A bi-ligand co-functionalized gold nanoparticles-based calcium ion probe and its application to the detection of calcium ions in serum†
Min Sik
Eom
a,
Woojeong
Jang
b,
Yoon Seo
Lee
a,
Gildon
Choi
c,
Yong-Uk
Kwon
*b and
Min Su
Han
*a
aDepartment of Chemistry, Chung-Ang University, Seoul 156-756, Republic of Korea. E-mail: E-mail: mshan@cau.ac.kr; Fax: +82-2-825-4736; Tel: +82-2-820-5198
bDepartment of Chemistry and Nano Science, Ewha Womans University, Seoul 120-750, Republic of Korea. E-mail: yukwon@ewha.ac.kr; Fax: +82-2-3277-2384; Tel: +82-2-3277-6685
cDivision of Drug Discovery, Korea Research Institute of Chemical Technology, Yuseong-gu, Daejeon 305-600, Republic of Korea
First published on 3rd April 2012
Abstract
In this study, the use of bi-ligand co-functionalized gold nanoparticles in a highly selective and sensitive colorimetric probe for Ca2+ ions is demonstrated and this probe also determined the concentrations of Ca2+ ions in serum samples.
Recognizing and detecting metal ions has been the aim of much important research in recent years. Several materials, such as organic dyes, proteins, magnetic nanoparticles, and nanomaterials, have been used to design sensing probes.1 Among these materials, gold nanoparticles (AuNPs) have been widely used to create sensing probes because they are easily synthesized and functionalized.2 Gold nanoparticles exhibit 3–5 orders of magnitude higher extinction coefficients than organic dye molecules. They also demonstrate distance-dependent optical properties that make them useful in colorimetric detection systems for metal ions.3 Most of these probes have been based on gold nanoparticles functionalized with mono-species ligands. However, some of these probes exhibited low selectivity and sensitivity. To address these problems, mixed ligands were introduced on AuNPs to provide cooperative recognition sites or imprinted recognition sites for analytes.4 For example, AuNPs functionalized with a combination of thiotic acid moieties and crown ether moieties can recognize K+ more efficiently than AuNPs functionalized with only a crown ether moiety.4a
The simple detection of Ca2+ among various metal ions is important because calcium ions are essential for regulating numerous biological processes such as excitability, neurotransmitter release, gene transcription, cell proliferation, synaptic plasticity, and hormone secretion.5 Recently, colorimetric probes for calcium ions have been developed using AuNPs functionalized with oligosaccharide and calsequestrin, respectively, and AuNPs stabilized with cytidine triphosphate.6–8 Oligosaccharides are well-known to chelate Ca2+ ions and the interaction enhances the oligosaccharide–oligosaccharide interaction.9 This property has been used to develop AuNP-based colorimetric probes to detect Ca2+.6 However, these probes exhibited interference from the presence of Mg2+ ions and low sensitivity in mM ranges. We speculated that these problems can be solved by introducing a combination of carboxylic acid and oligosaccharide groups into AuNPs. The carboxylic acid group can relax the steric constraints of the recognition moiety in saccharides and provide additional cooperative binding sites.4,10 Although saccharides and carboxylic acids have weak binding affinities for Ca2+ ions, self-assembly of the two ligands on the AuNP surface can provide a cooperative effect for Ca2+ recognition. This results in a AuNP-based colorimetric probe with high sensitivity and selectivity. In this study, bi-ligand functionalized-AuNPs were prepared as a colorimetric probe for Ca2+ ions more efficiently than AuNPs functionalized with carboxylic acid groups and oligosaccharide groups. To synthesize bi-ligand functionalized AuNPs, 1-thiohexyl β-D-lactopyranoside was prepared from lactose in 5 steps (see ESI†). Various bi-ligand functionalized AuNPs were synthesized by mixing citric acid-stabilized AuNPs with different ratios of 1-thiohexyl carboxylic acid (named A) and 1-thiohexyl β-D-lactopyranoside (named S), as shown in Scheme 1. These bi-ligand functionalized AuNPs were symbolized by AmSn-AuNPs where m and n represent different ratios of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside. For example, AuNPs functionalized with a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside were named A1S3-AuNPs. Also, AuNPs functionalized with a 1
1 mixture of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside were named A1S3-AuNPs. Also, AuNPs functionalized with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 mixture of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside were named A1S3-AuNPs. The S-AuNP preparations functionalized with only 1-thiohexyl β-D-lactopyranoside failed because AuNPs aggregated in the synthetic process. However, other AuNPs were prepared easily (see ESI†).
3 mixture of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside were named A1S3-AuNPs. The S-AuNP preparations functionalized with only 1-thiohexyl β-D-lactopyranoside failed because AuNPs aggregated in the synthetic process. However, other AuNPs were prepared easily (see ESI†).
 | ||
| Scheme 1 Synthetic routes to bifunctionalized AuNPs. | ||
To select an optimal ratio of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside in bi-ligand functionalized AuNPs, we evaluated how the AuNPs respond to Ca2+ and Mg2+. Selectivity of Ca2+ over Mg2+ in various metal ions was important because both Ca2+ and Mg2+ are major divalent metal ions in serum, and most Ca2+ probes involve Mg2+ interference.1a,6,11 Absorbance changes caused by Ca2+ and Mg2+ in these AuNPs were measured to evaluate Ca2+ selectivity. The UV/Vis spectra of bi-ligand functionalized AuNPs were recorded 5 min after adding two different concentrations (50 μM and 10 μM) of two metal ions, respectively. With an increasing ratio of thiohexyl β-D-lactopyranoside in the bi-ligand mixture, the selectivities of bi-ligand functionalized AuNPs over Mg2+ enhanced while responses caused by Ca2+ decreased. The selectivity and sensitivity of bi-ligand functionalized AuNPs were dependent on the ratio of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside. For example, A-AuNPs and A5S1-AuNPs were sensitive to Ca2+ but also significantly responded to Mg2+, as shown in Fig. 1. A1S5-AuNPs and A1S3-AuNPs were insensitive to Mg2+ but absorbance changes induced by Ca2+ were small. The A2S1-AuNPs did not respond to Mg2+ under the given conditions and exhibited large absorbance changes in the presence of Ca2+. Based on these results, A2S1-AuNPs were chosen for the Ca2+ colorimetric probe. The results suggest that carboxylic acid and oligosaccharide provided cooperative binding sites for selective recognition of Ca2+.
 | ||
| Fig. 1 (A) Absorbance changes of mixed-ligand functionalized AuNPs solutions in the presence of Mg2+ (50 μM) and Ca2+ (50 μM), respectively. (B) Absorbance changes of mixed-ligand functionalized AuNPs solutions in the presence of Mg2+(10 μM) and Ca2+ (10 μM), respectively. 0.1 mL of 500 μM and 100 μM metal ions were added to 0.9 mL AuNPs solutions, and the absorbance of the assay solution was recorded 5 min after the addition of metal ions. | ||
To understand the recognition abilities of these AuNPs, the sensing mechanism of this method was investigated. The UV/Vis spectrum and transmission electron microscopy (TEM) provided evidence that AuNPs aggregated upon Ca2+ addition. The surface plasmon resonance (SPR) band of the AuNPs red shifted from 520 to 590 nm in the presence of Ca2+, as shown in Fig. 2A. The red shift is a well-known phenomenon used to confirm the formation of AuNPs aggregates.3 AuNPs aggregation was also confirmed by TEM (Fig. 2B). This result suggests that bi-ligands provide a binding site for Ca2+. Consequently, Ca2+ interconnected the bi-ligands on one AuNP to bi-ligands on other AuNPs. To confirm that Ca2+-mediated bi-ligand interconnection caused the aggregation of AuNPs, 20 μM of EDTA was added to the aggregate. Immediately, aggregates dispersed and exhibited a SPR absorption change from 590 nm to 520 nm. The dispersion of A2S1-AuNPs was confirmed by the appearance of a SPR band at 520 nm in the UV/Vis spectrum and by TEM (Fig. 2C). The sensing mechanism of this method was proposed by combining these observations, as shown in Scheme 2.
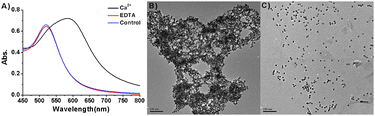 | ||
| Fig. 2 (A) The absorbance spectra of A2S1-AuNPs in the presence of Ca2+ (20 μM) followed by EDTA addition (20 μM). (B) TEM image of A2S1-AuNPs (3 nM) after the addition of Ca2+ (20 μM). (C) TEM image of re-dispersion of Ca2+-A2S1-AuNPs in the presence of EDTA (20 μM). Scale bar represents 100 nm. | ||
 | ||
| Scheme 2 Schematic illustration of AuNPs aggregation in the presence of Ca2+. | ||
To evaluate the sensitivity of this method, UV/Vis spectra of the A2S1-AuNPs solution were recorded after adding various concentrations of Ca2+. A stock solution of Ca2+ ions was added to the assay solution AuNPs (3 nM) in a pH 7.4 buffer solution, such that the final concentration was between 0 μM and 30 μM. Changes in the absorbance of the assay mixtures were measured 3 minutes after adding the Ca2+ ions. The addition of Ca2+ induced an increase in absorbance at 590 nm. As shown in Fig. 3, the observed absorbance intensity was almost proportional to the Ca2+ concentration and it saturated around 20 μM. From the titration data, the Ca2+ detection limit of this method was determined to be 1.9 μM (see Fig. S1 in ESI†). The combination of carboxylic acid and the oligosaccharide enhanced the sensitivity by >100 times than previous mono-ligand systems.6 In addition this method is fully sensitive for the detection of Ca2+ in serum because the normal level of calcium in serum is around 2 mM.
 | ||
| Fig. 3 (A) UV/Vis spectra of A2S1-AuNPs solution recorded 3 min after the addition of Ca2+ ions. (B) Plot of absorbance intensities of A2S1-AuNPs solutions recorded at 590 nm after the addition of Ca2+ ions versus Ca2+ concentration. | ||
There must be selectivity for Ca2+ over major serum metal ions to apply this probe to the analysis of Ca2+ ions in a serum sample. The levels of these ions are Ca2+ (∼2.45 mM), Na+ (∼150 mM), K+ (∼11.8 mM), Mg2+ (∼0.9 mM), Fe3+ (∼22 μM), Cu2+ (∼1 μM), and Zn2+ (∼10 μM) in human serum.12 The absorbance changes in A2S1-AuNPs solutions were recorded 3 min after adding other metal ions (20 μM). The addition of 20 μM of Zn2+ caused absorbance changes, while other metal ions and 2 μM of Zn2+ did not cause any significant changes, as shown in Fig. 4A and B. The A2S1-AuNPs had high selectivity for Ca2+ over Mg2+ in broad ranges (see Fig. S2 in ESI†). Although 20 μM of Zn2+ caused absorbance changes, the interference is not a serious problem for detecting Ca2+ in serum. The Zn2+ level in real samples will be ∼100 nM because serum can be diluted over 100 times with assay buffer. Therefore, the interference of Zn2+ in real samples is not significant.
 | ||
| Fig. 4 (A) UV/Vis spectra obtained 3 min after the addition of various cations to pH 7.4 buffer solution (Tris–HCl + 50 mM NaCl) containing A2S1-AuNPs (3 nM). (B) The color of the solution in the absence and presence of cations: from left to right; Control, Ca2+ (20 μM), Na+ (20 μM), K+ (20 μM), Mg2+ (20 μM), Fe3+ (20 μM), Cu2+ (20 μM), Zn2+ (2 μM), Zn2+ (20 μM). | ||
To demonstrate the ability of the developed probe to analyze Ca2+ ions in a practical sample, the concentrations of calcium in several serums were determined. Fetal bovine serum (FBS), horse, donkey, rat, mouse, and chicken serum were tested. The serums were acidified to pH 2 with HCl and incubated for 3 h at 80 °C because in serum, Ca2+ ions exit in both free form and bound form with proteins. After treatment, the solution was filtrated by using a spin filter with 3 kDa pore size to remove serum protein. The filtrate was diluted ∼10 times with assay buffer and then 0.1 mL of the diluted filtrate was added to 0.9 mL of the assay solution containing A2S1-AuNPs (3.3 nM). Changes in the absorbance of the assay mixtures were recorded at 590 nm 3 minutes after adding the diluted filtrate. The total Ca2+ ions in serum was determined based on these results and are summarized in Table 1. The determined concentrations of Ca2+ were in agreement with those obtained by using an inductively coupled plasma atomic emission spectrometer (ICP-AES). Titration curves from various concentrations of Ca2+ solutions made by using diluted filtrate from FBS also correlated well with the titration data in Fig. 3B (see Fig. S3 in ESI†). This indicates that the present method worked well in blood serum.
| ICP-AES (mM) | A2S1-AuNPs (mM) | |
|---|---|---|
| FBS | 2.70 ± 0.04 | 2.96 ± 0.13 |
| Horse serum | 3.79 ± 0.06 | 3.50 ± 0.06 |
| Donkey serum | 4.35 ± 0.04 | 4.62 ± 0.09 |
| Rat serum | 3.26 ± 0.03 | 3.32 ± 0.04 |
| Mouse serum | 1.56 ± 0.01 | 1.52 ± 0.01 |
| Chicken serum | 3.06 ± 0.03 | 3.32 ± 0.04 |
In conclusion, we selected AuNPs functionalized with a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside as a Ca2+ probe. This probe was highly selective for Ca2+ ions over other metal ions relevant to blood serum and it allowed assay of the Ca2+ ions up to 1.9 μM. Moreover, the probe determined Ca2+ concentrations in various serum samples and the results correlated well with those obtained by using ICP-AES.
1 ratio of 1-thiohexyl carboxylic acid and 1-thiohexyl β-D-lactopyranoside as a Ca2+ probe. This probe was highly selective for Ca2+ ions over other metal ions relevant to blood serum and it allowed assay of the Ca2+ ions up to 1.9 μM. Moreover, the probe determined Ca2+ concentrations in various serum samples and the results correlated well with those obtained by using ICP-AES.
This work was supported by the Korea Research Foundation Grant funded by the Korean Government (2011-0005747 and 2009-0068960) and Priority Research Centers Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2009-0093817).
Notes and references
- (a) A. P. D. Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef; (b) E. M. Nolan and S. J. Lippard, Chem. Rev., 2008, 108, 3443 CrossRef CAS; (c) H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465 RSC; (d) T. A. Kost, J. P. Condreay and D. L. Jarvis, Nat. Biotechnol., 2005, 23, 567 CrossRef CAS; (e) Y. Lu and J. Liu, Acc. Chem. Res., 2007, 40, 315 CrossRef CAS.
- (a) N. L. Rosi and C. A. Mirkin, Chem. Rev., 2005, 105, 1547 CrossRef CAS; (b) S. K. Ghosh and T. Pal, Chem. Rev., 2007, 107, 4797 CrossRef CAS.
- (a) Y. Kim, R. C. Johnson and J. T. Hupp, Nano Lett., 2001, 1, 165 CrossRef CAS; (b) S.-Y. Lin, S.-W. Liu, C.-M. Lin and C.-H. Chen, Anal. Chem., 2002, 74, 330 CrossRef CAS; (c) S. O. Obare, R. E. Hollowell and C. J. Murphy, Langmuir, 2002, 18, 10407 CrossRef CAS; (d) G. K. Darbha, A. K. Singh, U. S. Rai, E. Yu, H. Yu and P. C. Ray, J. Am. Chem. Soc., 2008, 130, 8038 CrossRef CAS; (e) X. Xue, F. Wang and X. Liu, J. Am. Chem. Soc., 2008, 130, 3244 CrossRef CAS; (f) J.-S. Lee, M. S. Han and C. A. Mirkin, Angew. Chem., Int. Ed., 2007, 46, 4093 CrossRef CAS; (g) C.-W. Liu, Y.-T. Hsieh, C.-C. Huang, Z.-H. Lina and H.-T. Chang, Chem. Commun., 2008, 2242 RSC; (h) Y.-W. Lin, C.-C. Huang and H.-T. Chang, Analyst, 2011, 136, 863 RSC.
- (a) S.-Y. Lin, C.-H. Chen, M.-C. Lin and H.-F. Hsu, Anal. Chem., 2005, 77, 4821 CrossRef CAS; (b) Y. Yao, D. Tian and H. Li, ACS Appl. Mater. Interfaces, 2010, 2, 684 CrossRef CAS; (c) Y. Ben-Ben-Amram, R. Tel-Vered, M. Riskin, Z.-G. Wang and I. Willner, Chem. Sci., 2012, 3, 162 RSC.
- (a) A. Marty, Trends Neurosci., 1989, 12, 420 CrossRef CAS; (b) R. C. Malenka, J. A. Kauer, D. J. Perkel and R. A. Nicoll, Trends Neurosci., 1989, 12, 444 CrossRef CAS; (c) S. Orrenius, B. Zhivotovsky and Nicotera, Nat. Rev. Mol. Cell Biol., 2003, 4, 552 CrossRef CAS.
- (a) J. M. de la Fuente, A. G. Barrientos, T. C. Rojas, J. Rojo, J. Cañada, A. Fernández and S. Penadés, Angew. Chem., Int. Ed., 2001, 40, 2257 CrossRef; (b) M. J. Hernáiz, J. M. de la Fuente, Á. G. Barrientos and S. Penadés, Angew. Chem., Int. Ed., 2001, 41, 1554 CrossRef; (c) Á. G. Barrientos, J. M. de la Fuente, T. C. Rojas, A. Fernández and S. Penadés, Chem.–Eur. J., 2003, 9, 1909 CrossRef; (d) A. J. Reynolds, A. H. Haines and D. A. Russell, Langmuir, 2006, 22, 1156 CrossRef CAS; (e) A. C. de Souza, K. M. Halkes, J. D. Meeldijk, A. J. Verkleij, J. F. G. Vliegenthart and J. P. Kamerling, ChemBioChem, 2005, 6, 828 CrossRef.
- S. Kim, J. W. Park, D. Kim, D. Kim, I.-H. Lee and S. Jon, Angew. Chem., Int. Ed., 2007, 46, 4093 CrossRef.
- S. Kim, J. Kim, N. H. Lee, H. H. Jang and M. S. Han, Chem. Commun., 2011, 47, 10299 RSC.
- C. E. Bugg, J. Am. Chem. Soc., 1973, 95, 908 CrossRef CAS.
- X. Liu, Y. Hu and F. Stellacci, Small, 2011, 7, 1961 CrossRef CAS.
- (a) R. Y. Tsien, Biochemistry, 1980, 19, 2396 CrossRef CAS; (b) G. Grynkiewicz, M. Poenie and R. Y. Tsien, J. Biol. Chem., 1985, 260, 3440 CAS; (c) H. M. Kim, B. R. Kim, M. J. An, J. H. Hong, K. J. Lee and B. R. Cho, Chem.–Eur. J., 2008, 14, 2075 CrossRef CAS; (d) E. Chapoteau, B. P. Czech and W. Zazulak, J. Inclusion Phenom. Mol. Recognit. Chem., 1995, 23, 147 CrossRef CAS.
- (a) P. M. May, P. W. Linder and D. R. Williams, J. Chem. Soc., Dalton Trans., 1977, 588 RSC; (b) A. R. McNeil, A. Gardner and S. Stables, Clin. Chem., 1999, 45, 135 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details for the synthetic method of 1-thiohexyl β-D-lactopyranoside and mixed-ligand functionalized AuNPs. UV-Vis spectra of bi-ligand co-functionalized AuNPs in the presence of Ca2+ and Mg2+. Details for the Ca2+ detection limit for this method. Titration curves from various concentrations of Ca2+ solutions made by using diluted filtrate from FBS. UV-Vis spectra of A2S1-AuNPs in the presence of alkaline earth metal ions. See DOI: 10.1039/c2cc31724a |
| This journal is © The Royal Society of Chemistry 2012 |
