when NiX2 salts are used as reagents. In the same hydrothermal condition, when CuO was added in these systems, the M5L6 structure can be kept only with a tail added (Scheme 1).35,36 But if CuO is used as the only metal source, no crystal can be obtained. However, when only CuBr2 existed, we observed that Cu2+ could catalyse the decomposition of ligand TDB to in situ ligand II, single benzimidazole, and be deoxidized to mixed valence ions in certain hydrothermal conditions, then obtain the title Cu(I,II)–bromine complex. This indicates that Cu(II) ions play a unique catalytic role in the decomposition and deoxidation procedure. The reaction mechanism remains unclear, but the decomposition reaction may occur at the C–C bond between benzimidazole and ethanediol. Similar examples were also observed in hydrothermal synthesis.42,43
 | ||
| Scheme 1 Synthetic routes and summary of different configurations formed by the same ligand in the presence of different metal salts. | ||
We also tried the different anions and valence states of copper salts as reagents, like CuCl, CuBr, CuI and CuCl2, none of the crystals can be obtained from monovalent Cu salts, and with CuCl2 as reagent we could not obtain a suitable single crystal for X-ray diffraction analysis. We computed experimental powder XRD of complex 1 by the CPMD program and constructed an optimized analogous structure of (C7H5N2)(CuICuIICl2), and attempted to deduce the series structure through calculation of experimental powder XRD, elemental analysis and thermogravimetric analyses, but the calculation indicated that CuCl2 as a reagent seems to result in other products rather than the analogous structure of 1. These results suggest that different category and dissolubility of metal salts are pivotal in the reaction containing ligand TDB under certain hydrothermal conditions.
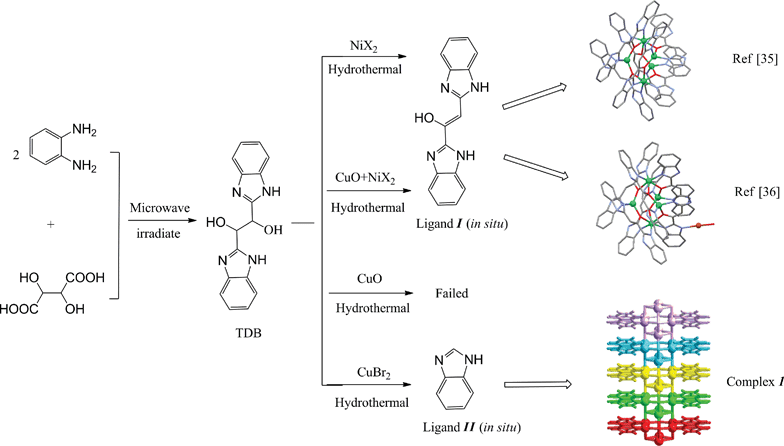
Complex 1 is synthesized by hydrothermal reactions, and single-crystal X-ray diffraction§ demonstrated that it crystallized in the C2/m space group. This complex exhibits an interesting reticular structure, in which the symmetric unit is constructed by mixed valence copper ions. In each symmetric unit there are one monovalent Cu cation, one divalent Cu cation, two Br− anions and one benzimidazole anion (Fig. 1). Here we assigned the Cu1 coordinated to N atom as being divalent and the other Cu2 as monovalent. Both monovalent and divalent copper ions have tetrahedral coordinated geometries and the valence state of the Cu atom is determined by its structural comparison as well as the neutral molecule as a whole and calculation of bond valence model below. Despite bearing the identical tetrahedron coordination environment, Cu1 is coordinated to two μ6-Br, one μ4-Br and one N from benzimidazole, while Cu2 is surrounded by two μ6-Br and two μ4-Br atoms (Fig. 2a). The Cu–Br contact distances span a wider range by far, from 2.372(4)Å to 2.764(5)Å, average length 2.5345 Å, and the Br–Cu–Br angle ranges 96.54(2)°–114.31(7)°, which are similar to those found in coordination polymers.44–46
![The structure of complex 1, with displacement ellipsoids drawn at the 30% probability level and H atoms elimination. Cu atoms are shown in cyan, C atoms in gray, Br atoms in red, N atoms in blue. [Symmetry codes: (i) x, −y + 1, z; (ii) −x + 2, y, −z; (iii) −x + 2, −y + 1, −z; (iv) −x + 2, −y + 2, −y + 1; (v) −x + 2, y, −z + 1.]](/image/article/2012/CE/c1ce06215h/c1ce06215h-f1.gif) | ||
| Fig. 1 The structure of complex 1, with displacement ellipsoids drawn at the 30% probability level and H atoms elimination. Cu atoms are shown in cyan, C atoms in gray, Br atoms in red, N atoms in blue. [Symmetry codes: (i) x, −y + 1, z; (ii) −x + 2, y, −z; (iii) −x + 2, −y + 1, −z; (iv) −x + 2, −y + 2, −y + 1; (v) −x + 2, y, −z + 1.] | ||
 | ||
| Fig. 2 (a) Tetrahedron coordination environment of divalent Cu ions; (b) tetragonal pyramid μ4 bridging geometries of Br ions and the related Cu planes. Cu atoms are shown in red and Br atoms in orange. | ||
In the case of the coordination of the Br− ions, they have two types of coordination modes, hexadentate bridge and quadridentate bridge, Br1 forms the μ6 bridges with the bridging angles ranging from 64.98(9)° to 179.11(2)°. Br2 assumes the tetragonal pyramid μ4 bridging geometries and lies 1.0884Å above the related Cu planes (Fig. 2b). The μ4 bridging Cu–Br–Cu angles are in the range of 71.92(12)–118.1(2)°. It is worth noting that the existence of more acute Cu–Br–Cu angles leads to the presence of short Cu⋯Cu contacts. Dancey et al. reported a direct Cu(I)–Cu(II) bond with the distance of 2.445(4) Å.47 A delocalized mixed-valent Cu(I/II)–Cu(I/II) bond with the bond length of 2.5Å was characterized for cytochrome c oxidase.48 Therefore, the long distance of Cu⋯Cu (2.841–3.000 Å) in the title complex suggests that there is no metal–metal bond.
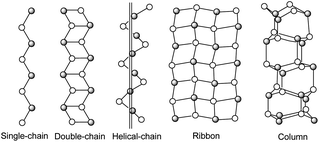
Compound 1 can also be better described as such a sheet that consists of the 1D [(C7H5N2)CuICuIIBr2]∞ chains as shown in Fig. 2b linked by the column of piled copper(I,II)–bromine clusters as the bridges. The inorganic [Cu(I)Cu(II)Br2]∞ columnar framework motif can be described as a series of perpendicularly stacked rhombic dodecahedral clusters [CuI2CuII2Br4]∞ (Fig. 2a), linked by Cu–Br contacts. Copper(I) halides are famous in forming diverse structures due to the flexibility of the Cu(I) coordination geometry and the versatile bridging ability of the halide anions, some of which are shown in Scheme 2. In this compound, the [CuI2CuII2Br4] unit is centrosymmetric crystallographically, resulting in only half of the oligomers being independent crystallographically. The [CuI2CuII2Br4]∞clusters adopt rhombic dodecahedral configuration which represents a new mode in the [CuX]∞cluster family and has not been reported before. Eight Cu atoms lie on the vertex of a cube, and six Br atoms are located in the salient points of each face (Fig. 3). A columnar framework is formed through overlap of a face of the dodecahedral and interspace of the columns is about 2 Å diameter, which is shown in Fig. 4a. Each [CuI2CuII2Br4]∞ unit is connected with four in situ formed ligands which are like steps against the column. The 3.5636(7) Å of planar distance indicates the existence of π⋯π interactions between neighbouring benzimidazole and imidazole rings (Fig. 4a). Thus, a two-dimensional framework formed as shown in Fig. 4b.
 | ||
| Scheme 2 Some representative 1D copper(I) halide aggregates. | ||
 | ||
| Fig. 3 The rhombic dodecahedral configuration of the CuBr cluster. | ||
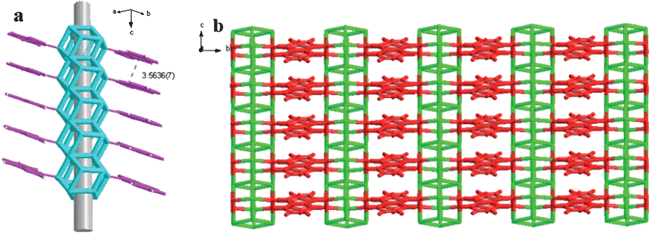 | ||
| Fig. 4 (a) Steps configuration and (b) two-dimensional framework of complex 1. | ||
To assign the oxidation state of the copper centers in 1, we have taken recourse to the calculation based on the bond valence sum (BVS) model.49 In this method, the valence S of a bond between two atoms i and j is related by an empirical expression (eqn (1)) where Rij is the length of the bond (expressed in Å) and R0 a parameter characteristic of the bond.
| Sij = exp[(R0 − Rij)/0.37] | (1) |
This R0, known as bond valence parameter, is however geometry and coordination number specific. The oxidation number Ni of the atom i is simply the algebraic sum (eqn (2)) of these S values of all the bonds (n) around the atom i.
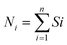 | (2) |
Brown had refined R0 values for Cu(I)–N, Cu(II)–N, Cu(I)–Br and Cu(II)–Br bonds as 1.571, 1.731, 1.967 and 2.134 Å respectively.49 Taking these R0 values, when we apply this BVS method (Table 1) to our copper compound following eqn (1), the BVS values for copper atoms in 1 are found to be 1.759 for Cu1 and 0.967 for Cu2 respectively, which agreed with our theoretical ones.
| Bond type | Bond distance/Å | Bond valence I (1.571, 1.976) | Bond valence II (1.731, 2.134) | Bond valance sum |
|---|---|---|---|---|
| a (i) x, y, z − 1; (ii) −x + 2, −y + 2, −z; (iii) −x + 2, −y + 2, −z + 1; (iv) x, y, z + 1. | ||||
| Cu1–N1 | 1.84(1) | 0.7448 | 1.759 for Cu1 | |
| Cu1–Br1 | 2.764(5) | 0.1822 | ||
| Cu1–Br1i | 2.572(4) | 0.3061 | ||
| Cu1–Br2ii | 2.372(4) | 0.5256 | ||
| Cu2–Br1 | 2.5096(19) | 0.2364 | 0.967 for Cu2 | |
| Cu2–Br1iii | 2.5096(19) | 0.2364 | ||
| Cu2–Br2 | 2.525(3) | 0.2268 | ||
| Cu2–Br2iv | 2.464(3) | 0.2674 |
Powder XRD data on the material are determined to detect other species present that escaped analysis by the single crystal X-ray study (Fig. S1†). The experimental PXRD pattern is consistent with the simulation on the basis of the single crystal structure, respectively, indicating phase purity.
In order to prove the coexistence of monovalent and divalent copper, we tried different methods. First, electronic spectra can support the existence of monovalent copper. Fig. 5a shows the UV-Vis absorption spectra of the ligand as well as complex 1 in DMF ([C] = 0.5 × 10−4 M). The absorption spectra of the ligand and complex have similar features. Both of them are characterized by three absorption bands at 267, 274–275 and 281 nm, respectively, which may be attributed to the π–π* transition of the coordinated benzimidazole ligand.50Cu(II) complexes generally exhibit a broad but very weak copper-centered d–d transition band in the region of 500–800 nm, related to the coordination environment of central Cu(II).51 So another broad band for complex 1 at 754 nm has been assigned to Cu(II) d–d transition (inset) due to the distorted tetrahedral Cu(II) centre.52
![(a) UV-Vis spectra of the ligand and complex 1 in DMF ([C] = 0.5 × 10−4 M, inset [C] = 1.0 × 10−2 M); (b) transformation of UV-Vis spectra of complex 1 in DMF along 54 minutes ([C] = 1.0 × 10−2 M and 2 minute spacing interval).](/image/article/2012/CE/c1ce06215h/c1ce06215h-f5.gif) | ||
| Fig. 5 (a) UV-Vis spectra of the ligand and complex 1 in DMF ([C] = 0.5 × 10−4 M, inset [C] = 1.0 × 10−2 M); (b) transformation of UV-Vis spectra of complex 1 in DMF along 54 minutes ([C] = 1.0 × 10−2 M and 2 minute spacing interval). | ||
Interestingly, the characteristic of Cu(II) d–d transition is being strengthened as time goes on, indicating that there are monovalent copper cations in this complex because monovalent copper will be oxidised under air. Fig. 5b records this process along 54 minutes, with 2 minutes spacing interval. Complex 1 is not photoluminescent. So we consider copper cations may quench the fluorescence of the benzimidazole fragment,53,54 because free benzimidazole itself emits in an energy maximum of 391 nm.55
Furthermore, the powder EPR (Fig. S2†) at room temperature can also support the existence of divalent copper. The experimental spectrum was reproduced using the parameters in an axial symmetry of S = 1/2, g‖ = 2.59, and g⊥ = 2.08. Magnetic susceptibility measurements indicated diamagnetism, which is in agreement with the weak EPR signal.56 The diamagnetic property of complex 1 may be ascribed to the antiferromagnetic coupling between the spins on the CuII atoms57 coming from the symmetry of the crystal structure.
Thermogravimetric analyses of the complex are shown in Fig. S3†. The thermogravimetric analysis (TGA) of complex 1 was conducted under an N2 atmosphere and it was heated to 800 °C with a 10 °C min−1 temperature ramp. From TGA results, a total weight loss of 81.6% occurred in the range of 200–750 °C, corresponding to the removal of the organic species and the bromine ions. The weight loss from 200 to 410 °C may result from the decomposition of benzimidazole, experimental weight loss (12.2%) is in agreement with the theoretical one (12.9%). This polymeric complex has excellent thermal stability, which may be due to the highly polarized M–N bond58,59 and inorganic column–organic hybrid.
Electrochemistry is also performed and the free benzimidazole ligand shows no electrochemical signal. Fig. 6 depicts the cyclic voltammogram of complex 1 in DMSO solution containing 0.1 M sodium perchlorate at a scan rate of 100 mV s−1, with N2 deoxygenation. Only one pair of redox peaks was obtained, and can be ascribed to the CuI/CuII. This is an irreversible one-electron redox couple with ΔE = 180 mV and ia/ic = 1.36 (ia and ic are the anodic and cathodic currents, respectively). Multicycle scanning confirms that the curve is repeatable.
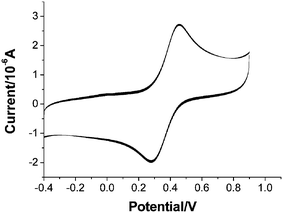 | ||
| Fig. 6 Cyclic voltammogram of complex 1. | ||
In summary, a mixed-valent cluster [CuI2CuII2Br4]∞ with the in situ formed benzimidazole ligand was observed in the solids of complex 1. The successfully prepared new 2D network is constructed from a novel mixed-valent rhombic dodecahedron-shaped [CuI2CuII2Br4]∞ linker and 1D [(C7H5N2)CuICuIIBr2]∞ chain structure. It provides the first example of rhombic dodecahedron-shaped copper–bromine configuration and the first Cu(I,II)Br species with benzimidazole ligand and verifies that Cu(II) is necessary and may play the catalyst role for the decomposition of the original ligand. Further studies on related Cu(I,II) halide frameworks with other N-containing ligands are in progress.
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China (20471033 and 21171109), the Natural Science Foundation of Shanxi Province (2010011011-2, 2011011009-1 and 2010021010-1 for Young Scientists), the Scientific Research Foundation for the Returned Overseas Chinese Scholars, State Education Ministry (20093602), the Shanxi Scholarship Council of China in 2008, Doctor Startup Foundation of Shanxi University of China, Students Scientific Practice Foundation of Shanxi University of China.Notes and references
- A. C. Sudik, A. R. Millward, N. W. Ockwig, A. P. Côté, J. Kim and O. M. Yaghi, J. Am. Chem. Soc., 2005, 127, 7110 CrossRef CAS.
- A. Lan, K. Li, H. Wu, M. Hong and J. Li, Angew. Chem., Int. Ed., 2009, 48, 2334 CAS.
- B. Chen, L. Wang, Y. Xiao, F. R. Fronczek, M. Xue, Y. Cui and G. Qian, Angew. Chem., Int. Ed., 2009, 48, 500 CrossRef CAS.
- B. Chen, L. Wang, F. Zapata, G. Qian and E. B. A. Lobkovsky, J. Am. Chem. Soc., 2008, 130, 6718 CrossRef CAS.
- Z. Xie, L. Ma, K. E. deKrafft, A. Jin and W. Lin, J. Am. Chem. Soc., 2010, 132, 922 CrossRef CAS.
- X. X. Xu, X. L. Bai, Y. Lu, E. B. Wang and Y. Ma, Inorg. Chem. Commun., 2006, 9, 872 CrossRef CAS.
- L. Subramanian and R. Hoffmann, Inorg. Chem., 1992, 31, 1021 CrossRef CAS.
- Q. Ye, X. S. Wang, H. Zhao and R. G. Xiong, Chem. Soc. Rev., 2005, 34, 208 RSC.
- J. Y. Lu, Coord. Chem. Rev., 2003, 246, 327 CrossRef CAS.
- J. K. Cheng, Y. B. Chen, L. Wu, J. Zhang, Y. H. Wen, Z. J. Li and Y. G. Yao, Inorg. Chem., 2005, 44, 3386 CrossRef CAS.
- G. H. Li, Z. Shi, X. M. Liu, Z. M. Dai and S. H. Feng, Inorg. Chem., 2004, 43, 6884 CrossRef CAS.
- J. D. Martin, J. C. Yang and A. M. Dattelbaum, Chem. Mater., 2001, 13, 392 CrossRef CAS.
- J. J. Hou, C. H. Guo and X. M. Zhang, Inorg. Chim. Acta, 2006, 359, 3991 CrossRef CAS.
- Y. Liu, Z. G. Zhao, Z. L. Fang, R. M. Yu and C. Z. Lu, Inorg. Chem. Commun., 2009, 12, 599 CrossRef CAS.
- T. Li, H. Zhou, P. Lin and S. W. Du, Inorg. Chem. Commun., 2006, 9, 1263 CrossRef CAS.
- K. H. Kim, T. Okubo, N. Tanaka, N. Mimura, M. Maekawa and T. Kuroda-Sowa, Chem. Lett., 2010, 39, 792 CrossRef CAS.
- T. Okubo, N. Tanaka, K. H. Kim, H. Yone, M. Maekawa and T. Kuroda-Sowa, Inorg. Chem., 2010, 49, 3700 CrossRef CAS.
- R. Peng, M. Li and D. Li, Coord. Chem. Rev., 2010, 254, 1 CrossRef CAS.
- J. H. Yu, H. Y. Bie, J. Q. Xu, J. Lu and X. Zhang, Inorg. Chem. Commun., 2004, 7, 1205 CrossRef CAS.
- A. J. Blake, N. R. Brooks, N. R. Champness, M. Crew, A. Deveson, D. Fenske, D. H. Gregory, L. R. Hanton, P. Hubberstey and M. Schroder, Chem. Commun., 2001, 1432 RSC.
- H. Ohi, Y. Tachi, T. Kunimoto and S. Itoh, Dalton Trans., 2005, 3146 RSC.
- S. Q. Liu, H. Konaka, T. Kuroda-Sowa, Y. Suenaga, H. Ito, G. L. Ning and M. Munakata, Inorg. Chim. Acta, 2004, 357, 3621 CrossRef CAS.
- J. Y. Lu, B. R. Cabrera, R. J. Wang and J. Li, Inorg. Chem., 1998, 37, 4480 CrossRef CAS.
- D. J. Chesnut, A. Kusnetzow, R. R. Birge and J. Zubieta, Inorg. Chem., 1999, 38, 2663 CrossRef CAS.
- E. Cariati, D. Roberto, R. Ugo, P. C. Ford, S. Galli and A. Sironi, Inorg. Chem., 2005, 44, 4077 CrossRef CAS.
- P. V. Solntsev, J. Sieler, H. Krautscheid and K. V. Domasevitch, Dalton Trans., 2004, 1153 RSC.
- R. D. Willett, Inorg. Chem., 2001, 40, 966 CrossRef CAS.
- J. K. Cheng, Y. B. Chen, L. Wu, J. Zhang, Y. H. Wen, Z. J. Li and Y. G. Yao, Inorg. Chem., 2005, 44, 3386 CrossRef CAS.
- J. R. D. DeBord, Y. J. Lu, C. J. Warren, R. C. Haushalter and J. Zubieta, Chem. Commun., 1997, 1365 RSC.
- S. H. Feng and R. Xu, Acc. Chem. Res., 2001, 34, 239 CrossRef CAS.
- J. Y. Lu, Coord. Chem. Rev., 2003, 246, 327 CrossRef CAS.
- J. Y. Lu, B. R. Cabrera, R. J. Wang and J. Li, Inorg. Chem., 1998, 37, 4480 CrossRef CAS.
- J. Y. Lu and A. M. Babb, Inorg. Chem., 2001, 40, 3261 CrossRef CAS.
- P. A. Prasad, S. Neeraj, S. Natarajan and C. N. R. Rao, Chem. Commun., 2001, 1250 Search PubMed.
- S. S. Feng, M. L. Zhu, L. P. Lu, L. Du, Y. B. Zhang and T. W. Wang, Dalton Trans., 2009, 6385 RSC.
- S. S. Feng, M. L. Zhu, L. P. Lu and M. L. Guo, Chem. Commun., 2007, 4785 RSC.
- S. S. Feng, L. P. Lu, H. M. Zhang, S. D. Qin, X. M. Li and M. L. Zhu, Acta Crystallogr., Sect. E: Struct. Rep. Online, 2005, 61, m659 Search PubMed.
- O. M. Yaghi and H. L. Li, J. Am. Chem. Soc., 1995, 117, 10401 CrossRef CAS.
- D. D. LeCloux, R. Davydov and S. J. Lippard, J. Am. Chem. Soc., 1998, 120, 6810 CrossRef CAS.
- R. P. Houser, V. G. Young and J. W. B. Tolman, J. Am. Chem. Soc., 1996, 118, 2101 CrossRef CAS.
- Y. H. Wen, J. K. Cheng, J. Zhang, Z. J. Li, Y. Kang and Y. G. Yao, Inorg. Chem. Commun., 2004, 7, 1120 CrossRef CAS.
- Y. Yan, C. D. Wu and C. Z. Lu, Z. Anorg. Allg. Chem., 2003, 629, 1991 CrossRef CAS.
- X. M. Zhang, Coord. Chem. Rev., 2005, 249, 1201 CrossRef CAS.
- F. Olbrich, J. Kopf and E. Weiss, Angew. Chem., Int. Ed. Engl., 1993, 32, 1077 CrossRef.
- J. C. Dyason, P. C. Healy, L. M. Engelhardt, C. Pakawatchai, V. A. Patrick, C. L. Raston and A. H. White, J. Chem. Soc., Dalton Trans., 1985, 831 RSC.
- C. Farren, S. FitzGerald, A. Beeby and M. R. Bryce, Chem. Commun., 2002, 572 RSC.
- K. P. Dancey, P. A. Tasker, R. Price, W. E. Hatfield and D. Brower, J. Chem. Soc., Chem. Commun., 1980, 1248 RSC.
- N. J. Blackburn, S. de Vries, M. E. Barr, R. P. Houser, W. B. Tolman, D. Sanders and J. A. Fee, J. Am. Chem. Soc., 1997, 119, 6135 CrossRef CAS.
- I. D. Brown, Chem. Rev., 2009, 109, 6858 CrossRef CAS.
- J. Yang, J. F. Ma, Y. C. Liu, G. L. Zheng, L. Li and J. F. Liu, J. Mol. Struct., 2003, 646, 55 CrossRef CAS.
- Y. Sun, Y. J. Hou, Q. X. Zhou, W. H. Lei, J. R. Chen, X. S. Wang and B. W. Zhang, Inorg. Chem., 2010, 49, 10108 CrossRef CAS.
- Z. L. Lu, T. Ladrak, O. Roubeau, J. V. D. Toorn, S. J. Teat, C. Massera, P. Gamez and J. Reedijk, Dalton Trans., 2009, 3559 RSC.
- Y. C. Wang, L. Z. Liu, Y. M. Pan and H. S. Wang, Molecules, 2011, 16, 100 CrossRef CAS.
- H. J. Jung, N. Singh, D. Y. Lee and D. O. Jang, Tetrahedron Lett., 2009, 50, 5555 CrossRef CAS.
- T. Wu, D. Li and S. W. Ng, CrystEngComm, 2005, 7, 514 RSC.
- G. A. V. Albada, M. G. V. D. Horst, I. Mutikainen, U. Turpeinen and J. Reedijk, J. Chem. Crystallogr., 2009, 39, 358 CrossRef CAS.
- P. Chen, D. E. Root, C. Campochiaro, K. Fujisawa and E. I. Solomon, J. Am. Chem. Soc., 2003, 125, 466 CrossRef CAS.
- Q. Wu, M. Esteghamatian, N. X. Hu, Z. D. Popovic, G. Enright and S. R. Breeze, Chem. Mater., 2001, 20, 79 Search PubMed.
- Y. Zhou, C. F. Zhong, Y. He, L. F. Xiao, Y. Liu and H. L. Zhang, J. Inorg. Organomet. Polym. Mater., 2009, 19, 328 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Crystal data (CIF files), experimental details, PXRD, EPR and TG spectra for 1. CCDC reference number 810807. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ce06215h |
| ‡ Student from College of Chemistry and Chemical Engineering, Shanxi University, and doing Scientific Practice in Institute of Molecular Science, Key Laboratory of Chemical Biology and Molecular Engineering of the Education Ministry, Shanxi University. |
| § Crystal data for 1: [C7H5Br2CuICuIIN2], monoclinic, space groupC2/m, a = 25.745(6), b = 9.538(2), c = 3.984(1) Å, β = 95.72(1)°, V = 973.6(4) Å3, Z = 4, Dc = 2.756 g cm−3, T = 298(2) K, crystal size 0.20 × 0.10 × 0.05 mm3, R = 0.057, wR = 0.1604, GOF = 1.127 with I > 2r(I). |
| This journal is © The Royal Society of Chemistry 2012 |
