Electrogenerated upconverted emission from doped organic nanowires†
Qing
Li
,
Chuang
Zhang
,
Jian Yao
Zheng
,
Yong Sheng
Zhao
* and
Jiannian
Yao
*
Beijing National Laboratory for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China. E-mail: yszhao@iccas.ac.cn; jnyao@iccas.ac.cn; Fax: +86-10-62652029; Tel: +86-10-62652029
First published on 4th November 2011
Abstract
The electrogenerated upconversion was achieved in the uniformly doped organic nanowires based on triplet energy transfer from tris(2,2′-bipyridyl)ruthenium(II) to 9,10-diphenylanthracene.
Due to their unique optoelectronic properties1,2 and high photoluminescence (PL) efficiency,3 organic nanomaterials have attracted great attention in the past decade. Organic composite nanomaterials, for example the uniformly doped organic nanowires, are good models for the investigation of intermolecular interactions between different components, such as fluorescence resonance energy transfer (FRET),4,5 photoinduced electron transfer (PET),6etc. As an important kind of intermolecular interaction, triplet–triplet energy transfer (TTET) and the relevant upconverted emission based on triplet–triplet annihilation (TTA) have been actively studied.7,8 In our recent work,9 the TTA upconversion was observed from the core–shell nanomaterials, which ensured a high energy transfer efficiency due to the proper molecular space distance. This sensitized TTA based upconversion can be conveniently performed by low-power excitation and it is now an emerging technique with possible applications in opto-electronic devices.10
Electrochemiluminescence (ECL), the production of light from intermediates generated during electrolysis,11–13 occurs when the energy liberated by chemical reactions is sufficient to generate a species in an electronically excited form. The phenomenon of ECL has long been of interest since it provides a simple route to photon production.14 The nanomaterials could produce stable ECL in aqueous solutions.15–21 The efficient nature of ECL, coupled with the simplicity of photonic detection, has led to its applications in trace analytical methods22–24 and energy-transfer studies.25–27 This kind of electron transfer reaction of electrogenerated species could supply the excitation energy for the TTA based upconversions, since the TTA processes are independent of the coherence. Moreover, no light excitation source is needed in the ECL upconversion system, which helps to avoid the interferences from scattered light and luminescent impurities.
Herein, we report the upconverted emission based on TTA excited viaECL in the doped organic nanocomposite materials. The wires of 9,10-diphenylanthracene (DPA) uniformly doped with a small amount of tris(2,2′-bipyridyl)ruthenium(II) (Ru(bpy)32+) (Fig. S1, ESI†) were fabricated first. The triplet energy level of Ru(bpy)32+ is higher than that of DPA. Therefore, Ru(bpy)32+ can act as the triplet energy donor, while DPA acts as the triplet energy acceptor as well as the annihilator in the TTA system. DPA has a strong luminescence and no anodic ECL emission in a positive potential range, which make it serve as a sensitive energy acceptor of an anodic ECL donor (Ru(bpy)32+) with interference-free from ECL emission generated by itself. In the binary wires, the ∼440 nm blue emission from DPA was detected on the excitation of the Ru(bpy)32+ triplet by electron transfer reaction between electrogenerated species (Ru(bpy)33+ and the excited state of tripropylamine (TPrA)).
The wires with different doping ratios were prepared via a simple reprecipitation method (ESI†). Fig. 1A illustrates the formation process of the doped wires. First, Ru(bpy)32+/DPA solutions with different molar ratios were added into water in the presence of CTAB as soft templates. The Ru(bpy)32+ and DPA molecules would dissolve into the hydrophobic core of spherical CTAB micelles to induce the formation of rod-like micelles, which then facilitate the 1D growth of the Ru(bpy)32+ doped DPA wires.28 The prepared wires were centrifuged and washed three times with water to remove the CTAB. The final aqueous dispersion was used to prepare samples by drop-casting for further characterizations.
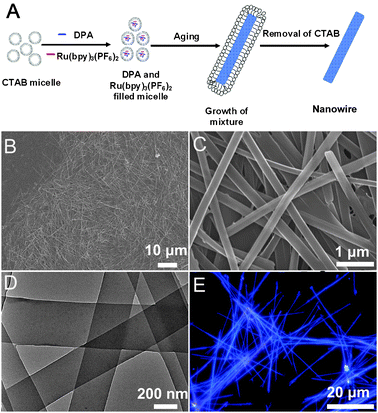 | ||
Fig. 1 (A) Schematic illustration for the fabrication of the Ru(bpy)32+ @DPA wires. (B and C) SEM and (D) TEM images of the wires with a Ru(bpy)32+/DPA molecular ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. (E) Fluorescence microscopy images of the wires excited with the UV band (330–380 nm) of a mercury lamp. 50. (E) Fluorescence microscopy images of the wires excited with the UV band (330–380 nm) of a mercury lamp. | ||
Fig. 1B–D show the typical SEM and TEM images of the wires with a Ru(bpy)32+/DPA molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. The images indicate that the products are 1D wire-like nanostructures with smooth surfaces. As shown in Fig. 1C, the average width of the wires is about 330 nm, and the length about 40 μm, which gives an aspect ratio of ∼120. By changing the concentration of DPA in the stock solutions, organic wires with different doping ratios can be obtained. The different molar ratios of Ru(bpy)32+/DPA do not have noticeable influence on the morphology of wires as shown in Fig. S2 (ESI†). The compositions of the doped wires were proven by EDX measurements (Fig. S3, ESI†). The morphology of the wires was further confirmed by TEM, which indicates that the uniform two-component structures are solid wires (Fig. 1D). Fig. 1E shows the fluorescence microscopy images of the wires drop cast onto a quartz wafer, in which the blue-light emission of DPA was observed from the wires. The homogeneous emission manifests that the Ru(bpy)32+ molecules are uniformly dispersed in the DPA matrices in each wire. The absorption spectra of the doped wires, DPA wires and Ru(bpy)3(PF6)2 were measured (Fig. S1C, ESI†), which indicated that there was no interactions between Ru(bpy)3(PF6)2 and DPA in the ground state.
50. The images indicate that the products are 1D wire-like nanostructures with smooth surfaces. As shown in Fig. 1C, the average width of the wires is about 330 nm, and the length about 40 μm, which gives an aspect ratio of ∼120. By changing the concentration of DPA in the stock solutions, organic wires with different doping ratios can be obtained. The different molar ratios of Ru(bpy)32+/DPA do not have noticeable influence on the morphology of wires as shown in Fig. S2 (ESI†). The compositions of the doped wires were proven by EDX measurements (Fig. S3, ESI†). The morphology of the wires was further confirmed by TEM, which indicates that the uniform two-component structures are solid wires (Fig. 1D). Fig. 1E shows the fluorescence microscopy images of the wires drop cast onto a quartz wafer, in which the blue-light emission of DPA was observed from the wires. The homogeneous emission manifests that the Ru(bpy)32+ molecules are uniformly dispersed in the DPA matrices in each wire. The absorption spectra of the doped wires, DPA wires and Ru(bpy)3(PF6)2 were measured (Fig. S1C, ESI†), which indicated that there was no interactions between Ru(bpy)3(PF6)2 and DPA in the ground state.
The energy levels of Ru(bpy)32+ and DPA are shown in Table S1 (ESI†). The lower limit of the Ru(bpy)32+ triplet energy level is estimated to be ∼2.03 eV,29 whereas that of DPA is ∼1.77 eV,7 which is located at the appropriate position to ensure the energy transfer. The fluorescence spectra in Fig. S4 (ESI†) were measured by exciting the doped wires with a 510 nm Xe lamp. Fig. S5 (ESI†) indicates that the doped wires contain DPA, while the pure DPA wires are not luminescent under the excitation of 510 nm. However, the blue emission of DPA at 400–480 nm was achieved from the doped organic wires with a longer wavelength excitation. Although the relative intensity of the upconverted DPA emission increases at the expense of Ru(bpy)32+ emission with the increase of DPA amount, there is an intensely scattered light at 510 nm, which will be one of the interference factors in future research and influence the application of this energy unconverted system. ECL upconversion could help in avoiding this interference, because the excitation energy is supplied by the electrochemical reaction to generate ECL, which replaces the absorption of light to give rise to the photoluminescence.
To choose an appropriate potential range in the investigation of the electrogenerated upconversion of the doped wires, we measured the cyclic voltammograms (CV) of pure Ru(bpy)32+ and undoped DPA wires first. Ru(bpy)32+ was dissolved into the electrolyte and the suspension of DPA wires was dropped onto a cleaned glass carbon electrode (GCE). The electrolyte, 0.20 M PBS (pH = 8.0) containing 0.10 mM TPrA, was carefully deoxygenated by bubbling high purity nitrogen. In Fig. 2A, a pair of oxidation and reduction peaks appear at 1.10 V and 1.05 V for Ru(bpy)32+. At about 1.10 V,30Ru(bpy)32+ can be electro-oxidized to Ru(bpy)33+, followed by the reaction with a reductant (TPrA) to form excited Ru(bpy)32+* resulting in light emission at about 610 nm. However, the oxidation potential of DPA is much more positive than that of Ru(bpy)32+, and the ECL mechanisms (Scheme S1, ESI†)31 of Ru(bpy)32+ and DPA are totally different. Therefore, the cyclic voltammetric potential started at 0.60 V and ended at 1.30 V (versusAg/AgCl) in the ECL study, which ensures the occurrence of Ru(bpy)32+ electrochemical reaction and prevents the oxidation of DPA.
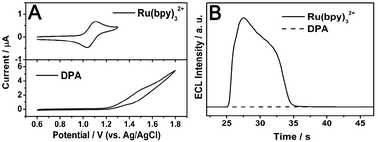 | ||
| Fig. 2 (A) Cyclic voltammograms of Ru(bpy)32+ (0.5 mM) (top) and DPA wires (bottom) in 0.20 M, pH 8.0, PBS containing 0.10 mM TPrA. Scan rate, 100 mV s−1. Scan range, 0.6 to 1.30 V (top) and 0.6 to 1.8 V (bottom). (B) ECL intensities of Ru(bpy)32+ and pure DPA wires obtained by stepping the potential between 0.6 and 1.30 V. Other experimental conditions are consistent with (A). | ||
The suspension of doped wires was dropped onto the cleaned GCE and dried in air, which is used to investigate ECL induced upconversion. The experimental conditions of ECL were consistent with that of the CV measurements. Fig. 2B displays the time-resolved ECL behaviors of pure Ru(bpy)32+ and undoped pure DPA wires. The stable ECL is produced upon concomitant oxidation of Ru(bpy)32+ and TPrA, while no ECL response is observed for pure DPA in this positive scan range from 0.6 to 1.3 V.
The ECL spectra of the doped wires are shown in Fig. 3A. It is revealed that there is no ECL response in the spectral range from 360 nm to 640 nm for the electrode coated with pure DPA wires. In the absence of DPA, the electrogenerated Ru(bpy)32+ emission at 540–640 nm can be clearly observed. For the doped materials, a new emission peak appeared in the range of 380–500 nm, which is identical to the fluorescence emission spectrum (Fig. S1, ESI†) of DPA. It can be observed that the orange-red emission from Ru(bpy)32+ decreases sharply while the blue from DPA increases gradually when the molar ratio of Ru(bpy)32+/DPA decreased from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 to 1
3 to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10, indicating the triplet–triplet energy transfer from Ru(bpy)32+ to DPA and the relevant TTA based upconverted emission. Fig. 3B shows the effect of the doping ratio on the weight of the emission intensities from the two compositions (I440 nm/I605 nm). The ratio of the blue emission intensity grew gradually with the increase of DPA molar ratio, which provides strong evidence for efficient TTET from a donor to an acceptor in the doped wires.
10, indicating the triplet–triplet energy transfer from Ru(bpy)32+ to DPA and the relevant TTA based upconverted emission. Fig. 3B shows the effect of the doping ratio on the weight of the emission intensities from the two compositions (I440 nm/I605 nm). The ratio of the blue emission intensity grew gradually with the increase of DPA molar ratio, which provides strong evidence for efficient TTET from a donor to an acceptor in the doped wires.
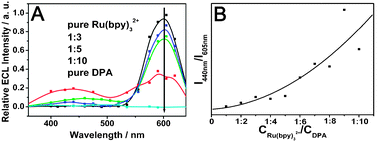 | ||
| Fig. 3 (A) ECL spectra of the doped wires with different molar ratios of Ru(bpy)32+/DPA. The experimental conditions are the same as Fig. 2B. (B) Plot of the ECL intensity ratios of DPA (440 nm) to Ru(bpy)32+ (605 nm) as a function of the molar ratio in the doped wires. | ||
The energy level diagram of the electron process in the doped wires is depicted in Scheme S2 (ESI†). The fluorescence decay profiles of DPA wires and the doped wires are shown in Fig. S6 (ESI†). The doped wires lifetime is much more elongated than that of DPA wires, as it is temporally limited by the Ru(bpy)32+ decay-time32 and the diffusion. The result confirms the upconversion in the doped wires. Ru(bpy)32+ is first excited to its higher vibrational energy level of the singlet excited state 1MLCT′ by the reaction between electro-oxidized Ru(bpy)32+ and TPrA, which then transits to the 0 level via vibrational relaxation (VR). The lowest singlet state level transits to the triplet state level (2.03 eV) via an effective intersystem crossing (ISC). The triplet state level of Ru(bpy)32+ is high enough to facilitate the TTET to populate the triplet state of DPA (1.77 eV). Two of such triplet 3DPA* molecules may undergo a TTA process and generate one singlet 1DPA* molecule, which emits the characteristic blue fluorescence of DPA with a high quantum yield.
In conclusion, the electrogenerated upconverted emission was observed from the Ru(bpy)32+ @DPA wires successfully. The suitable pair of donor (Ru(bpy)32+)–acceptor (DPA) was applied to investigate the ECL TTET and TTA upconversion, due to the energy level matching between their excited triplet states and their different ECL properties. Upconverted blue emissions of DPA were detected from the wires via exciting them with electron transfer reaction of Ru(bpy)33+. This ECL upconversion system helps to avoid the interference from scattered light and luminescent impurities, which might be an important advantage in applications such as chemical or biological sensing and imaging.
This work was supported by National Natural Science Foundation of China (Nos. 51073164, 91022022), the Chinese Academy of Sciences, and the National Basic Research 973 Program of China.
Notes and references
- Y. S. Zhao, H. B. Fu, A. D. Peng, Y. Ma, D. B. Xiao and J. N. Yao, Adv. Mater., 2008, 20, 2859–2876 CrossRef CAS.
- L. Zang, Y. Che and J. S. Moore, Acc. Chem. Res., 2008, 41, 1596–1608 CrossRef CAS.
- Y. S. Zhao, H. B. Fu, A. D. Peng, Y. Ma, Q. Liao and J. N. Yao, Acc. Chem. Res., 2010, 43, 409–418 CrossRef CAS.
- Y. S. Zhao, H. B. Fu, F. Q. Hu, A. D. Peng, W. S. Yang and J. N. Yao, Adv. Mater., 2008, 20, 79–83 CrossRef CAS.
- A. Peng, D. Xiao, Y. Ma, W. Yang and J. Yao, Adv. Mater., 2005, 17, 2070–2073 CrossRef CAS.
- F. Shen, A. Peng, Y. Chen, Y. Dong, Z. Jiang, Y. Wang, H. Fu and J. Yao, J. Phys. Chem. A, 2008, 112, 2206–2210 CrossRef CAS.
- H. C. Chen, C. Y. Hung, K. H. Wang, H. L. Chen, W. S. Fann, F. C. Chien, P. Chen, T. J. Chow, C. P. Hsu and S. S. Sun, Chem. Commun., 2009, 4064–4066 RSC.
- R. R. Islangulov, J. Lott, C. Weder and F. N. Castellano, J. Am. Chem. Soc., 2007, 129, 12652–12653 CrossRef CAS.
- C. Zhang, J. Y. Zheng, Y. S. Zhao and J. N. Yao, Chem. Commun., 2010, 46, 4959–4961 RSC.
- P. Ceroni, Chem.–Eur. J., 2011, 17, 9560–9564 CrossRef CAS.
- M. M. Richter, Chem. Rev., 2004, 104, 3003–3036 CrossRef CAS.
- L. R. Faulkner, Int. Rev. Sci.: Phys. Chem., Ser. Two, 1975, 9, 213–263 Search PubMed.
- L. R. Faulkner and A. J. Bard, Electroanalytical Chemistry, Marcel Dekker, New York, vol. 10, 1977 Search PubMed.
- E. L. Ritchie, P. Pastore and R. M. Wightman, J. Am. Chem. Soc., 1997, 119, 11920–11925 CrossRef CAS.
- J. Suk, Z. Wu, L. Wang and A. J. Bard, J. Am. Chem. Soc, 2011, 133, 14675–14685 CrossRef CAS.
- K. M. Omer and A. J. Bard, J. Phys. Chem. C, 2009, 113, 11575–11578 CAS.
- Y. Wang, J. Lu, L. H. Tang, H. X. Chang and J. H. Li, Anal. Chem., 2009, 81, 9710–9715 CrossRef CAS.
- C. Z. Wang, Y. F. E, L. Z. Fan, S. H. Yang and Y. L. Li, J. Mater. Chem, 2009, 19, 3841–3846 RSC.
- C. Z. Wang, E. F. Yifeng, L. Z. Fan, Z. H. Wang, H. B. Liu, Y. L. Li and S. H. Yang, Adv. Mater., 2007, 19, 3677–3681 CrossRef CAS.
- Y. M. Fang, J. Song, J. Li, Y. W. Wang, H. H. Yang, J. J. Sun and G.-N. Chen, Chem. Commun., 2011, 47, 2369–2371 RSC.
- L. L. Li, K. P. Liu, G. H. Yang, C. M. Wang, J. R. Zhang and J. J. Zhu, Adv. Funct. Mater., 2011, 21, 869–878 CrossRef CAS.
- A. W. Knight and G. M. Greenway, Anal. Commun., 1996, 33, 171–174 RSC.
- H. N. Choi, S. H. Cho and W. Y. Lee, Anal. Chem., 2003, 75, 4250–4256 CrossRef CAS.
- M. M. Collinson and R. M. Wightman, Science, 1995, 268, 1883–1885 CAS.
- Y. Shan, J. J. Xu and H. Y. Chen, Chem. Commun., 2009, 905–907 RSC.
- M. S. Wu, H. W. Shi, J. J. Xu and H. Y. Chen, Chem. Commun., 2011, 47, 7752–7754 RSC.
- B. B. Kulkarni, V. S. Swayambunathan and K. S. V. Santhanam, Bioelectrochem. Bioenerg., 1992, 27, 141–151 CrossRef CAS.
- Y. Lei, Q. Liao, H. Fu and J. Yao, J. Am. Chem. Soc., 2010, 132, 1742–1743 CrossRef CAS.
- B. Schlicke, P. Belser, L. De Cola, E. Sabbioni and V. Balzani, J. Am. Chem. Soc., 1999, 121, 4207–4214 CrossRef CAS.
- Y. Du, H. Wei, J. Z. Kang, J. L. Yan, X. B. Yin, X. R. Yang and E. K. Wang, Anal. Chem., 2005, 77, 7993–7997 CrossRef CAS.
- T. Horiuchi, O. Niwa and N. Hatakenaka, Nature, 1998, 394, 659–661 CrossRef CAS.
- A. J. Bard, Electrogenerated Chemiluminescence, Marcel Dekker, New York, 1st edn, 2004 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details; structures of DPA and Ru(bpy)32+; absorption and PL spectra of DPA and Ru(bpy)32+ monomers; SEM images of the wires with different molar ratios of Ru(bpy)32+/DPA; EDX spectra of the doped organic wires; physicochemical properties of Ru(bpy)32+ and DPA; fluorescence spectra of the doped wires; PL spectra of pure DPA wires; fluorescence decay profiles of DPA wires and the doped wires; the ECL mechanism of Ru(bpy)32+ and DPA; the energy level diagram of the electron process in the doped wires. See DOI: 10.1039/c1cc15632b |
| This journal is © The Royal Society of Chemistry 2012 |
