Oxygen bridged neutral annulenes: a novel class of materials for organic field-effect transistors†
Kamaljit
Singh
*a,
Tarunpreet Singh
Virk
a,
Jing
Zhang
b,
Wei
Xu
b and
Daoben
Zhu
*b
aOrganic Synthesis Laboratory, Department of Applied Chemical Sciences and Technology, Guru Nanak Dev University, Amritsar 143005, India. E-mail: kamaljit19in@yahoo.co.in; Fax: +91-2258819/20
bBeijing National Laboratory for Molecular Science, CAS Key Laboratory of Organic Solids, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100080, P. R. China. E-mail: wxu@iccas.ac.cn
First published on 20th October 2011
Abstract
New, neutral, meso-substituted tetraoxa[22]annulene[2,1,2,1] aromatic macrocycles are synthesised and disclosed as new p-type semiconductors with reproducible bulk-like carrier mobility (as high as 0.40 cm2 V−1 s−1) on highly crystalline thin films deposited on octadecyltrichlorosilane modified SiO2.
In the course of development of cyclic conjugated chemical entities, structural variations of the fundamental tetrapyrrolic porphyrinoids 1, by way of replacing pyrrole with other heterocyclic species and/or changing the topology of the macrocyclic framework in a manner in which the π-electron conjugation pathway is maintained, have led to the synthesis of potentially rich families of neutral as well as charged heteroannulenes 2–8 (Fig. 1).1 The propensity of these neutral π-systems to take up additional electrons to form radical anions or dianions or to lose two electrons to form dications etc. is a distinctive feature implicated in the understanding of the electronic structure and physical properties of the ions and neutral molecules2a tailored for applications ranging from medicine to material science.2b Compared to oligothiophenes, attractive features of linear oligofurans: superior solubility, tighter packing, higher HOMO, fluorescence and biodegradability, have led to their use as p-type OFETs with hole mobility significantly inferior to that of oligothiophenes.3 In this context, we have previously, in the case of meso-aryl tetrathia[22]annulene[2,1,2,1] 6, disclosed the electronic structure and demonstrated its use as thin film organic field-effect transistors (OFETs)4 with mobility higher than those of porphyrins,5 and copper phthalocyanine6 as well as α-oligofurans and α-oligothiophenes. This is believed to be benefited from the extended π-conjugation, which lowered the reorganization energy and enhanced intermolecular π–π overlapping.
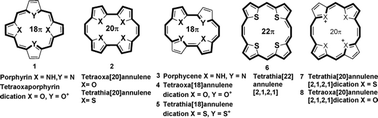 | ||
| Fig. 1 Neutral and charged heteroannulenes 1–8. | ||
The neutral alkene linked tetraoxa[20]annulene 2 represents a 20π antiaromatic system, which though slightly puckered is considerably stable compared to 22,24-dihydroporphycene and revealed pronounced paramagnetic ring currents in its 1H NMR spectrum. Its dicationic counterpart, 4,7 is (4n + 2)π aromatic and planar as revealed from its 1H NMR spectrum. The UV data of both 4 and 1 suggest conjugation, accompanied by reduction in splitting of both Soret and especially the Q-type bands in the latter.8 The 1H NMR spectral data and X-ray structure analysis of 4nπ tetraoxa[24]annulene[2,2,2,2]9 point to a cis, trans, cis, trans configuration, maintained in its oxidized aromatic C2 symmetric, 22π dication also, and exhibit paratropic ring current [ethylenic protons: 12.38 ppm (inner); 13.60 ppm (outer)]. The latter in its 1H NMR spectrum depicted diatropic ring current with the inner core ethylenic protons observing considerable upfield shift (Δδ, 20.46 ppm) to appear at −8.08 ppm, compared to the outer ring protons which appear at 5.30 ppm (Δδ, 8.36 ppm). In the UV-visible spectrum of the dication (in 70% perchloric acid), all absorptions were red-shifted (approximately 100 nm), compared to those of the tetraoxa[24] system, and exhibited significantly greater molar extinction coefficients. The C2h symmetric, planar tetraoxa[22]annulene[2,1,2,1]10 depicted high sensitivity to oxygen. Thus, a stable tetraoxa[22]annulene[2,1,2,1] still awaits synthesis and makes it attractive synthetic target. In this communication, we report the first study of semiconducting properties of stable tetraoxa[22]annulene[2,1,2,1] which shows hole mobility significantly higher than those of both α-oligofurans and oligothiophenes.
As the method of choice for the synthesis of the tetraoxa[22]annulene[2,1,2,1] 12 (Scheme 1), the two electron oxidation of the dihydrotetraoxaannulenes 11, in analogy with synthesis of 6 presented itself. Ready oxidizability of 11 to 12 appeared to be facile using DDQ, in view of the observation that 11a upon exposure to air and chromatographic purification was partly transformed to 12a. However, exceptionally stable [22]annulenes 12a,b were isolated as purple leaflets (from dichloromethane/toluene, 12a, and o-dichlorobenzene, 12b). The dihydro macrocycles 11a as well as 11b were synthesized by reductive coupling reaction of the diformyl bis(furan-2-yl)methane derivatives 10a and 10b (S2, ESI†), using a low valent titanium compound (McMurry coupling). Aldehydes 10a,b in turn were synthesized by Vilsmeier–Haack reaction of 9 with POCl3/DMF (S1–S4, ESI†).
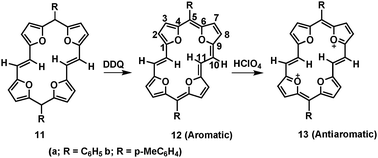 | ||
| Scheme 1 Synthesis of 12a/12b and oxidation to 13. | ||
The 1H NMR spectra of 11a and 11b (Table S1, ESI†), as expected, consisting of two doublets, one each for H2/H8 and H3/H7 protons, and a singlet for H10/H11, appeared at a relatively high field. The magnitude of the vicinal coupling constants, which are very similar to those of furan, indicates thermodynamically more stable double-bond character of the four furan units in 11a and 11b. The (4n + 2)π tetraoxa[22]annulene[2,1,2,1], 12a and 12b, which ought to be planar and aromatic systems is established very convincingly from the 1H NMR spectrum (Fig. S19 and S22, ESI†). Thus, the AB system of the furans in 12a,b appeared quite downfield (Table S1, ESI†) compared to that in 11 and none of the proton signals of the macrocyclic framework appeared upfield to 9.01 ppm. Only the ethylenic proton pairs of 12a and 12b, directed inside the cavity of the macrocycle, under the influence of ring currents appeared at −5.42 ppm and −5.40 ppm, respectively. That 12a and 12b, in contrast to 11a and 11b, are delocalized is revealed not only by the chemical shifts, but also by a partial equalization of the vicinal coupling constants of the AB system of the furan rings.
As a quantitative probe of aromaticity, we have calculated Nucleus-Independent Chemical Shift, NICS(1),11 values (Fig. S26, S30 and S33, ESI†) using ab initio quantum mechanical DFT calculations at the B3LYP/6-311G(d) level using the Gauge Independent Atomic Orbitals (GIAO) method. The large negative NICS(1) values of 12a (−13.17 ppm) and 12b (−13.17 ppm), calculated at centres of the molecules, indicate a greater degree of aromaticity, reflecting the enhanced ring current effects of a planar geometry. The shapes of the plots of the chemical shifts as a function of the distance of the NICS probe (bq) from the molecular plane gave a clear indication of occurrence of diamagnetic ring currents.
The aromaticity of 12a (and 12b, Fig. S42a, ESI†) was further evidenced by the UV-visible absorption spectra, recorded in DCM. There are sharp and strong absorption maxima for 12a at 415 and 433 nm (ε/dm3 mol−1 cm−1, 200![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 687 and 161
687 and 161![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 940, respectively), compared to 11a (Fig. 2a), and several weaker absorptions at longer wavelengths [510, 558, 587, 718 and 785 nm (ε/dm3 mol−1 cm−1 5840, 13
940, respectively), compared to 11a (Fig. 2a), and several weaker absorptions at longer wavelengths [510, 558, 587, 718 and 785 nm (ε/dm3 mol−1 cm−1 5840, 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833, 104
833, 104![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 833, 1940, 10
833, 1940, 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 687, respectively)]. The split Soret band at 415 and 433 nm of the lower symmetry (C2) 12a is analogous to the unsplit Soret band of the high (D2h) symmetry 6 while the Q-type bands appeared slightly red shifted than those of porphycenes. In analogy with 6,4 the 22π annulenes 12a and 12b were found to dissolve in 70% perchloric acid to give a reddish-violet solution, indicative of formation of dication 13a (disappearance of sharp Soret bands as well as broadening of absorption bands). Also 13a depicted a considerably positive (19.59 ppm) NICS(1) value (Fig. S36, ESI†). The UV-vis data of thin films (Fig. S42b, ESI†) showed good correlation with the solution absorption spectra. In the IR (KBr) spectra medium-strong bands are found at
687, respectively)]. The split Soret band at 415 and 433 nm of the lower symmetry (C2) 12a is analogous to the unsplit Soret band of the high (D2h) symmetry 6 while the Q-type bands appeared slightly red shifted than those of porphycenes. In analogy with 6,4 the 22π annulenes 12a and 12b were found to dissolve in 70% perchloric acid to give a reddish-violet solution, indicative of formation of dication 13a (disappearance of sharp Soret bands as well as broadening of absorption bands). Also 13a depicted a considerably positive (19.59 ppm) NICS(1) value (Fig. S36, ESI†). The UV-vis data of thin films (Fig. S42b, ESI†) showed good correlation with the solution absorption spectra. In the IR (KBr) spectra medium-strong bands are found at ![[small upsilon, Greek, macron]](https://www.rsc.org/images/entities/i_char_e0d5.gif) = 779, 1175, 1299, 1654, 3100 cm−1 (12a); 779, 1176, 1301, 1645, 3100 cm−1 (12b) (Fig. S21 and S24, ESI†).
= 779, 1175, 1299, 1654, 3100 cm−1 (12a); 779, 1176, 1301, 1645, 3100 cm−1 (12b) (Fig. S21 and S24, ESI†).
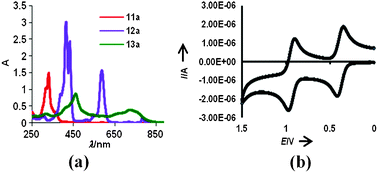 | ||
| Fig. 2 (a) UV-vis spectra of 11a (DCM), 12a (DCM) and 13a (HClO4), (b) cyclic voltammogram (CV) for 12a. | ||
The HOMO–LUMO gap of 1.56 eV, determined from the UV-vis absorption edge of the solution spectrum, compared favorably with 2.05 eV, obtained from DFT calculations at B3LYP/6-311G(d). Visualization of HOMOs and LUMOs (Fig. S38 and S39, ESI†) revealed π-delocalization over the annulene rings of both 12a and 12b, supporting the observed aromatic character and greater contribution of oxygen atoms to HOMO compared to LUMO. The cyclic voltammogram of 12a (Fig. 2b) shows two reversible oxidation peaks at 0.418 V and 0.970 V (0.408 V and 0.965 V for 12b, Fig. S45, ESI†). The lower value of the first oxidation potential of 12a compared to that of the sulfur analogue (0.552 V)4 hints at ease in oxidation of 12a to the dication 13a upon treatment with HClO4.
The good thermal stability of 12a and 12b was indicated by the onset thermal decomposition temperatures of 374 °C and 380 °C, respectively in the thermogravimetric analysis (Fig. S46 and S47, ESI†).
Compounds 12a and 12b crystallize in monoclinic P21/c (12a) and orthorhombic pbca (12b) space groups, respectively. The unit cell parameters are reported in Tables S6 and S7 (ESI†). The four oxygen atoms in the macrocycles 12a and 12b are in the same plane, as are the furan rings, much similar to thiophene analogues, and both conform to a “heteroannulene ring system”. The following structural features are evident. (i) Both 12a (Fig. 3) and 12b (Fig. S48, ESI†) are practically planar, as evident from the torsion angles and deviation of the C and O atoms from the mean plane (3.97° and 7.46°, respectively, in 12a and 12b). (ii) The O4 core of both 12a and 12b adopts almost rectangular shape.
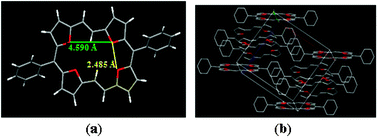 | ||
| Fig. 3 Crystal structure and stacking pattern of 12a (H atoms are omitted for clarity). | ||
Of the two sets of oxygen atoms in 12a (Fig. 3), the ones intercepted by four-carbon atoms are at a distance of 4.590 Å apart, while the distance between the neighboring oxygen atoms separated by a three-carbon bridge is 2.485 Å. These distances change to 4.624 Å and 2.493 Å in the case of 12b. This is in contrast to the almost square shape in tetrathia[22]annulene where the two S⋯S distances were 3.110 Å and 3.018 Å.4 This is presumably because of the trans-geometry of the ethylenic bridge connecting the two neighboring furan rings, compared to cis-geometry in the tetrathia[22]annulene. (iii) As expected, bond alternation is not evident, consistent with a planar aromatic system with electron delocalization. This fact has also been verified from the NICS(1) values, which, in contrast to the that of isolated furan (−10.35), are considerably negative (−20.01, −18.65, −15.15 and −16.61) for the four furans of the fully conjugated 12a (Fig. S28 and S32, ESI†). Changing the meso substituent from phenyl in 12a to p-tolyl in 12b does not induce any ring puckering of the annulene core and/or affect aromaticity. In the packing diagrams, the molecules of both 12a and 12b stacked in a standard layer-by-layer herringbone fashion. In 12a the molecules stacked into layers along the bc-plane with a tilt angle of 52.79° between the annulene core and the bc-plane. This is significantly different from their sulfur analogues, where a shifted face-to-face pattern was observed.4
Electrical transport properties of 12a and 12b were characterized on vacuum deposited thin films, both of these compounds displayed p-type semiconductor behavior. Thin-film transistors were fabricated on OTS or PMMA/SiO2/Si substrates in a top-contact configuration using Au as source and drain electrodes. The transfer and output curves for the devices of 12a and 12b are shown in Fig. S49 (ESI) and detailed performance is given in Table 1.
| Compound | T s/°C | μ/cm2 V−1 s−1 | I on/Ioff | V T/V |
|---|---|---|---|---|
| a PMMA surface modified SiO2/Si substrate (25 nm). b The maximum value of mobility is presented in brackets beside the average value. | ||||
| 12a | 20 | 0.10 | 0.8 × 103 | 20.3 |
| 20 | 0.02a | 1.2 × 104 | 6.0 | |
| 60 | 0.35 (0.40)b | 3.51 × 103 | 20.0 | |
| 100 | 0.14 | 1 × 103 | 15.7 | |
| 12b | 20 | 0.28 × 10−3 | 28 | 24.8 |
| 60 | 0.1 | 1.6 × 102 | 24.8 | |
| 100 | 0.11 | 102 | 24.4 |
The morphology of the thin films (Fig. 4) changed with temperature (20 °C to 100 °C). While changing the temperature from 20 °C to 60 °C increased the grain size as well as yielded more ordered films, at 100 °C, the films developed cracks. The highest performance with a mobility as high as 0.40 cm2 V−1 s−1 was observed on thin films of 12a deposited on OTS modified SiO2 at the substrate temperature of 60 °C (Table 1). The more positive value12 of VT, observed for the OTS modified devices, may arise from the assembling of charge on the OTS surface during the operation, while the device of 12a based on a PMMA surface modified SiO2/Si substrate yielded a rather higher on/off ratio and a favorable VT.
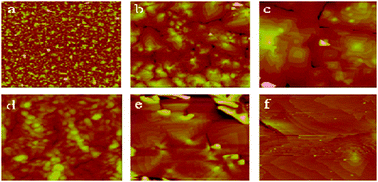 | ||
| Fig. 4 AFM images of 50 nm thick films deposited on an OTS modified SiO2/Si substrate of 12a (5 μm × 5 μm) at 25 °C (a), 60 °C (b) and 100 °C (c) and 12b (2 μm × 2 μm) at 25 °C (d), 60 °C (e) and 100 °C (f). | ||
In summary, this work is a desolate example of the synthesis, structure and application of new meso-aryl substituted air-stable oxygen bridged neutral annulenes as promising active materials for OFETs.
KS thanks DST, New Delhi, for the Research grant SR/S1/OC-27/2009 and UGC (SAP), New Delhi and National single crystal X-ray facility, IIT Mumbai.
Notes and references
- (a) J. L. Sessler and D. Seidel, Angew. Chem., Int. Ed. Engl., 2003, 42, 5134–5175 CrossRef CAS; (b) J. M. Lim, Z. S. Yoon, J. Y. Shin, K. S. Kim, M. C. Yoon and D. Kim, Chem. Commun., 2009, 261–273 RSC; (c) D. S. Gracia and J. L. Sessler, Chem. Soc. Rev., 2008, 37, 215–232 RSC.
- (a) R. Bachmann, F. Gerson, G. Gescheidt and E. Vogel, J. Am. Chem. Soc., 1992, 114, 10855–10860 CrossRef CAS; (b) J. L. Sessler and S. J. Weghorn, Expanded, Contracted and Isomeric Porphyrins, Tetrahedron Organic Chemistry Series 15, Pergamon Elsevier, Amsterdam, 1997 Search PubMed.
- O. Gidron, A. Dadvand, Y. Sheynin, M. Bendikov and D. F. Perepichka, Chem. Commun., 2011, 47, 1976–1978 RSC.
- (a) K. Singh, A. Sharma, J. Zhang, W. Xu and D. Zhu, Chem. Commun., 2011, 47, 905–907 RSC; (b) T. Zhao, Z. Wei, Y. Song, W. Xu, W. Hu and D. Zhu, J. Mater. Chem., 2007, 17, 4377–4381 RSC.
- (a) P. Checcoli, G. Conte, S. Salvatori, R. Paolesse, A. Bolognesi, M. Berliocchi, F. Brunetti, A. D'Amico, A. Di Carlo and P. Lugl, Synth. Met., 2003, 138, 261–266 CrossRef CAS; (b) Y. Y. Noh, J. J. Kim, Y. Yoshida and K. Yase, Adv. Mater., 2003, 15, 699–702 CrossRef CAS.
- Z. Bao, A. J. Lovinger and A. Dodabalapur, Appl. Phys. Lett., 1996, 69, 3066–3068 CrossRef CAS.
- E. Vogel, M. Sicken, P. Rohrig, H. Schmickler, J. Lex and O. Ermer, Angew. Chem., Int. Ed. Engl., 1988, 27, 411–414 CrossRef.
- W. Haas, B. Knipp, M. Sicken, J. Lex and E. Vogel, Angew. Chem., Int. Ed. Engl., 1988, 27, 409–411 CrossRef.
- G. Markl, H. Sauer, P. Kreitmeier, T. Burgemeister, F. Kastner, G. Adolin, H. Noth and K. Polborn, Angew. Chem., Int. Ed. Engl., 1994, 33, 1151–1153 CrossRef.
- G. Markl, R. Ehrl and P. Kreitmeier, Helv. Chim. Acta, 1998, 81, 93–108 CrossRef.
- A. Stanger, J. Org. Chem., 2006, 71, 883–893 CrossRef CAS.
- Y. Wang, E. Zhou, Y. Liu, H. Xi, S. Ye, W. Wu, Y. Guo, C. Di, Y. Sun, G. Yu and Y. Li, Chem. Mater., 2007, 19, 3361–3363 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Details of experimental procedures, full characterization. CCDC 825048 and 825049. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1cc16344b |
| This journal is © The Royal Society of Chemistry 2012 |
