DOI:
10.1039/C2AY25061F
(Paper)
Anal. Methods, 2012,
4, 1259-1264
Colorimetric detection of Cr3+ using tripolyphosphate modified gold nanoparticles in aqueous solutions
Received
17th January 2012
, Accepted 25th February 2012
First published on 22nd March 2012
Abstract
A sensitive and selective colorimetric assay method for the detection of Cr3+ has been developed using tripolyphosphate functionalized gold nanoparticles (P3O105−–AuNPs). Infrared (IR) spectra and energy dispersive X-ray spectroscopy (EDS) confirmed that tripolyphosphates capped on the surfaces of gold nanoparticles (AuNPs). Gold nanoparticles were prepared by reducing HAuCl4 with sodium borohydride (NaBH4) in the presence of sodium tripolyphosphate (Na5P3O10). Upon exposure to Cr3+, the color of the gold nanoparticle solution changed from red to violet, which was in response to the surface plasmon absorption of dispersed and aggregated nanoparticles. The P3O105−–AuNPs were bound by Cr3+ and showed excellent selectivity compared to other ions (Hg2+, Fe3+, Cr6+, Mn2+, Cd2+, Ni2+, Pb2+, Ba2+, Co2+, Cu2+, Ca2+, Mg2+, Zn2+, Al3+, Na+, Cl−, SO42−, PO43−, CO32−, NO3−, and all of the above interfering ions with the exception of Cr3+) and had a detection limit of 10−7 M by the naked eye in this way. Most importantly, the P3O105−–AuNPs can be stored at room temperature over half a year. In addition, the P3O105−–AuNPs were also used to detect Cr3+ in real environmental water samples, with low interference.
Introduction
During the past decade, gold nanoparticles (AuNPs) have been widely used in a range of applications including: sensing, electronics, and surface enhanced Raman spectroscopy (SERS), as they exhibit strong surface plasmon resonance (SPR) absorption with extremely high extinction coefficients (108–1010 M−1 cm−1) in the visible wavelength range.1 The high SPR is dependent on the composition, size, and shape of the gold nanoparticles and sensitivity to the inter-particle distances. In view of this advantage, lots of colorimetric sensors have been developed for the detection of metal ions,2–7 proteins,8 oligonucleotides,9 and organic molecules,10–13 most of which can be distinguished as aggregation sensors based on cross-linking and electrostatic absorption. In these assay methods, the introduction of ligands onto the surfaces of gold nanoparticles not only stabilize AuNPs in solution, but also can interact with metal ions through a coordination reaction. Furthermore, analyte-triggered aggregation of these functionalized AuNPs leads to a red shift in the SPR absorption band resulting in a red-to-gray color change. Above all, the distance-dependent SPR absorption of AuNPs has become a useful tool for the development of colorimetric sensing with various analytes.
It is known that trivalent chromium (Cr(III)) is an integral part of the glucose tolerance factor, and it plays an important role on the regulation of sugar and lipid metabolism. Moreover, Cr(III) can prevent certain mutations of genetic materials in cells as a nucleic acid stabilizer; thereby it can prevent the occurrence of cancer. Many cases have been reported where the complete removal of Cr(III) from the diet has caused chromium deficiency. However, larger amounts and different forms of chromium can be toxic and carcinogenic. Most importantly, as we all know Cr(III) is less harmful than hexavalent chromium (Cr(VI)), in the aqueous solution containing Cr(VI), by adding a certain amount of reducing agent, making the solution containing only trivalent chromium, then it could be used as a useful method for detecting the content of Cr(VI). Besides, through the determination and control the content of trivalent chromium, not only to understand the performance of the bath, the conductivity of the solution, the situation of the anode, etc., but also to ensure the normal electroplating with chrome. Above all, the content of trivalent chromium is essential to the quality of electroplating chromium coatings. Nowadays, chromium has been increasingly used in a number of industrial processes including chrome plating, dye and pigment fabrication, leather tanning, and wood preserving. Due to the increasing threat of chromium exposure in the environment, there has been a growing interest in the development of highly sensitive and selective assay methods for the determination of chromium over the past few years. Various sensor systems have been reported.14–22 Most of these systems, however, have either limitations with respect to simplicity, selectivity, and time consuming for Cr3+ determination using voltammetric, electrothermal atomic absorption spectrometry, and surface plasmon field-enhanced resonance light scattering. Besides, the diphenylcarbazide colorimetric method is simple and fast, but certain substances react with the 1,5-diphenylcarbazide reagent to form a colored product which is absorbed at 520–540 nm that may obscure or interfere with the quantization of the chromate peak.23 Therefore, the development of a sensor that is not only sensitive and selective but also simple and practical for Cr3+ determination remains a challenge.
In this report, a new strategy for the efficient recognition and detection of Cr3+ in the aqueous solutions through tripolyphosphate modified AuNPs has been reported. Herein, AuNPs were prepared by reducing HAuCl4 with NaBH4 in the presence of Na5P3O10. These AuNPs have been coated with tripolyphosphates to selectively bind Cr3+ (Scheme 1). Also, the IR spectrum and EDS suggest that tripolyphosphates were capped on the surfaces of gold nanoparticles. In the presence of Cr3+, these modified AuNPs interacted with Cr3+ through the cooperative metal–ligand reaction, which led to the aggregation of AuNPs quickly resulting in excellent sensitivity and high selectivity of the detection of Cr3+. Moreover, the presence of Cr3+ can be monitored by a colorimetric response of functionalized AuNPs, and it has a detection limit of 1.0 × 10−7 M by the naked eye in this way, making it a promising rapid and sensitive technique for Cr3+ analysis.
 |
| | Scheme 1 An analytical process for Cr3+ detection using tripolyphosphate modified gold nanoparticles. | |
Experimental section
Materials and characterization
Hydrogen tetrachloroaurate (III) tetrahydrate and sodium borohydride were purchased from Shanghai Chemical Reagent Co. Ltd. without further purification. Sodium tripolyphosphate (Na5P3O10) was obtained from Aladdin. All of the chemicals were used as received. UV-vis spectra were performed using a Lambda 950 from Perkin Elmer. Transmission electron microscopy (TEM) images and EDS data were recorded from a Tecnai F20 instrument and were operated at 200 kV. IR data were obtained on Nicolet 6700 Fourier-Transform Infrared Spectrometer. ICP-AES data were recorded on an ICP-AES Perkin-Elmer, Optima 2100.
Preparation of P3O105−–AuNPs
AuNPs were prepared by reducing HAuCl4 with NaBH4 in the presence of Na5P3O10. All the glassware was washed with aqua regia (HCl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 = 3
HNO3 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), aqua regia - prepare immediately before use by carefully adding one volume of conc. HNO3 to three volume of conc. HCl in a glass container (it is very corrosive!). Wear chemical splash goggles and thick gloves when using the aqua regia solution in a fumehood. The spent solution is neutralized with sodium bicarbonate and disposed via the drain, followed by flushing with copious amounts of water) and then rinsed with Milli-Q water. Briefly, 20 mL of 5 mM HAuCl4 was added rapidly to the solution of Na5P3O10 (1 mM, 100 mL), and then sodium borohydride (0.1 M, 5 mL) was slowly added into the mixed solutions and stirred for 30 min. The color of the gold nanoparticle solution turned a vivid wine-red. Finally, the prepared solution was stored at room temperature for future use and kept there for three months at least. As shown in Fig. 1, these P3O105−–AuNPs with an average size of 11.13 nm have good dispersion properties.
1 (v/v), aqua regia - prepare immediately before use by carefully adding one volume of conc. HNO3 to three volume of conc. HCl in a glass container (it is very corrosive!). Wear chemical splash goggles and thick gloves when using the aqua regia solution in a fumehood. The spent solution is neutralized with sodium bicarbonate and disposed via the drain, followed by flushing with copious amounts of water) and then rinsed with Milli-Q water. Briefly, 20 mL of 5 mM HAuCl4 was added rapidly to the solution of Na5P3O10 (1 mM, 100 mL), and then sodium borohydride (0.1 M, 5 mL) was slowly added into the mixed solutions and stirred for 30 min. The color of the gold nanoparticle solution turned a vivid wine-red. Finally, the prepared solution was stored at room temperature for future use and kept there for three months at least. As shown in Fig. 1, these P3O105−–AuNPs with an average size of 11.13 nm have good dispersion properties.
 |
| | Fig. 1 (Top) A typical TEM image and (bottom) histogram of particle size distribution of P3O105−–AuNPs. | |
Colorimetric detection of Cr3+ ions
For the detection of Cr3+ by using the tripolyphosphate modified gold nanoparticles, different concentrations of Cr3+ aqueous solutions were added separately into a 2.0 mL of solution containing P3O105−–AuNPs, and adjusted the pH value of the mixed solutions to 3.25. Then the mixtures were maintained at room temperature for several minutes, and then the color changed from red to gray-blue.
Results and discussion
Sensing strategy
Scheme 1 outlined a possible sensing mechanism of P3O105−–AuNPs selectively binding Cr3+. The strong peaks at 889.73 cm−1, 1159.33 cm−1 and 1211.29 cm−1 were assigned to P–O stretches in Na5P3O1024 and the P3O105−–AuNPs were also characterized by IR spectroscopy as shown in Fig. 2. However, the P3O105−–AuNPs had only one weak peak at 1122.3 cm−1. The energy dispersive X-ray spectra (EDS) result (Fig. 3) confirms that the peaks of P and O were attributed to the P3O105−–AuNPs after complete centrifugation. These are evidences for the existence of P3O105−–AuNPs. It was shown that P3O105−–AuNPs were aggregated in the presence of Cr3+ shown in Fig. 4. Therefore, the Na5P3O10 was absorbed on the surfaces of the AuNPs and the P3O105−–AuNPs were aggregated in the presence of Cr3+ due to binding with a chelating ligand. It could be explained that coordination compounds were formed by Cr3+ with P3O105−. Therefore, this approach could be a rapid and sensitive assay method for the detection of Cr3+.
 |
| | Fig. 4 A typical TEM image of P3O105−–AuNPs in the presence of Cr3+ ions (10 μM). | |
Effects of Na5P3O10 and pH
In this report, further experiments revealed that many factors such as the concentration of Na5P3O10 and pH of the aqueous solution, which had influences on the modified gold nanoparticles in the recognition of Cr3+. Although higher concentration of Na5P3O10 benefited for the homogeneity, dispersibility and size of P3O105−–AuNPs and the recognition of Cr3+, however, the P3O105−–AuNPs would aggregate together when the concentration of Na5P3O10 reached 3 mM. Moreover, the concentration of Na5P3O10 was less responsible for the instability of AuNPs than pH. The P3O105−–AuNPs were not stable in one hour when the pH of the aqueous solution was less than 2.50 and the color did not change obviously even the concentration of Cr3+ was 10−5 M when the pH of the aqueous solution was higher than 4. Above all, it was the reason why the concentration of Na5P3O10 was 0.8 mM and the pH of the aqueous solution at 3.25 was used in this experiment. Under these optimized conditions, the recognition of Cr3+ with P3O105−–AuNPs showed a significant color change by this colorimetric assay method.
Selectivity and sensitivity for Cr3+
To investigate the influence of other ions in which P3O105−–AuNPs can effectively detect Cr3+, competitive experiments were carried out in the presence of Cr3+ (50 μM) with Hg2+, Fe3+, Cr6+, Mn2+, Cd2+, Ni2+, Pb2+, Ba2+, Co2+, Cu2+, Ca2+, Mg2+, Zn2+, Al3+, Na+, Cl−, SO42−, PO43−, CO32−, NO3− and Mn+ at 50 μM. Fig. 5 shows that the photographic images and UV-vis spectra of P3O105−–AuNPs when various types of ions were added to P3O105−–AuNPs aqueous solutions within 1 min, it was clearly shown that only when the sample was added did Cr3+ ions induced a distinct color change from red to violet (Fig. 5, top image). The SPR absorption of P3O105−–AuNPs was measured with a UV-vis spectrophotometer and its maximum absorption was located at 520 nm. Moreover, Cr3+ was also the only ion which resulted in an absorption peak red shift from 520 nm to 725 nm (Fig. 5, bottom image). This red shift was also observed as a color change from red to violet. These results could be evidence for the aggregation of P3O105−–AuNPs triggered by Cr3+, which agrees with the TEM images shown in Fig. 4. Effectively, Cr3+ triggered the aggregation of P3O105−–AuNPs as Cr3+ functioned as a bridge among these modified nanoparticles. Other ions did not influence the absorption spectra and indicating that other ions do not interfere in the binding of P3O105−–AuNPs with Cr3+. The extremely high selectivity toward Cr3+ was attributed to the small Ksp (the solubility product constant), and it had a solubility product constant with CrPO4 (Ksp = 2.4 × 10−23) over several orders of magnitude more than the other ions. Most importantly, this finding could be a basis for the detection of Cr3+ with P3O105−–AuNPs as even if all of the above interfering ions were added that did not influence the detection of Cr3+. It was suggested that this method could be suitable for various environmental samples.
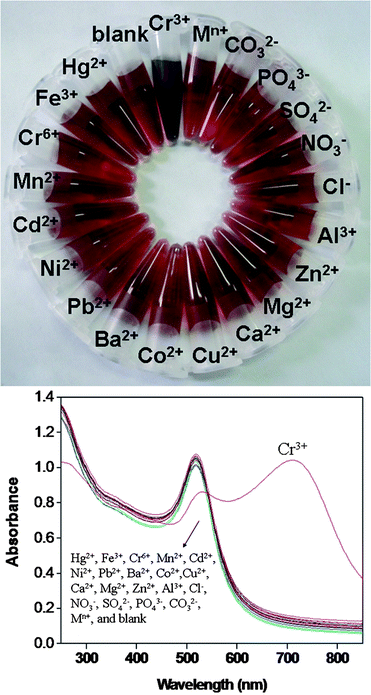 |
| | Fig. 5 (Top) Photographic images of P3O105−–AuNPs in the presence of various types of ions. (Bottom) UV-vis spectra of P3O105−–AuNPs in the presence of different ions (50 μM). Mn+ contains all of the above interfering ions (50 μM) with the exception of Cr3+. pH = 3.25. | |
Fig. 6 shows the various colors of the P3O105−–AuNPs aqueous solutions after the addition of different concentrations of Cr3+. The P3O105−–AuNPs were utilized for the colorimetric assay of Cr3+ under these optimized conditions. As the incorporating concentration of Cr3+ increased from 10−7 M to 10−3 M, the color of these aqueous solutions gradually changed from deep red to gray-blue. As shown in Fig. 4, the color change could be clearly observed when the concentration of Cr3+ was higher than 1.0 × 10−7 M, in other words, the detection limit of Cr3+ was 1.0 × 10−7 M or so according to this approach of colorimetric assay by naked eye.
 |
| | Fig. 6 Various colors of different concentrations of Cr3+ were added to the P3O105−–AuNPs aqueous solutions. | |
According to the above optimized conditions, the minimum detectable concentration of Cr3+ was investigated by the color change. Also, UV-vis spectroscopy allowed the detection of low concentrations of Cr3+ for the quantitative assay. As shown in Fig. 7, a wide range of Cr3+ concentrations from 0.02 μM to 22 μM could be successfully detected in the experiments using UV-vis spectra. Moreover, the surface plasmon resonance absorption of P3O105−–AuNPs in the presence of different concentrations of Cr3+ had two distinct peaks (Fig. 7a), the left absorption peaks had formed due to the dispersion of the modified AuNPs, and the absorption peaks on the right were triggered by the aggregation of P3O105−–AuNPs. Above all, a good linear relationship (R = 0.9945) in the range of the concentration of Cr3+ from 3 μM to 12 μM was obtained by A0/A from the left absorption peaks at 520 nm shown in Fig. 7b. Moreover, the limit of quantitative detection (3σ) for Cr3+ was found to be 1.2 μM. Furthermore, the colors change of P3O105−–AuNPs in the presence of 2 μM Cr3+ within 5 min is shown in Fig. 8.
 |
| | Fig. 7 (a) Surface plasmon resonance absorption change of P3O105−–AuNPs in the presence of different concentrations of Cr3+. (b) The calibration curve for the detection of Cr3+ by P3O105−–AuNPs. The ratio (A0/A) was plotted against different concentrations of Cr3+. Where A0 is the UV-vis absorption of the P3O105−–AuNPs and A is the UV-vis absorption of P3O105−–AuNPs in the presence of different concentrations of Cr3+ at 520 nm. | |
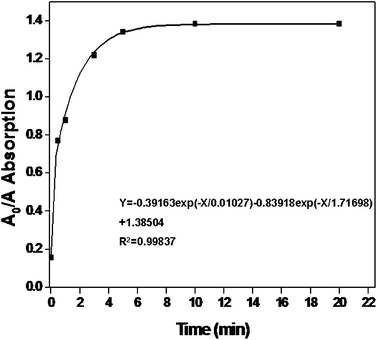 |
| | Fig. 8 The aggregation time of P3O105−–AuNPs in the presence of Cr3+ (2 μM). It shows that the detection time was rapid within 5 min. | |
Application of P3O105−–AuNPs for the analysis of real water samples
To confirm the practical application of P3O105−–AuNPs, the Cr3+ concentration of real environmental water samples (Ningbo Environment Monitoring Center, Ningbo, China) was determined by applying this assay method. Firstly, 100 mL water sample was treated with a certain amount of Na2S2O3 to convert Cr6+ to Cr3+ and stirred for 30 min at room temperature. Then the pH of the treated water sample was adjusted to 3.25 with HCl before use. A calibration curve of P3O105−–AuNPs SPR shifted in the presence of different concentrations of Cr3+ was prepared (see Fig. 7). The concentration of Cr3+ of the real water sample (Ningbo Environment Monitoring Center, Ningbo, China) was from 8.10 μM to 8.20 μM based on the detection results, which was generally in good agreement with the detection result of Cr3+ (8.15 μM) by the ICP-AES-based method. Thus, the result demonstrates that the designed colorimetric method is applicable for Cr3+ rapid detection in the real water samples.
Conclusions
A highly sensitive, selective and stable colorimetric sensor for the detection of Cr3+ has been developed based on the aggregation of P3O105−–AuNPs induced by Cr3+. Cr3+ was the only ion which induced the aggregation of P3O105−–AuNPs which brought a color change of the sample solution from pink to gray-blue and enhanced the absorption of the SPR of gold nanoparticles. The optimal pH value using P3O105−–AuNPs was determined to be 3.25. A test of the Cr3+ concentration in real environmental samples was done and the results are consistent with the ICP-AES-based method. In principle, this method could provide a promising sensor for on-line detection of Cr3+ in real water samples later.
Acknowledgements
This work was supported by the Program of Zhejiang Provincial Natural Science Foundation of China under Grant No. R5110230, Natural Science Foundation of China under Grant Nos: 31170964, 31128007, and 51102251; Hundred Talents and Academy-Locality Cooperation Programs of Chinese Academy of Sciences, and Ningbo Science and Technology Bureau (Grants Nos 2011C50009, 2011A610140, 2011A610121, 2009B21005, and 2010A610159). The Projects Sponsored by the Scientific Research Foundation for the Returned Overseas Chinese Scholars, States of Ministry of Human Resources and Social Security and Ministry of Education. The authors also thank Prof. Yanbo Weng and Dr Xiaoqin Fu from Ningbo Environment Monitoring Center, Ningbo, China, for the real environmental samples.
References
- H. Wang, D. W. Brandl, P. Nordlander and N. Halas, Acc. Chem. Res., 2007, 40, 53–62 CrossRef CAS.
- J. R. Kalluri, T. Arbneshi, S. A. Khan, A. Neely, P. Candice, B. Varisli, M. Washington, S. McAfee, B. Robinson, S. Banerjee, A. K. Singh, D. Senapati and P. C. Ray, Angew. Chem., Int. Ed., 2009, 48, 9668–9671 CrossRef CAS.
- F. Q. Zhang, l. Y. Zeng, Y. X. Zhang, H. Y. Wang and A. G. Wu, Nanoscale, 2011, 3, 2150–2154 RSC.
- C. Y. Lin, C. J. Yu, Y. H. Lin and W. L. Tseng, Anal. Chem., 2010, 82, 6830–6837 CrossRef CAS.
- C. E. Lisowski and J. E. Hutchison, Anal. Chem., 2009, 81, 10246–10253 CrossRef CAS.
- N. Xiao and C. X. Yu, Anal. Chem., 2010, 82, 3659–3663 CrossRef CAS.
- F. Q. Zhang, L. Y. Zeng, C. Yang, J. W. Xin, H. Y. Wang and A. G. Wu, Analyst, 2011, 136, 2825–2830 RSC.
-
(a) C. S. Tsai, T. B. Yu and C. T. Chen, Chem. Commun., 2005, 4273–4275 RSC;
(b) X. Xu, M. S. Han and C. A. Mirkin, Angew. Chem., Int. Ed., 2007, 46, 3468–3470 CrossRef CAS;
(c) A. Laromiane, L. Koh, M. Murugesan, R. V. Ulijin and M. M. Stevens, J. Am. Chem. Soc., 2007, 129, 4156–4157 CrossRef.
-
(a) C. A. Mirkin, R. L. Letsinger, R. C. Mucic and J. J. Storhoff, Nature, 1996, 382, 607–609 CrossRef CAS;
(b) H. X. Li and L. Rothberg, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 14036–14039 CrossRef CAS.
- L. B. Wang, Y. Y. Zhu, L. G. Xu, W. Chen, H. Kuang, L. Q. Liu, A. Agarwal, C. Xu and N. A. Kotov, Angew. Chem., Int. Ed., 2010, 49, 1–5 CrossRef CAS.
- H. Chi, B. H. Liu, G. J. Guan, Z. P. Zhang and M. Y. Han, Analyst, 2010, 135, 1070–1075 RSC.
- K. L. Ai, Y. L. Liu and L. H. Lu, J. Am. Chem. Soc., 2009, 131, 9496–9497 CrossRef CAS.
- J. Liu and Y. Lu, Angew. Chem., Int. Ed., 2006, 45, 90–94 CrossRef CAS.
- E. Margui, C. Fontas, M. Toribio, M. Guillem, M. Hidalgo and I. Queralt, Appl. Spectrosc., 2010, 64, 547–551 CrossRef CAS.
- Z. Q. Han, L. Qi, G. Y. Shen, W. Liu and Y. Chen, Anal. Chem., 2007, 79, 5862–5868 CrossRef CAS.
- O. D. Uluozlu, M. Tuzen, D. Mendil, B. Kahveci and M. Soylak, J. Hazard. Mater., 2009, 172, 395–399 CrossRef CAS.
- M. S. Hosseini and F. Belador, J. Hazard. Mater., 2009, 165, 1062–1067 CrossRef CAS.
- Y. Xiang, L. Mei, N. Li and A. J. Tong, Anal. Chim. Acta, 2007, 581, 132–136 CrossRef CAS.
- M. F. Bergamini, D. P. dos Santos and M. V. B. Zanoni, Sens. Actuators, B, 2007, 123, 902–908 CrossRef.
- M. Jakubowska, J. Hazard. Mater., 2010, 176, 540–548 CrossRef CAS.
- H. Chen, P. Du, J. Chen, S. H. Hu, S. Q. Li and H. L. Liu, Talanta, 2010, 81, 176–179 CrossRef CAS.
- K. Sasaki, S. Oguma, Y. Namiki and N. Ohmura, Anal. Chem., 2009, 81, 4005–4009 CrossRef CAS.
- California Environmental Protection Agency, Method 425, 1997 Search PubMed.
- H. Bih, I. Saadoune, H. Ehrenberg and H. Fuess, J. Solid State Chem., 2009, 182, 821–826 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2012 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HNO3 = 3
HNO3 = 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v/v), aqua regia - prepare immediately before use by carefully adding one volume of conc. HNO3 to three volume of conc. HCl in a glass container (it is very corrosive!). Wear chemical splash goggles and thick gloves when using the aqua regia solution in a fumehood. The spent solution is neutralized with sodium bicarbonate and disposed via the drain, followed by flushing with copious amounts of water) and then rinsed with Milli-Q water. Briefly, 20 mL of 5 mM HAuCl4 was added rapidly to the solution of Na5P3O10 (1 mM, 100 mL), and then sodium borohydride (0.1 M, 5 mL) was slowly added into the mixed solutions and stirred for 30 min. The color of the gold nanoparticle solution turned a vivid wine-red. Finally, the prepared solution was stored at room temperature for future use and kept there for three months at least. As shown in Fig. 1, these P3O105−–AuNPs with an average size of 11.13 nm have good dispersion properties.
1 (v/v), aqua regia - prepare immediately before use by carefully adding one volume of conc. HNO3 to three volume of conc. HCl in a glass container (it is very corrosive!). Wear chemical splash goggles and thick gloves when using the aqua regia solution in a fumehood. The spent solution is neutralized with sodium bicarbonate and disposed via the drain, followed by flushing with copious amounts of water) and then rinsed with Milli-Q water. Briefly, 20 mL of 5 mM HAuCl4 was added rapidly to the solution of Na5P3O10 (1 mM, 100 mL), and then sodium borohydride (0.1 M, 5 mL) was slowly added into the mixed solutions and stirred for 30 min. The color of the gold nanoparticle solution turned a vivid wine-red. Finally, the prepared solution was stored at room temperature for future use and kept there for three months at least. As shown in Fig. 1, these P3O105−–AuNPs with an average size of 11.13 nm have good dispersion properties.








