High-Tc ferromagnetism in a Co-doped ZnO system dominated by the formation of a zinc-blende type Co-rich ZnCoO phase†
Lin-Juan
Zhang
a,
Jian-Qiang
Wang
b,
Jiong
Li
b,
Jing
Zhou
a,
Wu-Peng
Cai
c,
Jie
Cheng
d,
Wei
Xu
a,
Guangzhi
Yin
b,
Xiang
Wu
e,
Zheng
Jiang
b,
Shuo
Zhang
*b and
Zi-Yu
Wu
*ad
aBeijing Synchrotron Radiation Facility, Institute of High Energy Physics, Chinese Academy of Sciences, Beijing 100049, China. E-mail: wuzy@ustc.edu.cn
bShanghai Synchrotron Radiation Facility, Shanghai Institute of Applied Physics, Chinese Academy of Sciences, Shanghai 201204, China. E-mail: zhangshuo@sinap.ac.cn
cLaboratory of Advanced Materials, Department of Material Science and Engineering, Tsinghua University, Beijing 100084, China
dNational Synchrotron Radiation Laboratory, University of Science and Technology of China, Hefei, Anhui 230029, China
eKey Laboratory of Orogenic Belts and Crustal Evolution, MOE, Peking University and School of Earth and Space Sciences, Peking University, Beijing 100871, China
First published on 7th November 2011
Abstract
The modulation of the distribution of magnetic ions embedded in the host is crucial for the functionality of dilute magnetic semiconductors. Through an element-specific structural characterization, we observe the formation and enhancement of an unrevealed Co-doped ZnO phase and consequently magnetic properties from paramagnetism to ferromagnetism are controlled by surface-modification.
Dilute magnetic semiconductors (DMSs) are base ingredients for a new generation of semiconductor devices that may simultaneously control both spin and charge of carriers.1,2 New fabrication methods for materials with unique characteristics and understanding of the mechanism inducing a magnetic order at room temperature are of considerable interest. Recently, there has been an increasing amount of evidence that the magnetic ions could aggregate to form magnetic nanocrystals embedded in the paramagnetic matrix in a synthesis process.3–6 This finding suggests that a separated phase plays an important role in the observed room-temperature ferromagnetism (RTF) of these systems. Therefore, controlling and characterizing these special phases are crucial for the functionality of the materials. In addition, several reports have shown that the ferromagnetism could be activated by a surface-modified approach in nano-structures.7–9 This result implies a possible correlation between surface-modification and distribution of doping atoms.
The wurtzite Co-doped ZnO has been intensively investigated as an ideal dilute magnetic oxide. However, recent research indicates that no stable ferromagnetic phases may exist when cobalt atoms are homogeneously distributed in the wurtzite (WZ) ZnO matrix.10,11 The aggregation of cobalt ions forming a wurtzite CoO phase in ultrathin ZnCoO films was observed, but no signal of RTF has been detected.12 Actually, among the potential host materials for DMSs, zinc-blende (ZB) type ZnO has long been predicted to be a promising host material to achieve RTF.13 Nevertheless, no successful experimental data have been presented to date.
In this research, we report the synthesis and investigation by X-ray absorption spectra (XAS) combined with XRD of a Zn0.98Co0.02O nanostructure system. Through modulation of the content of the capping molecule, we observed the formation and the enhancement of zinc-blende type Co-rich ZnCoO nanoclusters and consequently evolution of magnetic behavior from paramagnetism to ferromagnetism. The first-principles calculation revealed that the RTF arose from the ferromagnetic super-exchange interaction of this special phase.
Capping of the Co-doped ZnO with various contents of oleic acid (OA) produces the surface-modified samples with the following Zn(II)/OA molar ratios: 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5
3, 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, and 5
6, and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, denoted by samples A1, A2 and A3 (ESI 1†). The variations of the magnetization at 300 K under an applied magnetic field from −10 to 10 kOe are displayed in the lower part of Fig. 1(a). With the increasing OA concentration, a magnetic transition from paramagnetism to ferromagnetism is observed (S3†). Both the TEM image and analysis of XRD data (S2†) indicate that the average particle sizes of all samples are about 20 nm, showing that the content of OA surfactant has little influence on the size of our sample.
9, denoted by samples A1, A2 and A3 (ESI 1†). The variations of the magnetization at 300 K under an applied magnetic field from −10 to 10 kOe are displayed in the lower part of Fig. 1(a). With the increasing OA concentration, a magnetic transition from paramagnetism to ferromagnetism is observed (S3†). Both the TEM image and analysis of XRD data (S2†) indicate that the average particle sizes of all samples are about 20 nm, showing that the content of OA surfactant has little influence on the size of our sample.
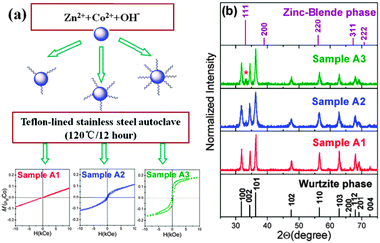 | ||
| Fig. 1 (a) The scheme of the split-batch experiment and the dependence of the magnetization at room temperature vs. the applied magnetic field (M vs. H) for the samples A1, A2, and A3, respectively. (b) X-Ray diffraction pattern of Zn0.98Co0.02O nanoparticles with different contents of oleic acid. Vertical lines indicate the diffraction peaks of the bulk wurtzite ZnO and the zinc-blende ZnO phases. | ||
As shown in Fig. 1(b), sample A1 can be readily indexed into a wurtzite ZnO structure (JCPDS card No. 89-0511, a = b = 3.249 Å and c = 5.205 Å) with no impurity clusters. An additional peak (marked by *) appears in A2 and becomes more evident in sample A3. It cannot be assigned to simple cobalt oxides such as cubic Co3O4, cubic CoO, wurtzite CoO and ZB CoO as initially considered. After a careful comparison we assigned the feature to the (111) peak of the ZB-type ZnO phase. From the analysis of XRD data we also point out that, upon increasing the concentration of OA the cluster of the ZB phase increases and a strong correlation between RTF and ZB-type nanoclusters occurs. However, the information regarding the distribution of magnetic ions could not be extracted from XRD data.
Previous studies found that the capping molecule has a significant impact on the magnetic properties even without any transition metal atom doping,14,15 owing to the defect or boundary effects. In comparison, we also present control experiments where other conditions remain the same, however, without Co dopants. Only diamagnetic behaviour can be observed in these OA-modified ZnO samples (Fig. S3†). Such a result suggests that defect or boundary effects do not contribute to ferromagnetism in our system.
Synchrotron radiation X-ray absorption near-edge structure (XANES) data were used to obtain local structural information around specific elements. The presence of cobalt oxides such as CoO (cubic), Co3O4 and Co2O3 can be ruled out through comparison of their experimental spectra in agreement with those reported in a previous study16 (ESI 4†). The effect of the OA content on the local structure around Co atoms is evident from the comparison of the spectra shown in the lower part of Fig. 2(a). Two significant changes occur upon increasing the OA content: (1) the intensity of the “white line” (peak C) increases and (2) the resonance peak D weakens almost disappearing in sample A3 (see the inset of Fig. 2(a)). Actually, all features in this energy region mainly originate from photoelectron multi-scattering (MS) contributions, being strongly sensitive to changes of the local structures around absorbers. They may involve structural rearrangements17, disorders18 and defects.19 In particular, the raise of the peak C can be understood as a rearrangement of the CoO4 tetrahedron.20 Similarly, a comparison of XRD data shows changes in the local environment around Co atoms as a function of the OA content. Therefore, we could speculate that cobalt atoms probably aggregate in the ZB-type phase upon increasing the OA content.
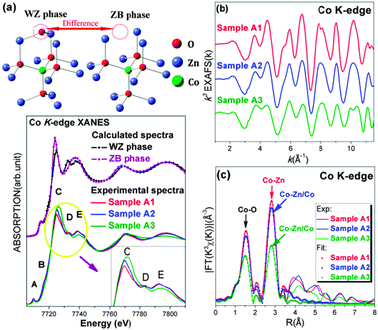 | ||
| Fig. 2 (a) Upper panel: two ZnO-phase local structures. Lower panel: a comparison of the theoretical XANES spectra at the Co K-edge of the ZB-type and the WZ-type phases and experimental spectra of different Zn0.98Co0.02O samples. The inset shows a zoom of the experimental white-line region. (b) The k-weighted EXAFS χ(k) function at the Co K-edge for the three samples. (c) Experimental Fourier Transform at the Co K-edge of EXAFS data of the Zn0.98Co0.02O samples and the corresponding fits. | ||
In order to validate the hypothesis, we carried out XANES simulations at the Co K-edge in the framework of the MS theory by using the FEFF 8.4 code.21 In the top panel of Fig. 2(a) we compare the two structural models of the WZ-type structure and of the ZB-type phase, including only the first and the second coordination shells around the central Co substitutional atom. Main differences observed correspond to an O missing in the second shell and to a symmetry change. These differences imply significant changes in the calculated spectra of the WZ-type and the ZB-type phases (Fig. 2(a)). In the latter while feature C enhances significantly feature D misses completely, in good agreement with the experimental data. The increase in the intensity of feature C can be assigned to a symmetry enhancement of the ZB-type phase that has four coordinated Co–O distances. If a coherent phase of backscattering photoelectrons occurs, a sharper resonance has to be observed.22 The disappearance of feature D is associated with the lack of an oxygen atom in the second coordination shell of the ZB-type phase structure. The important role of the missing oxygen in the feature D has been already pointed out in a previous investigation on annealing effects on the local structure around cobalt atoms.19 Hence, by MS calculations we can provide a strong evidence for the occurrence of a phase separation in sample A3, i.e., a ZB-type Co-rich ZnCoO nanocluster, which should be responsible for the RTF in this system.
As shown in Fig. 2(b), structural changes could also be seen in the k range of 3–7 Å−1 of the χ(k) function. Quantitative information about the local structures was further obtained by fitting EXAFS data using the IFEFFIT23 package. Data are listed in Table S1 (ESI†) and the fits are plotted in Fig. 2(c). Coordination numbers NCo–O and NCo–Zn(Co) are both close to standard data pointing out that cobalt atoms do not distribute at the nanoparticle surface, otherwise these values would obviously be smaller. RCo–O and RCo–Zn values in sample A1 are also in good agreement with those reported in previous research.24 Thanks to these data, we may easily rule out the presence of wurtzite CoO clusters, previously reported with an unusually long axial bond distance of 2.164 Å and three shorter distances of 1.923 Å.25 From our analysis we may summarize that cobalt atoms in sample A1 do not aggregate while distributing homogeneously in the matrix. In sample A3, we observe a contraction of the Co–O distance (1.96 Å), a value slightly shorter than ZB-type CoO data (1.97 Å).26 The origin of this contraction is probably due to the presence of this cluster embedded in the matrix and unavoidably constrained by the host lattice.
A possible correlation between the magnetic behavior and the local structures is outlined in Fig. 3. In the low OA concentration case characterized by a paramagnetic behavior, cobalt atoms are randomly distributed in the wurtzite ZnO matrix, in agreement with previous investigation.10 For the highest level capping sample, almost all cobalt atoms are incorporated into ZB-type nanoclusters. Actually, a robust RTF is observed in this sample and we assigned it to the contribution of Co doped ZB-type nanoclusters. In the intermediate sample a coexistence of both structures is detected and the sample exhibits both paramagnetism and a weak ferromagnetism.
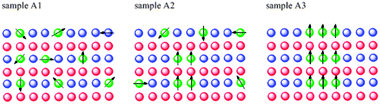 | ||
| Fig. 3 A schematic evolution of the Co atoms distribution and of the underlying magnetic order. | ||
In order to explain this structure, a band coupling model27 has been considered. According to such a model, the magnetic order is dominated by the position and the occupancy of the Co 3d sub-band near the Fermi-level and a competition between the ferromagnetic super-exchange, arising from the hybridization of the occupied eg↓ states with the empty t2↓ states, and an anti-ferromagnetic super-exchange due to the hybridization of the occupied t2↑ states with the empty t2↓ states should be taken into account (Fig. 4(b)). Therefore, we carried out a first-principles calculation based on the density functional theory (DFT) (S6†) and the related projected density of state (DOS) is shown in Fig. 4(a). The results indicated a FM ground state with ΔEFM–AFM = −37 meV, showing that FM super-exchange interaction is dominant in this system. This finding may trigger more comprehensive theoretical research and give an opportunity to reconsider the contribution of the short-range exchange interaction to the magnetic order in this system.
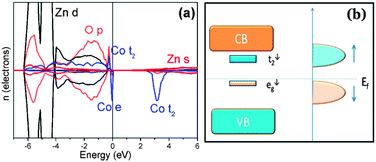 | ||
| Fig. 4 (a) The DFT calculated density of states of the Zn30Co2O32 sample in a ferromagnetic configuration. The Co states are ×2 for clarity. (b) The schematic band level diagram and the Fermi level are indicated by the horizontal line. | ||
In summary, we presented data regarding Co-doped ZnO nanoparticles with a ferromagnetic character at room-temperature whose synthesis route is controlled by a simple solvothermal process. The mechanism underlying their magnetic properties has been investigated by XRD and XAS methods. The results indicate that the RTF behavior is associated with the formation of zinc-blende type Co-rich ZnCoO nanoclusters, a special phase addressed in previous theoretical studies. The results appear to be relevant in the context of the existing theoretical frameworks describing the magnetic properties of dilute magnetic semiconductors but also for a much larger class of transition-metal semiconductor materials.
This work was partly supported by the Academy of Sciences (KJCX2-YW-N42), the Key Important Project of the National Natural Science Foundation of China (10734070), the National Natural Science Foundation of China (grant No. 11005145 and No. 20901055), the National Basic Research Program of China (2010CB934501) and the Graduate Student Innovation Fund of NSRL (20090622S). Sincere thanks are due to the staff of the beamlines 14B of the Shanghai Synchrotron Radiation Facility (SSRF) and beamlines U19 of National Synchrotron Radiation Laboratory (NSRL) for their support during experimental runs.
Notes and references
- H. Ohno, Science, 1998, 281, 951–956 CrossRef CAS.
- S. A. Wolf, D. D. Awschalom, R. A. Buhrman, J. M. Daughton, S. von Molnar, M. L. Roukes, A. Y. Chtchelkanova and D. M. Treger, Science, 2001, 294, 1488–1495 CrossRef CAS.
- S. Kuroda, N. Nishizawa, K. Takita, M. Mitome, Y. Bando, K. Osuch and T. Dietl, Nat. Mater., 2007, 6, 440–446 CrossRef CAS.
- T. Dietl, Nat. Mater., 2006, 5, 673 CrossRef CAS.
- A. Bonanni, A. Navarro-Quezada, T. Li, M. Wegscheider, Z. Matecaronj, V. Holý, R. T. Lechner, G. Bauer, M. Rovezzi, F. D'Acapito, M. Kiecana, M. Sawicki and T. Dietl, Phys. Rev. Lett., 2008, 101, 135502 CrossRef CAS.
- R. Lardé, E. Talbot, P. Pareige, H. Bieber, G. Schmerber, S. Colis, V. r. Pierron-Bohnes and A. Dinia, J. Am. Chem. Soc., 2011, 133, 1451 CrossRef.
- K. R. Kittilstved and D. R. Gamelin, J. Am. Chem. Soc., 2005, 127, 5292–5293 CrossRef CAS.
- N. S. Norberg, K. R. Kittilstved, J. E. Amonette, R. K. Kukkadapu, D. A. Schwartz and D. R. Gamelin, J. Am. Chem. Soc., 2004, 126, 9387–9388 CrossRef CAS.
- B. D. Yuhas, S. Fakra, M. A. Marcus and P. Yang, Nano Lett., 2007, 7, 905–909 CrossRef CAS.
- A. Ney, K. Ollefs, S. Ye, T. Kammermeier, V. Ney, T. C. Kaspar, S. A. Chambers, F. Wilhelm and A. Rogalev, Phys. Rev. Lett., 2008, 100, 157201 CrossRef CAS.
- P. Sati, C. Deparis, C. Morhain, S. Schäfer and A. Stepanov, Phys. Rev. Lett., 2007, 98, 137204 CrossRef CAS.
- H. L. Meyerheim, C. Tusche, A. Ernst, S. Ostanin, I. V. Maznichenko, K. Mohseni, N. Jedrecy, J. Zegenhagen, J. Roy, I. Mertig and J. Kirschner, Phys. Rev. Lett., 2009, 102, 156102 CrossRef CAS.
- T. Dietl, H. Ohno, F. Matsukura, J. Cibert and D. Ferrand, Science, 2000, 287, 1019–1022 CrossRef CAS.
- A. P. Thurber, G. L. Beausoleil II, G. A. Alanko, J. J. Anghel, M. S. Jones, L. M. Johnson, J. Zhang, C. B. Hanna, D. A. Tenne and A. Punnoose, J. Appl. Phys., 2011, 109, 07C305 CrossRef.
- M. A. Garcia, J. M. Merino, E. Fernandez Pinel, A. Quesada, J. de la Venta, M. L. Ruiz Gonzalez, G. R. Castro, P. Crespo, J. Llopis, J. M. Gonzalez-Calbet and A. Hernando, Nano Lett., 2007, 7, 1489–1494 CrossRef CAS.
- W. Li, Q. Kang, Z. Lin, W. Chu, D. Chen, Z. Wu, Y. Yan, D. Chen and F. Huang, Appl. Phys. Lett., 2006, 89, 112507 CrossRef.
- T. Liu, H. Xu, W. S. Chin, Z. Yong and A. T. S. Wee, J. Phys. Chem. C, 2008, 112, 3489–3495 CAS.
- K. S. Hamad, R. Roth, J. Rockenberger, T. van Buuren and A. P. Alivisatos, Phys. Rev. Lett., 1999, 83, 3474–3477 CrossRef CAS.
- S. Zhang, L. Zhang, H. Li, J. Li, Z. Jiang, W. Chu, Y. Huang, J. Wang and Z. Wu, J. Synchrotron Radiat., 2010, 17, 600–605 CrossRef CAS.
- S. Zhang, Y. Du, H. Li, W. Chu, J. Li, W. Yan, S. Wei, C. Yan and Z. Wu, J. Phys. Chem. C, 2009, 113, 4263–4269 CAS.
- A. L. Ankudinov, B. Ravel, J. J. Rehr and S. D. Conradson, Phys. Rev. B, 1998, 58, 7565–7576 CrossRef CAS.
- J. J. Rehr and R. C. Albers, Rev. Mod. Phys., 2000, 72, 621 CrossRef CAS.
- M. Newville, J. Synchrotron Radiat., 2001, 8, 322–324 CrossRef CAS.
- T. Shi, S. Zhu, Z. Sun, S. Wei and W. Liu, Appl. Phys. Lett., 2007, 90, 102108 CrossRef.
- A. S. Risbud, L. P. Snedeker, M. M. Elcombe, A. K. Cheetham and R. Seshadri, Chem. Mater., 2005, 17, 834–838 CrossRef CAS.
- M. J. Redman and E. G. Steward, Nature, 1962, 193, 867 CrossRef CAS.
- B. Belhadji, L. Bergqvist, R. Zeller, P. H. Dederichs, K. Sato and H. Katayama-Yoshida, J. Phys.: Condens. Matter, 2007, 19, 436227 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental and calculated details. See DOI: 10.1039/c1cc15622e |
| This journal is © The Royal Society of Chemistry 2012 |
