Conjugated polymers containing a 2,2′-biimidazole moiety—a novel fluorescent sensing platform†
Yinyin
Bao
,
Qianbiao
Li
,
Bin
Liu
,
Fanfan
Du
,
Jiao
Tian
,
Hu
Wang
,
Yanxue
Wang
and
Ruke
Bai
*
CAS Key Laboratory of Soft Matter Chemistry, Department of Polymer Science and Engineering, University of Science and Technology of China, Hefei, P. R. China 230026. E-mail: bairk@ustc.edu.cn; Fax: +0086-551-3631760; Tel: +0086-551-3600722
First published on 4th November 2011
Abstract
Two novel conjugated polymers containing a 2,2′-biimidazole moiety have been designed, synthesized, and demonstrated to be used as an effective fluorescent sensing platform for detection of Ag+ and cysteine. This is the first example utilizing a fluorescent conjugated polymer–Ag+ complex to selectively detect Cys with a nanomolar range detection limit.
Fluorescent conjugated polymers have been widely utilized as highly sensitive chemosensors for detection of many analytes in recent years due to the signal amplification effect through the “molecular wire” of the conjugated backbone.1 Among these polymers, several N-heterocyclic aromatic structures, such as pyridine,2bipyridyl,3terpyridyl,4 and phenanthroline,5 have been introduced into the backbone as coordination sites for metal ions. When the interaction occurs between the coordination sites and metal ions, the conjugated polymers exhibit fluorescence quenching or emission red/blue-shift, this is generally attributed to electron density variations on the main chains or conjugation enhancement along the polymer backbone.3a,b However, the selectivity of polymer sensors based on N-heterocycles, especially for the bipyridyl analogue, remains unsatisfactory compared with that of the small molecule sensors.6 In order to improve the properties of the polymer sensors, the design and synthesis of the conjugated polymer sensors with a new aromatic N-heterocycle as a receptor are still a quite important and intriguing theme.
Imidazole is an important aromatic N-heterocycle because of its significant role in biosystems and attractive chemical properties.7 Most recently, the conjugated polymers with imidazole or imidazolium as the side groups exhibit outstanding sensing properties to metal ions, anions, nitric oxide, and amino acids.82,2′-Biimidazole, the dimeric analogue of imidazole, is one of the most important derivatives of imidazole and plays a particular role in crystal engineering because of the excellent coordination ability and diverse coordination modes.9 However, only very few papers have been devoted to the study of the conjugated polymers containing a biimidazole moiety. Yamamoto et al. prepared three 2,2′-biimidazole homopolymers by the dehalogenative polycondensation using a zerovalent nickel catalyst.10a Then MacLean et al. reported conjugated polymers of 2,2′-biimidazole obtained through electrochemical polymerization.10b The authors did not investigate the fluorescence properties of the polymers, they focused on the structure and chemical properties.
In order to explore and design new conjugated polymer sensors with excellent properties, we designed and prepared 2,2′-biimidazole based conjugated polymers, and studied the fluorescence sensing property of the polymers for the detection of metal ions and amino acids. Two polymers P1 and P2 were synthesized through Suzuki condensation and the synthetic routes are illustrated in Scheme 1. 2,2′-Biimidazole was first prepared according to the published procedure,11a and then was reacted with n-butyl acrylate and P1 was conveniently prepared by the Suzuki coupling reaction between M1 and M2, and P2 was obtained in a similar approach. P1 and P2 were purified by precipitation from hexane and collected as slightly gray and red solids, respectively. The purified polymers were characterized by 1H NMR and FTIR (see ESI†), providing conclusive evidence for the well-controlled structure as we expected by the incorporation of the monomers into the polymer backbone. Both polymers readily dissolve in common organic solvents, such as toluene, CH2Cl2, THF, and dioxane. The molecular weights (Mn) and polydispersity index (PDI) of the polymers were determined by gel permeation chromatography (GPC) with polystyrene as the reference standard (Table S1, ESI†).
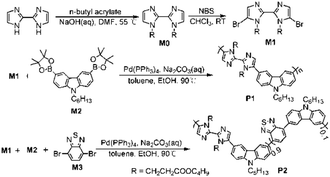 | ||
| Scheme 1 Synthesis of monomers and polymers. | ||
The absorption and emission spectra of the two polymers were measured in dioxane at room temperature (Fig. 1). P1 exhibits the absorption maximum at 312 nm and its photoluminescence spectrum peaked at 416 nm. In comparison with P1, P2 displays a main absorption peak at 310 nm and the other weak peak at 450 nm. Similarly in the PL spectrum, P2 shows two peaks centred at 416 and 560 nm, which are due to the emission of carbazole–biimidazole and carbazole–benzothiazole units, respectively. Simultaneously, a Förster resonance energy transfer (FRET) process exists in P2, because the weak absorption peak of the carbazole–benzothiazole unit was found to overlap with the emission peak of the carbazole–biimidazole unit (Fig. 1). Both the polymers are emissive in the visible region under UV irradiation (365 nm), P1 shows a blue-violet color whereas P2 is a reddish orange color. The fluorescence quantum yields of P1 and P2 were determined to be 0.2 and 0.17, respectively.
![UV-vis absorption and fluorescence spectra of P1 (blue line) and P2 (red line) in dioxane. [P1] = [P2] = 8.0 μM. λex = 333 nm.](/image/article/2012/CC/c1cc15787f/c1cc15787f-f1.gif) | ||
| Fig. 1 UV-vis absorption and fluorescence spectra of P1 (blue line) and P2 (red line) in dioxane. [P1] = [P2] = 8.0 μM. λex = 333 nm. | ||
The ion responsive properties of the polymers were studied by fluorescence emission spectroscopy in dioxane at a concentration of 8.0 μM (Fig. S1–S4, ESI†). It was found that both of the polymers have no response to the addition of alkali as well as alkaline earth metal ions. However, upon the addition of Ag+, the emission peaks at 416 nm exhibit a red shift of 40 nm with a decreased intensity for both of the polymers, which causes obvious changes of the fluorescence color. Besides the Ag+ ion, Zn2+ induces a smaller red shift, while Fe3+, Ni2+, Cu2+, and Co2+ ions can cause only fluorescence quenching in various degrees with no wavelength changes. The other metal ions examined lead to negligible changes of the fluorescence. Therefore, Ag+ can be readily distinguished from the other metal ions by the emission shift. As shown in Fig. S5 (ESI†), upon interaction with Ag+, the emission peak of P1 gradually red-shifts to the wavelength of 456 nm along with the concentration of Ag+, and the fluorescence color of the solution changes from blue-violet to pale blue (Fig. S16a, ESI†). However, compared with P1, P2 exhibits a notable difference during the titration experiment because of the FRET process. It was noticed that the emission peak of P2 at 560 nm shows a weaker fluorescence quenching than that at 416 nm upon the addition of Ag+. As a result, the intensity ratio F560/F416 gradually increases with Ag+ concentration (Fig. S6, ESI†). Simultaneously, the fluorescence color of P2 changes from reddish orange to yellow orange (Fig. S16b, ESI†). The coordination reaction has been confirmed by a UV-Vis titration experiment (Fig. S23 and S24, ESI†).
It is obvious that the ion responsive properties of P1 and P2 are quite different from those of the conjugated polymers with the oligopyridyl moieties in their backbones and also with the imidazole moieties in their side chains, which may be due to the large M–N (metal–nitrogen) bond lengths12 and different molecular rigidities of the polymer chains. It is presumed that the distinguishing responsive characteristic of the polymers to Ag+ ions is attributed to both electron density variations on the backbone and chain aggregation induced by coordination. The initial fluorescence quenching of the polymers may be attributed to the electron transfer interactions between the polymer backbone and Ag+ ions. Then the later quenching can be induced by the chain aggregation,13,14 which was confirmed by the experiment of water induced aggregation (Fig. S17, ESI†). This means that the FRET effect was enhanced because of the Ag+ induced aggregation. The interesting phenomenon was not observed in the previous reports about the conjugated polymer sensor for Ag+ ions.15 The possible mechanism is illustrated in Scheme S1, ESI.† The Stern–Volmer quenching constants Ksv of P1–Ag+ and P2–Ag+ systems were determined to be 2.2 × 105 M−1 and 4.6 × 105 M−1, respectively.
Since cysteine (Cys) can bind Ag+ through the thiol–Ag+ interaction,16 this provides an approach to detect Cys by utilizing the polymer–Ag+ complex as a novel sensor. There are some papers published on the study of Cys fluorescent sensors based on the Ag+ complexes of the different ligand species, such as calix[4]arene,17aamino acids,17b and DNA.17c–e Herein, we prepared the Ag+ complexes of the conjugated polymers containing 2,2′-biimidazole moieties in situ by mixing P1 and P2 with Ag+ salt, respectively, and studied their properties as Cys fluorescent sensors. We found that the emission spectra of the complexes exhibit remarkable changes upon interaction with Cys (Fig. 2 and Fig. S7 (ESI†)). As shown in Fig. 2a, the emission peak of the P1–Ag+ complex gradually blue-shifts from 456 nm to 416 nm, accompanied by an increase in the fluorescence intensity upon increasing the concentration of Cys. Moreover, we noticed that the ratio of emission intensities (F416nm/F456nm) varies from 0.52 to 2.05 and it exhibits a good linear response to a Cys concentration change from 0 to 8.0 μM (Fig. 2b). The detection limit of Cys was determined to be as low as 90 nM by a fluorimetric assay (Fig. S8, ESI†). Similarly, the intensity ratio (F416nm/F560nm) of P2–Ag+ also varies linearly with the concentration of Cys and the detection limit was calculated to be about 150 nM by the titration experiment (Fig. S9, ESI†). These results indicate that the chemosensing complexes are excellent ratiometric fluorescent sensors for Cys with a high sensitivity. In addition, the fluorescence color changes under a UV lamp can be distinctly observed by the naked eye (Fig. S16, ESI†).
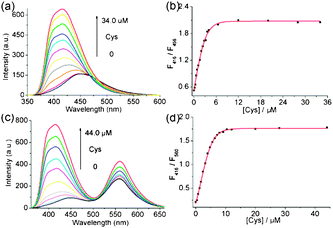 | ||
| Fig. 2 Fluorescence spectra of (a) P1–Ag+ and (c) P2–Ag+ upon the titration of Cys. (b) Fluorescence intensity of P1–Ag+ and (d) P2–Ag+ as a function of Cys concentration. λex = 333 nm. | ||
To determine the selectivity of the Cys-sensing systems, the fluorescence responses of P1–Ag+ and P2–Ag+ complexes were further examined with 15 different amino acids. The experiment results show that the other amino acids exhibit only a weak binding affinity to the Ag+ ion and lead to negligible changes in the emission properties of the complexes. Therefore, Cys can be easily differentiated from other amino acids either by the fluorescence intensity or by the intensity ratio (Fig. 3 and Fig. S12–S15 (ESI†)). For comparison, the interactions of glutathione (GSH) with P1–Ag+ and P2–Ag+ complexes were also studied, which exhibit a similar effect compared to Cys (Fig. S18, ESI†). This phenomenon has been also observed in the previous reports.18
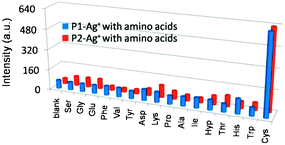 | ||
| Fig. 3 Amino acid selectivity profiles of P1–Ag+ and P2–Ag+. | ||
The reversibility of the conjugated polymer sensing systems was also examined and the results are shown, respectively, in Fig. S10b and S11b (ESI†). It can be seen that the sensing processes were reversible, and the emission intensity can be well recovered at the cyclic addition of Ag+ and Cys. The attenuation of emission intensity after several cycles may be a result of the adsorption of the Ag+–Cys complex to the cuvette surface. It should be pointed out that all of the fluorescence responses do not exhibit any appreciable time-dependent effects, which makes the detection of Cys more rapid and efficient.
In summary, we have successfully designed and synthesized two novel conjugated polymers containing a 2,2′-biimidazole moiety through the Suzuki coupling reaction, which was demonstrated to be an effective fluorescent sensing platform for ratiometric detection of Ag+ and cysteine. This is the first example utilizing a fluorescent conjugated polymer–Ag+ complex to selectively detect Cys with a nanomolar range detection limit and this work provides a new strategy for the development of Cys sensors.
The financial support from Ministry of Science and Technology of China (NO. 2007CB936401) is gratefully acknowledged.
Notes and references
- (a) D. T. McQuade, A. E. Pullen and T. M. Swager, Chem. Rev., 2000, 100, 2537 CrossRef CAS; (b) S. W. Thomas III, G. D. Joly and T. M. Swager, Chem. Rev., 2007, 107, 1339 CrossRef.
- (a) H. Huang, K. Wang, W. Tan, D. An, X. Yang, S. Huang, Q. Zhai, L. Zhou and Y. Jin, Angew. Chem., Int. Ed., 2004, 43, 5635 CrossRef CAS; (b) B. Liu, Y. Bao, F. Du, H. Wang, J. Tian and R. Bai, Chem. Commun., 2011, 47, 1731 RSC.
- (a) B. Wang and M. R. Wasielewski, J. Am. Chem. Soc., 1997, 119, 12 CrossRef CAS; (b) L. X. Chen, W. J. H. Ja1ger, D. J. Gosztola, M. P. Niemczyk and M. R. Wasielewski, J. Phys. Chem. B, 2000, 104, 1950 CrossRef CAS; (c) B. Liu, W.-L. Yu, J. Pei, S.-Y. Liu, Y.-H. Lai and W. Huang, Macromolecules, 2001, 34, 7932 CrossRef CAS; (d) R. C. Smith, A. G. Tennyson, M. H. Lim and S. J. Lippard, Org. Lett., 2005, 7, 3573 CrossRef CAS; (e) Y. Liu, S. Zhang, Q. Miao, L. Zheng, L. Zong and Y. Cheng, Macromolecules, 2007, 40, 4839 CrossRef CAS; (f) S. He, S. T. Iacono, S. M. Budy, A. E. Dennis, D. W. Smith and R. C. Smith, J. Mater. Chem., 2008, 18, 1970 RSC.
- (a) M. Kimura, T. Horai, K. Hanabusa and H. Shirai, Adv. Mater., 1998, 10, 459 CrossRef CAS; (b) Y. Zhang, C. B. Murphy and W. E. Jones, Macromolecules, 2002, 35, 630 CrossRef CAS.
- (a) T. Yasuda, I. Yamaguchi and T. Yamamoto, Adv. Mater., 2003, 15, 293 CrossRef CAS; (b) T. Yasuda and T. Yamamoto, Macromolecules, 2003, 36, 7513 CrossRef CAS; (c) M. Zhang, P. Lu, Y. Ma and J. Shen, J. Phys. Chem. B, 2003, 107, 6535 CrossRef CAS; (d) X. Liu, X. Zhou, X. Shu and J. Zhu, Macromolecules, 2009, 42, 7634 CrossRef CAS.
- (a) A. Ajayaghosh, P. Carol and S. Sreejith, J. Am. Chem. Soc., 2005, 127, 14962 CrossRef CAS; (b) A. E. Dennis and R. C. Smith, Chem. Commun., 2007, 4641 RSC; (c) S. Sreejith, K. P. Divya and A. Ajayaghosh, Chem. Commun., 2008, 2903 RSC; (d) X. Jiang, B. G. Park, J. A. Riddle, B. J. Zhang, M. Pink and D. Lee, Chem. Commun., 2008, 6028 RSC; (e) G. M. Cockrell, G. Zhang, D. G. VanDerveer, R. P. Thummel and R. D. Hancock, J. Am. Chem. Soc., 2008, 130, 1420 CrossRef CAS.
- S. W. Fox, Chem. Rev., 1943, 32, 47 CrossRef CAS.
- (a) H. A. Ho and M. Leclerc, J. Am. Chem. Soc., 2003, 125, 4412 CrossRef CAS; (b) X.-H. Zhou, J.-C. Yan and J. Pei, Macromolecules, 2004, 37, 7078 CrossRef CAS; (c) C. Xing, M. Yu, S. Wang, Z. Shi, Y. Li and D. Zhu, Macromol. Rapid Commun., 2007, 28, 241 CrossRef CAS; (d) Q. Zeng, P. Cai, Z. Li, J. Qina and B. Z. Tang, Chem. Commun., 2008, 1094 RSC; (e) Q. Zeng, C. K. W. Jim, J. W. Y. Lam, Y. Dong, Z. Li, J. Qin and B. Z. Tang, Macromol. Rapid Commun., 2009, 30, 170 CrossRef CAS; (f) A. Salinas-Castillo, M. Camprubí-Robles and R. Mallavia, Chem. Commun., 2010, 46, 1263 RSC.
- M. Tadokoro and K. Nakasuji, Coord. Chem. Rev., 2000, 198, 205 CrossRef CAS.
- (a) T. Yamamoto, T. Uemura, A. Tanimoto and S. Sasaki, Macromolecules, 2003, 36, 1047 CrossRef CAS; (b) D. T. Walker, C. D. Douglas and B. J. MacLean, Can. J. Chem., 2006, 84, 1218 CrossRef.
- (a) B. F. Fieselmann, D. N. Hendrickson and G. D. Stucky, Inorg. Chem., 1978, 17, 2078 CrossRef; (b) Y. Bao, B. Liu, H. Wang, F. Du and R. Bai, Anal. Methods, 2011, 3, 1274 RSC; (c) B. A. D. Neto, A. S. Lopes, G. Ebeling, R. S. Gonçalves, V. E. U. Costa, F. H. Quina and J. Dupont, Tetrahedron, 2005, 61, 10975 CrossRef.
- D. Buist, N. J. Williams, J. H. Reibenspies and R. D. Hancock, Inorg. Chem., 2010, 49, 5033 CrossRef CAS.
- X. Zhao and K. S. Schanze, Chem. Commun., 2010, 46, 6075 RSC.
- B. Bao, L. Yuwen, X. Zheng, L. Weng, X. Zhu, X. Zhan and L. Wang, J. Mater. Chem., 2010, 20, 9628 RSC.
- (a) H. Tong, L. Wang, X. Jing and F. Wang, Macromolecules, 2002, 35, 7169 CrossRef CAS; (b) C. Qin, W.-Y. Wong and L. Wang, Macromolecules, 2011, 44, 483 CrossRef CAS; (c) Y. Hu, Y. Xiao, H. Huang, D. Yin, X. Xiao and W. Tan, Chem.–Asian J., 2011, 6, 1500 CrossRef CAS.
- L. C. Gruen, Biochim. Biophys. Acta, 1975, 386, 270 CAS.
- (a) R. Joseph, B. Ramanujam, A. Acharya and C. P. Rao, J. Org. Chem., 2009, 74, 8181 CrossRef CAS; (b) J.-S. Shen, D.-H. Li, M.-B. Zhang, J. Zhou, H. Zhang and Y.-B. Jiang, Langmuir, 2011, 27, 481 CrossRef CAS; (c) J.-H. Guo, D.-M. Kong and H.-X. Shen, Biosens. Bioelectron., 2010, 26, 327 CrossRef CAS; (d) C. Zhao, K. Qu, Y. Song, C. Xu, J. Ren and X. Qu, Chem.–Eur. J., 2010, 16, 8147 CAS; (e) D.-Q. Feng, G. Liu, W. Zheng, J. Liu, T. Chen and D. Li, Chem. Commun., 2011, 47, 8557 RSC.
- (a) S. Sreejith, K. P. Divya and A. Ajayaghosh, Angew. Chem., Int. Ed., 2008, 47, 7883 CrossRef CAS; (b) Y.-B. Ruan, A.-F. Li, J.-S. Zhao, J.-S. Shen and Y.-B. Jiang, Chem. Commun., 2010, 46, 4938 RSC; (c) H. Xu and M. Hepel, Anal. Chem., 2011, 83, 813 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic procedures, experimental details, additional spectroscopic data. See DOI: 10.1039/c1cc15787f |
| This journal is © The Royal Society of Chemistry 2012 |
