Productivity enhancement of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e001.gif) C bioreductions by coupling the in situ substrate feeding product removal technology with isolated enzymes†
C bioreductions by coupling the in situ substrate feeding product removal technology with isolated enzymes†
Elisabetta
Brenna
a,
Francesco G.
Gatti
*a,
Daniela
Monti
*b,
Fabio
Parmeggiani
a and
Alessandro
Sacchetti
*a
aPolitecnico di Milano, Department of Chemistry, Materials and Chemical Engineering “Giulio Natta”, Piazza Leonardo Da Vinci 32, 20131, Milano, Italy. E-mail: francesco.gatti@polimi.it; alessandro.sacchetti@polimi.it; Tel: +39 02 23993070
bIstituto di Chimica del Riconoscimento Molecolare, C.N.R., Via Mario Bianco, 9, 20131, Milano, Italy. E-mail: daniela.monti@icrm.cnr.it; Tel: +39 02 28500038
First published on 9th November 2011
Abstract
To overcome the usually low productivities of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond bioreduction of α,β-unsaturated aldehydes we combined the in situ substrate feeding product removal (SFPR) technology with a cascade system comprising an isolated ene-reductase and a chemoselective alcohol dehydrogenase.
C bond bioreduction of α,β-unsaturated aldehydes we combined the in situ substrate feeding product removal (SFPR) technology with a cascade system comprising an isolated ene-reductase and a chemoselective alcohol dehydrogenase.
The baker's yeast (b.y.) mediated reduction of activated alkenes1 suffers from several drawbacks: low substrate loading, non-quantitative conversion, long reaction time, troublesome work-up, and competitive side reactions, that overall contribute to give modest productivities (<0.5 g L−1d−1).2 So far, two strategies have been adopted to address some of these issues.
The first one relies on the in situ substrate feeding product removal (SFPR) technology,3 which ensures, by adsorption of substrate and products on a hydrophobic resin, very low concentrations, preventing toxic effects. However, despite its application leading to an improvement of yield, work-up, and in certain cases of enantioselectivity,3b the productivities are still unsatisfactory.
Alternatively, the second strategy is based on isolated ene-reductases (ERs, e.g. the Old Yellow Enzymes OYE2–OYE3 of b.y.)4 which often allowed us to obtain quantitative conversions without side-products.5 Nevertheless, the productivities are usually too low, since high substrate concentrations cannot generally be employed (5–10 mM). Also the aqueous–organic biphasic systems have been tested for the reduction of a set of α-methylcinnamaldehyde derivatives.5e However, the effects were contrasting, indeed, the ee of products improved with respect to those achieved in the homogeneous phase, whereas the yields resulted worse. This example reveals another critical aspect of using the ERs for the reduction of α-substitued aldehydes since the products racemise in water even at almost neutral pH.
Thus, we envisioned that the goal of a substantial increase in productivity might be pursued by the combination of these two complementary strategies. For this purpose we compared their applications (either separated or combined) to the reduction of a set of α-substituted aldehydes (1a–4a, Scheme 1), which are for several reasons of some interest in our ongoing research program.
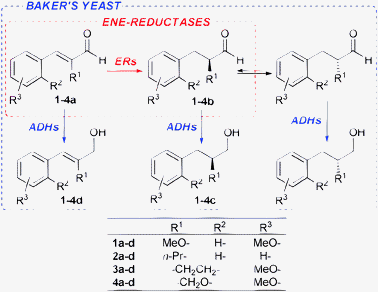 | ||
| Scheme 1 Enantioselective biocatalysed reductions of aldehydes 1a–4a. | ||
The b.y.-mediated reduction of 1a gave alcohol 1c (precursor of the antidiabetic Navaglitazar) in a modest yield of 36% and with a low ee of 55% (Table 1, entry 1). An improvement has been accomplished by applying the SFPR strategy (entry 2),1c however the productivity (0.39 g L−1d−1) remained way too low. The use of isolated OYEs in homogeneous phase, together with a glucose dehydrogenase (GDH from Bacillus megaterium)6 for NADPH cofactor regeneration, gave quantitative conversions, but disappointingly the ees of saturated aldehyde 1b resulted lower (90% and 86% ee with OYE2 and OYE3, respectively, entries 3 and 4) than those previously obtained. Instead, the SFPR technology combined with isolated OYEs, under conditions comparable to those used with whole cells, afforded 1b with a good ee of 96% and in a 65% yield, which allowed a 10-fold increase of productivity (entry 5). To further optimise the system, we assessed the effect of different resin/substrate ratios (Xr/s) and substrate loadings on enzyme performance (entries 6–9). It is noteworthy that high Xr/s values allow us to obtain 1b with a high ee but a lower conversion, whereas quantitative yields could be achieved with lower Xr/s at the expense of a slight decrease of optical purity. Under optimised conditions, an improved productivity of 29.1 g L−1d−1 was reached while maintaining a still good ee of 95% and a quantitative yield (entry 7). The crucial role of the SFPR approach was further emphasised by repeating the latter reaction with the same substrate loading, but in the absence of the resin and employing the portion-wise addition protocol.7 Indeed, with substrate 1a the yield decreased to an unacceptable 22% (entry 10). These results suggested the following conclusions: (i) the saturation concentrations of 1a and/or 1b somehow inhibit the enzymatic activity of ERs; (ii) the racemisation of 1b occurs during the biotransformation; (iii) the latter is slower than the b.y. mediated reduction of the carbonyl group to give the more stable alcohol 1c; and (iv) in the presence of the resin the optical purity of aldehyde 1b is preserved, since the racemisation is partially or completely suppressed. To verify the occurrence of racemisation, a sample of (S)-1b left under the homogeneous phase biotransformation conditions, but without any enzyme, showed an appreciable loss of optical purity after the same reaction time. In contrast, the same sample preserved its ee when it was adsorbed on the resin.
| Entry | Substrate | Biocatalyst | Product | Loading/g L−1 | X r/s/g g−1 | Time/d | Yielda [%] | eeb [%] | Productivity/g L−1d−1 |
|---|---|---|---|---|---|---|---|---|---|
| Standard expt. cond.: (i) for b.y.: 0.45 g mL−1 wet cells, 90 mg mL−1glucose, tap water, 30 °C; (ii) for OYE: 150 μg mL−1 OYE, 4 U mL−1 GDH, 0.1 mM NADP+, 4 eq. glucose, 50 mM phosphate buffer, pH 7.0, 30 °C, 160 rpm; (iii) for OYE + ADH: the same as (ii), 2 U mL−1 HLADH, 0.1 mM NAD+.a Determined by GC or after standard work-up.b Determined by chiral HPLC or GC.c Ref. 1c.d Ref. 7.e The starting material decomposed. | |||||||||
| 1 |
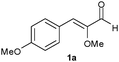
|
b.y. | 1c | 1 | — | 4 | 36 | 55 | 0.07 |
| 2 | b.y.c | 1c | 2 | 10 | 4 | 81 | 95 | 0.39 | |
| 3 | OYE2 | 1b | 1 | — | 0.5 | 100 | 90 | 1.90 | |
| 4 | OYE3 | 1b | 1 | — | 0.5 | 100 | 86 | 1.86 | |
| 5 | OYE2 | 1b | 15 | 10 | 0.5 | 65 | 96 | 19.11 | |
| 6 | OYE2 | 1b | 15 | 3 | 0.5 | 92 | 94 | 26.77 | |
| 7 | OYE2 | 1b | 15 | 1 | 0.5 | 100 | 95 | 29.10 | |
| 8 | OYE2 | 1b | 30 | 3 | 0.5 | 60 | 93 | 34.74 | |
| 9 | OYE2 | 1b | 30 | 1 | 0.5 | 82 | 94 | 47.72 | |
| 10 | OYE2 d | 1b | 15 | — | 0.5 | 22 | n.d. | — | |
| 11 |
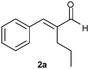
|
b.y. | 2c | 1 | 20 | 4 | 38 | 92 | 0.08 |
| 12 | OYE2 | 2b | 1 | — | 0.5 | —e | n.d. | — | |
| 13 | OYE3 | 2b | 1 | — | 0.5 | —e | n.d. | — | |
| 14 | OYE2 | 2b | 6 | 1 | 0.5 | 97 | 90 | 11.06 | |
| 15 | OYE3 | 2b | 6 | 1 | 0.5 | 94 | 90 | 10.72 | |
| 16 |
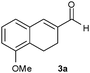
|
b.y. | 3c | 1 | 20 | 4 | 20 | 60 | 0.04 |
| 17 | OYE2 | 3b | 1 | — | 0.5 | 81 | 65 | 1.34 | |
| 18 | OYE3 | 3b | 1 | — | 0.5 | — | n.d. | — | |
| 19 | OYE2 | 3b | 10 | 3 | 0.5 | 29 | 83 | 5.31 | |
| 20 | OYE2 | 3b | 10 | 1 | 0.5 | 86 | 73 | 14.88 | |
| 21 | OYE2 + ADH | 3c | 10 | 1 | 0.5 | 94 | 99 | 18.71 | |
| 22 |

|
b.y. | 4c | 1 | 10 | 4 | 36 | 60 | 0.07 |
| 23 | b.y. | 4c | 1 | 15 | 4 | 29 | 85 | 0.07 | |
| 24 | b.y. | 4c | 1 | 20 | 4 | 23 | 99 | 0.06 | |
| 25 | OYE2 | 4b | 1 | — | 0.5 | 85 | 7 | 0.91 | |
| 26 | OYE3 | 4b | 1 | — | 0.5 | 87 | 11 | 0.97 | |
| 27 | OYE2 | 4b | 10 | 1 | 0.5 | 81 | 50 | 12.15 | |
| 28 | OYE2 | 4b | 10 | 3 | 0.5 | 58 | 69 | 9.80 | |
| 29 | OYE2 | 4b | 10 | 1 | 0.08 | 8 | 84 | 9.20 | |
| 30 | OYE3 | 4b | 10 | 1 | 0.08 | 9 | 80 | 10.13 | |
| 31 | OYE2 + ADH | 4c | 1 | — | 0.5 | 61 | 95 | 1.19 | |
| 32 | OYE2 + ADH | 4c | 10 | 1 | 0.5 | 88 | 99 | 17.42 | |
| 33 | OYE3 + ADH | 4c | 10 | 1 | 0.5 | 76 | 95 | 14.82 |
The reduction of aldehyde 2a illustrates another important feature of our methodology. In this case the reduction in homogeneous phase failed, since the starting material decomposed before being converted into the saturated aldehyde 2b (entries 12 and 13).8 Again, the combination of ERs with the SFPR technology led to a considerable improvement of productivity (two orders of magnitude) without any decomposition. Moreover, no appreciable loss of optical purity was observed, since the enantioselectivity achieved with both enzymes (ee 90%, entries 14 and 15) resulted comparable to that attained with b.y. (92% ee, entry 11).
Especially interesting is the reduction of aldehyde 3a to give either the alcohol 3c or the saturated aldehyde 3b, precursors of Rotigotine,9 a drug used for the treatment of Parkinson's disease. This substrate, in which the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C double bond is inserted into a six-membered ring, is much more sterically hindered than 1a and 2a. Indeed, its reduction with b.y. gave 3c in a lower yield (20%) and with a modest ee of 60% (entry 16). Unexpectedly, a very different substrate specificity was found, since the OYE3-catalysed reduction failed, whereas OYE2 gave 3b in a yield of 81% and with a slight enhancement of ee (entry 17). Even in this case the application of the combined strategy allowed a substantial improvement affording 3b with a better optical purity (73% ee) and productivity (entry 20 vs. entry 16).
C double bond is inserted into a six-membered ring, is much more sterically hindered than 1a and 2a. Indeed, its reduction with b.y. gave 3c in a lower yield (20%) and with a modest ee of 60% (entry 16). Unexpectedly, a very different substrate specificity was found, since the OYE3-catalysed reduction failed, whereas OYE2 gave 3b in a yield of 81% and with a slight enhancement of ee (entry 17). Even in this case the application of the combined strategy allowed a substantial improvement affording 3b with a better optical purity (73% ee) and productivity (entry 20 vs. entry 16).
Finally, despite its structural similarity to 3a, very different results were obtained in the case of 4a, a possible intermediate in the synthesis of the antidepressant Robalzotan.9 The reduction was first studied with b.y. and SFPR (entries 22–24). By increasing the Xr/s value the optical purity of alcohol 4c improved up to 99% ee, but at the expense of a lower yield (entry 24), since both substrate and product concentrations in water are lower, and consequentially the conversion rate and the racemisation rate decrease. The reduction with isolated ERs in homogeneous phase (entries 25 and 26) showed that a fast racemisation of aldehyde 4b occurred, since the latter was recovered almost racemic. Disappointingly, even our methodology turned out to be insufficient to suppress such a racemisation (entries 27 and 28). In an attempt to improve the optical purity we also tested a much shorter reaction time that provided better ees but too low conversions (entries 29 and 30).
Thus, we wondered whether the co-presence of alcohol dehydrogenases (ADHs) with ERs might play a determinant role in the achievement of higher ees of 4c. A screening of several commercially available ADHs allowed us to select the horse liver alcohol dehydrogenase (HLADH) as the most promising enzyme to be coupled with isolated ERs (data not shown). This cascade system (OYE2–3, HLADH, GDH, NADPH and NADH) was tested in homogeneous phase or coupled to the SFPR technology (Scheme 2). In the best case (OYE2, HLADH, SFPR, entry 32), as soon as the saturated aldehyde 4b was formed, the HLADH reduced chemoselectively the latter over the unsaturated aldehyde 4a to give the alcohol 4c with an excellent ee of 99%. The productivity was improved as well to 17.4 g L−1d−1, a 250-fold increase with respect to that achieved with yeast. Although a plethora of ADHs have been isolated and successfully employed for the reduction of many carbonyl compounds,10 such a chemoselectivity has never been reported; furthermore, very recently, an ADH has been combined with an ER in a telescopic sequence (one pot two steps in series) without taking advantage of such a selectivity,11 which might allow the set-up of a more appealing cascade system.
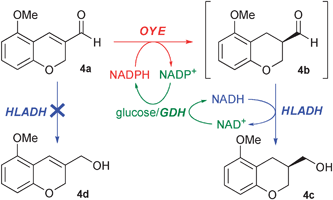 | ||
| Scheme 2 Cascade synthesis of 4c by coupling of an OYE with HLADH. | ||
In addition, since it is known that HLADH promotes the dynamic kinetic resolution (DKR) of α-arylpropionaldehydes in phosphate buffer (pH 7.4), to give the corresponding alcohols with good ees,12 we were concerned whether the high optical purity of 4c could be ascribed also to the HLADH or exclusively to the OYEs. Since the HLADH reduction of racemic 4b gave 4c without appreciable optical enrichment, we ruled out any DKR.
Finally, the synergic combination of this cascade system (OYE, ADH, GDH) together with the SFPR applied to 3a allowed us to obtain 3c with a 99% ee and a 470-fold productivity enhancement compared to b.y. (entry 21 vs. 16).
In conclusion, we have shown that the isolated enzymes (such as ERs, ADHs and GDH) are compatible13 with the in situSFPR technology. This methodology allows a substantial enhancement of productivity, opening a new perspective on the exploitation of recombinant ERs in preparative organic synthesis. Moreover, we found that the use of resin might be vital for the reduction of potentially unstable substrates, preventing either starting materials decomposition or products racemisation. In particular, the latter can be further minimised by coupling an ADH in cascade to an ER in such a way to reduce chemoselectively the unstable α-substitued aldehyde, as soon as it is formed, to the more robust alcohol.
Notes and references
- (a) R. Csuk and B. I. Glänzer, Chem. Rev., 1991, 91, 49 CrossRef CAS; (b) S. Serra, C. Fuganti and F. G. Gatti, Eur. J. Org. Chem., 2008, 1031 CrossRef CAS; (c) E. Brenna, C. Fuganti, F. G. Gatti and F. Parmeggiani, Tetrahedron: Asymmetry, 2009, 20, 2694 CrossRef CAS; (d) E. Brenna, C. Fuganti, F. G. Gatti, L. Malpezzi and S. Serra, Tetrahedron: Asymmetry, 2008, 19, 800 CrossRef CAS.
- Productivity = [Loading × Yield/100 × (ee + 100)/200]/Time.
- (a) J. T. Vicenzi, M. J. Zmijewski, M. R. Reinhard, B. E. Landen, W. L. Muth and P. G. Marler, Enzyme Microb. Technol., 1997, 20, 494 CrossRef CAS; (b) P. D'Arrigo, C. Fuganti, G. Pedrocchi Fantoni and S. Servi, Tetrahedron, 1998, 54, 15017 CrossRef CAS; (c) I. Hilker, M. C. Gutiérrez, V. Alphand, R. Wohlgemuth and R. Furstoss, Org. Lett., 2004, 6, 1955 CrossRef CAS; (d) I. Hilker, M. C. Gutierrez, R. Furstoss, J. Ward, R. Wohlgemuth and V. Alphand, Nat. Protoc., 2008, 3, 546 CrossRef CAS.
- (a) K. Stott, K. Saito, D. J. Thiele and V. Massey, J. Biol. Chem., 1993, 268, 6097–6106 CAS; (b) H. S. Toogood, J. M. Gardiner and N. S. Scrutton, ChemCatChem, 2010, 2, 892–914 CrossRef CAS.
- (a) C. Stueckler, M. Hall, H. Ehammer, E. Pointner, W. Kroutil, P. Macheroux and K. Faber, Org. Lett., 2007, 9, 5409 CrossRef CAS; (b) M. Hall, C. Stueckler, B. Hauer, R. Stuermer, T. Friederich, M. Breuer, W. Kroutil and K. Faber, Eur. J. Org. Chem., 2008, 1511 CrossRef CAS; (c) S. K. Padhi, D. J. Bougioukou and J. D. Stewart, J. Am. Chem. Soc., 2009, 131, 3271 CrossRef CAS; (d) J. F. Chaparro-Riggers, T. A. Rogers, E. Vazquez-Figueroa, K. M. Polizzi and A. S. Bommarius, Adv. Synth. Catal., 2007, 349, 1521 CrossRef CAS; (e) C. Stueckler, N. J. Mueller, C. K. Winkler, S. M. Glueck, K. Gruber, G. Steinkeller and K. Faber, Dalton Trans., 2010, 39, 8472 RSC; (f) Y. Yanto, C. K. Winkler, S. Lohr, M. Hall, K. Faber and A. S. Bommarius, Org. Lett., 2011, 13, 2540 CrossRef CAS.
- M. Bechtold, E. Brenna, C. Femmer, F. G. Gatti, S. Panke, F. Parmeggiani and A. Sacchetti, Org. Process Res. Dev., 2011 DOI:10.1021/op200085k.
- D. J. Bougioukou, A. Z. Walton and J. D. Stewart, Chem. Commun., 2010, 46, 8558 RSC.
- A sample of 2a (pH 7.0, 30 °C, 1% DMSO) completely decomposed after 12 hours.
- Rotigotine and Robalzotan synthesis will be reported elsewhere.
- M. M. Musa and R. S. Phillips, Catal. Sci. Technol., 2011, 1, 1311 CAS.
- M. Korpak and J. Pietruszka, Adv. Synth. Catal., 2011, 353, 1420 CrossRef CAS.
- D. Giacomini, P. Galletti, A. Quintavilla, G. Gucciardo and F. Paradisi, Chem. Commun., 2007, 4038 RSC.
- In a previous report the use of SFPR with isolated enzymes caused a loss of catalytic activity: F. Zambianchi, S. Raimondi, P. Pasta, G. Carrea, G. N. Caggero and J. M. Woodley, J. Mol. Catal. B: Enzym., 2004, 31, 165 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods, NMR spectra of products 1b, 2b, 3b and 4c, and representative chiral GC or HPLC chromatograms. See DOI: 10.1039/c1cc16014a |
| This journal is © The Royal Society of Chemistry 2012 |
