Study on the interaction behavior of catalase with cephalosporins by chemiluminescence with flow injection analysis†‡
Donghua
Chen
a,
Zhuming
Wang
ab,
Yun
Zhang
a,
Xunyu
Xiong
a and
Zhenghua
Song
*a
aKey Laboratory of Synthetic and Natural Functional Molecule Chemistry of Ministry of Education, College of Chemistry & Material Science, Northwest University, Xi'an, 710069, China. E-mail: songzhenghua@hotmail.com; Fax: (+86)029-88302604; Tel: (+86)029-88303798
bKey Laboratory of Western Mineral Resources and Geological Engineering Ministry of Education, College of Earth Sciences and Land Resources, Chang'an University, Xi'an, 710054, China
First published on 19th December 2011
Abstract
The interaction behavior of catalase with cephalosporins was first studied using luminol as luminescence probe. By chemiluminescence model of protein–drug interaction in flow injection system, the binding parameters of catalase with cephalosporins were obtained, giving the binding ability of cephalosporins to catalase and the thermodynamic parameters of catalase/cephalosporins association process.
Study of protein–drug interactions has become a hot spot in the fields of biology, medicine and chemistry in recent years. For example, study on the interaction of human serum albumin (HSA) with drugs used in the treatment of hypertension by flow injection (FI)–capillary electrophoresis (CE)–frontal analysis method,1 bovine serum albumin with flavones or aloe-emodin by fluorescence spectroscopy method,2,3 myoglobin with penicillins by thermodynamic and spectroscopy methods,4 catalase (CAT) with 4-aminoantipyrine using spectroscopic and molecular docking methods5 and lysozyme (Lys) with chloramphenicol using fluorescence spectroscopic method6etc. In this work, CAT is chosen to study the interaction behavior with cephalosporins. CAT (MW ∼ 240 kDa), one of the most common enzymes in plant and animal tissues, is present in the peroxisomes of nearly all aerobic cells and serves to protect the cell by catalyzing hydrogen peroxide decomposition into molecular oxygen and water without the production of free radicals.7 CAT exists as a dumbbell-shaped tetramer of four identical subunits, each of which is formed by a single polypeptide chain with heme as a prosthetic group well buried in a hydrophobic pocket and accessible through a hydrophobic channel. The heme is relevant to the enzymatic activity of CAT, and the residues of His74 and Asn147 on the distal side of the subunit are catalytically important.8,9 There is increasing evidence that CAT is a major factor in a variety of pathological states such as cancer, diabetes, aging, oxidative stress10,11etc. Thus, study on the interaction behavior of CAT with drugs is necessary in the fields of medicine and chemistry.12,13 Up to now few works have been reported on the interaction of CAT with cephalosporins by FI–chemiluminescence (CL) analysis.
Cefradine, cefadroxil, cefazolin, cefaclor, cefuroxime, cefotaxime, ceftriaxone and cefoperazone are eight typical cephalosporins belonging to the first, second and third generation respectively, and their structures are shown in Table S1, ESI†. In this paper, the interaction behavior of CAT–cephalosporins was first studied using luminol as luminescence probe. By fluorescence, it was found that CAT with luminol could form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (the number of binding site n = 0.97) with binding constant KD of 7.03 × 105 L mol−1via a static mechanism. The CAT/luminol complex could accelerate the electron transfer rate of excited oxidation product of luminol and enhance the CL intensity from luminol, based on which luminol–CAT CL system with FI analysis was first established. It was also found that cephalosporins could quench the CL intensity of luminol–CAT system, which proved the feasibility for study on the interaction behavior of CAT with cephalosporins using the FI–CL model of protein–drug interactions.14 The binding parameters (KD and n) of CAT with cephalosporins were given, and the thermodynamic parameters in the CAT–cephalosporins association process indicated that the binding forces of CAT with cephalosporins were mainly hydrophobic interaction force. The binding ability of the studied cephalosporins to CAT followed this pattern: the third generation (cefoperazone, ceftriaxone and cefotaxime) > the second generation (cefuroxime and cefaclor) > the first generation (cefazolin, cefadroxil and cefradine), which was consistent with their antibacterial ability.15 The luminol–CAT CL system was also successfully applied to the monitoring of excreted cefradine in human urine. Compared with the existing methods for the determination of cefradine such as colorimetry,16 spectrophotometry,17 spectrofluorimetry,18 near-infrared (NIR),19 high-performance liquid chromatography (HPLC),20,21 capillary zone electrophoresis (CZE)22 and CL methods23etc., the proposed method showed the advantages of simple sample pretreatment, short analysis time, wide linear range and low limit of detection (LOD), and the comparison was summarized in Table S2, ESI†.
1 complex (the number of binding site n = 0.97) with binding constant KD of 7.03 × 105 L mol−1via a static mechanism. The CAT/luminol complex could accelerate the electron transfer rate of excited oxidation product of luminol and enhance the CL intensity from luminol, based on which luminol–CAT CL system with FI analysis was first established. It was also found that cephalosporins could quench the CL intensity of luminol–CAT system, which proved the feasibility for study on the interaction behavior of CAT with cephalosporins using the FI–CL model of protein–drug interactions.14 The binding parameters (KD and n) of CAT with cephalosporins were given, and the thermodynamic parameters in the CAT–cephalosporins association process indicated that the binding forces of CAT with cephalosporins were mainly hydrophobic interaction force. The binding ability of the studied cephalosporins to CAT followed this pattern: the third generation (cefoperazone, ceftriaxone and cefotaxime) > the second generation (cefuroxime and cefaclor) > the first generation (cefazolin, cefadroxil and cefradine), which was consistent with their antibacterial ability.15 The luminol–CAT CL system was also successfully applied to the monitoring of excreted cefradine in human urine. Compared with the existing methods for the determination of cefradine such as colorimetry,16 spectrophotometry,17 spectrofluorimetry,18 near-infrared (NIR),19 high-performance liquid chromatography (HPLC),20,21 capillary zone electrophoresis (CZE)22 and CL methods23etc., the proposed method showed the advantages of simple sample pretreatment, short analysis time, wide linear range and low limit of detection (LOD), and the comparison was summarized in Table S2, ESI†.
The CL intensity-time profile of luminol–CAT–cefradine was tested with 25 μmol L−1 luminol, 200 nmol L−1 CAT and 10 nmol L−1 cefradine. It is shown in Fig. 1 that at a flow rate of 2.0 mL min−1, the maximum CL intensity (Imax) of luminol system (curve 3) was 93 at the time (Tmax) of 4.5 s; Imax of luminol–CAT system (curve 1) was increased from 93 to 349, and Tmax was shortened from 4.5 s to 3.5 s compared with curve 3; Imax of luminol–CAT–cefradine system (curve 2) was decreased from 349 to 311 compared with curve 1 with the same Tmax of 3.5 s.
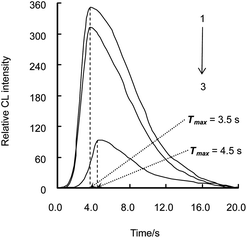 | ||
| Fig. 1 Relative CL intensity–time profile in different CL systems. 1. Luminol–CAT system; 2. Luminol–CAT–cefradine system; 3. Luminol system. The concentrations of luminol, CAT and cefradine were 2.5 × 10−5 mol L−1, 2.0 × 10−7 mol L−1 and 1.0 × 10−8 mol L−1, respectively. | ||
Under the optimal experimental conditions, a series of standard solutions of CAT were tested with luminol system, and a series of standard solutions of cephalosporins were determined by the luminol–CAT system. The increments of CL intensity from luminol system were proportional to the concentrations of CAT from 5 to 200 nmol L−1 with the linear equation of ΔI = 1.27CCAT + 0.94 (R = 0.9996) and the limit of detection (LOD) of 1.7 nmol L−1. In the presence of cephalosporins, the decrements of CL intensity from luminol–CAT system were proportional to the logarithm of cephalosporins concentrations and the linear equations, linear ranges and LODs are listed in Table S3, ESI†.
The interference of potentially interfering species was tested by analyzing a standard solution of cefradine (10 nmol L−1) into which increasing amounts of interferents were added. The tolerable ratios of interferents with respect to 10 nmol L−1 cefradine for interference less than 5.0% were over 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 for Ac−, I−, SO42−, PO43−, BrO3−, amylum, glucose, borate, malic acid, maltose, methanol and ethanol; 5000 for NH4+, Mg2+, Ca2+, Ba2+, oxalate, tartrate, sucrose, citrate and salicylic acid; 1000 for glutin, urea and dextrin; 100 for uric acid, respectively. Compounds abundant in human urine such as salt, lipid and proteins caused no obvious interference for the determination of cefradine. Therefore, the proposed method is suitable for the determination of cefradine in human urine without preliminary separation, saving a lot of time and eliminating the possibility of making mistakes due to separation procedure.
000 for Ac−, I−, SO42−, PO43−, BrO3−, amylum, glucose, borate, malic acid, maltose, methanol and ethanol; 5000 for NH4+, Mg2+, Ca2+, Ba2+, oxalate, tartrate, sucrose, citrate and salicylic acid; 1000 for glutin, urea and dextrin; 100 for uric acid, respectively. Compounds abundant in human urine such as salt, lipid and proteins caused no obvious interference for the determination of cefradine. Therefore, the proposed method is suitable for the determination of cefradine in human urine without preliminary separation, saving a lot of time and eliminating the possibility of making mistakes due to separation procedure.
By fluorescence method the interaction of CAT with luminol was studied. It was found that the intensity of luminol (λex/λem = 350 nm/425 nm) was quenched in the presence of CAT with its concentrations ranging from 5 to 100 nmol L−1, and the regression equation was F0/F = 1.00 + 1.12CCAT, (R = 0.9963), where F0 and F were the intensity of 125 nmol L−1 luminol in the absence and in the presence of CAT. According to Stern–Volmer quenching equation, the value of quenching rate constant Kq for CAT–luminol was 1.11 × 1014 L mol−1 s−1, far greater than the maximum scatter collision-quenching constant of various quenchers with fluorophors 2.0 × 1010 L mol−1 s−1.24 It was deduced that the quenching effect of CAT on the intensity of luminol was produced by forming a ground-state compound. According to the equation of lg[(F0 − F)/F] = lgKD + nlg[D], the binding constant KD and the number of binding site n of CAT–luminol were obtained as 7.03 × 105 L mol−1 and 0.98, respectively, which indicated that a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CAT/luminol complex was formed.
1 CAT/luminol complex was formed.
By CL method the interaction of CAT with cephalosporins was studied. Utilizing the established FI–CL model of protein–drug interaction lg[(I0 − I)/I] = lgKD + nlg[D],14 where I0 and I were the CL intensity of luminol–CAT system in the absence and in the presence of cephalosporins, the interaction parameters of CAT with cephalosporins at different temperatures could be obtained. The binding constants KD and the number of binding sites n of CAT with cephalosporins are listed in Table 1, which indicates that CAT and cephalosporins could form 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes. The binding ability of the studied cephalosporins to CAT followed this pattern: the third generation (cefoperazone, ceftriaxone and cefotaxime) > the second generation (cefuroxime and cefaclor) > the first generation (cefazolin, cefadroxil and cefradine), which was consistent with their antibacterial ability.25
1 complexes. The binding ability of the studied cephalosporins to CAT followed this pattern: the third generation (cefoperazone, ceftriaxone and cefotaxime) > the second generation (cefuroxime and cefaclor) > the first generation (cefazolin, cefadroxil and cefradine), which was consistent with their antibacterial ability.25
| Drug | T K | K D L mol−1 | n |
|---|---|---|---|
| Cefradine | 288 | 5.49 × 102 | 0.67 |
| 298 | 8.05 × 102 | 0.68 | |
| Cefadroxil | 288 | 7.81 × 102 | 0.65 |
| 298 | 1.23 × 103 | 0.68 | |
| Cefazolin | 288 | 1.78 × 103 | 0.70 |
| 298 | 5.59 × 103 | 0.78 | |
| Cefaclor | 288 | 3.79 × 103 | 0.76 |
| 298 | 4.49 × 103 | 0.80 | |
| Cefuroxime | 288 | 4.25 × 103 | 0.75 |
| 298 | 9.50 × 103 | 0.81 | |
| Cefotaxime | 288 | 1.37 × 104 | 0.85 |
| 298 | 2.65 × 104 | 0.90 | |
| Ceftriaxone | 288 | 5.75 × 104 | 0.89 |
| 298 | 1.05 × 105 | 0.93 | |
| Cefoperazone | 288 | 1.06 × 105 | 1.00 |
| 298 | 4.41 × 105 | 1.10 |
Based on the above studies, the possible interaction mechanism of luminol–CAT–cephalosporins was described in Fig. 2 and discussed as follows: it was hypothesized that luminol bound to the heme group of CAT, and the Fe(III) in the active center of CAT accelerated the electron transfer rate of excited oxidation product of luminol giving the enhancement of CL intensity of luminol. In the presence of cephalosporins, it might be that cephalosporins interacted with both His74 and Asn147 in the hydrophobic cavity of CAT with hydrophobic interaction force forming 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes online, led to a conformational change of CAT, blocked the interaction of CAT with luminol and resulted in the CL intensity of luminol–CAT being quenched.
1 complexes online, led to a conformational change of CAT, blocked the interaction of CAT with luminol and resulted in the CL intensity of luminol–CAT being quenched.
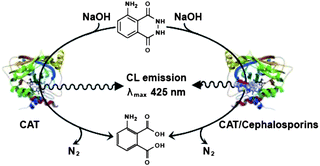 | ||
| Fig. 2 CL mechanism of luminol–CAT–cephalosporins. | ||
In this work, the proposed method was also applied to the determination of cefradine in human urine samples following the procedure described in ESI†. A healthy male volunteer ingested cefradine capsules (500 mg) orally with an empty stomach after an overnight fast. From then on, first-voided urine samples were collected in brown calibrated flasks after 0.5, 1, 2, 3, 4, 5, 6, 7 and 8 hours, respectively. The collected urine samples from the volunteer were diluted with purified water directly and sometimes supplemented with cefradine to test the recovery of the method. The results are summarized in Table S4, ESI†. It could be seen that the total excreted cefradine through urine was 397.8 mg in a total volume of 725 mL in 8 hours with the total cefradine excretive ratio of 79.6% and recoveries ranging from 90.0 to 106.7%.
By FI–CL, the thermodynamic parameters of protein interacting with drug could be obtained,14 which is consistent with the results obtained by fluorescence.26 In this work, with the binding constants KD of CAT–cephalosporins at different temperatures in Table 1, and using the Van't Hoff equation,27 the enthalpy change (ΔH°), entropy change (ΔS°) and free energy change (ΔG°) were obtained and are listed in Table 2. It is found that the signs for ΔG° and ΔH° are negative and positive, respectively, which means that the binding process is spontaneous and the formation of CAT–cephalosporins complex is an endothermic reaction. The positive ΔH° and ΔS° reveal that the hydrophobic interaction force plays a major role in the binding of cephalosporins to CAT. It is also found that the absolute value of ΔG° increases with the increase of cephalosporins generation, which is consistent with the antibacterial ability of cephalosporins.25
| Drug | T K | ΔH° kJ mol−1 | ΔS° J mol−1K−1 | ΔG° kJ mol−1 |
|---|---|---|---|---|
| Cefradine | 288 | 27.31 | 147.27 | −15.10 |
| 298 | −16.58 | |||
| Cefadroxil | 288 | 32.64 | 168.71 | −15.95 |
| 298 | −17.64 | |||
| Cefazolin | 288 | 81.68 | 345.84 | −17.92 |
| 298 | −21.38 | |||
| Cefaclor | 288 | 48.66 | 237.47 | −19.73 |
| 298 | −22.10 | |||
| Cefuroxime | 288 | 57.44 | 268.91 | −20.00 |
| 298 | −22.69 | |||
| Cefotaxime | 288 | 46.91 | 242.07 | −22.81 |
| 298 | −25.23 | |||
| Ceftriaxone | 288 | 43.39 | 241.78 | −26.24 |
| 298 | −28.66 | |||
| Cefoperazone | 288 | 101.68 | 449.29 | −27.71 |
| 298 | −32.20 |
Using luminol as luminescence probe, the interaction behavior of CAT–cephalosporins were studied by FI–CL method for the first time, giving the binding parameters of CAT with cephalosporins. The binding ability of the studied cephalosporins to CAT are consistent with their antibacterial ability, which offered the possibility for speculating drug action with the simple, reliable and automatic FI–CL method.
The authors gratefully acknowledge Prof. Hao Wu (Department of Biochemistry, Weill Medical College, Cornell University) for helpful assistance in fluorescence measurements. This work was supported by the Shaanxi Province Nature Science Foundation (No. 2006B05), the Northwest University (NWU) Graduate Innovation and Creativity Funds (No. 09YZZ45 and 10YZZ29) and NWU Graduate Experimental Research Funds (No. 09YSY18), China.
Notes and references
- X. M. Liu, S. Y. Li, J. S. Zhang and X. G. Chen, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2009, 877, 3144 Search PubMed.
- Q. Xu, D. D. Deng, Z. J. Cao, Q. Xie, J. Y. Liang and J. Z. Lu, Chin. J. Anal. Chem., 2010, 38, 483 Search PubMed.
- J. Z. Wang, J. X. He and C. Q. Jiang, Chin. J. Anal. Chem., 2001, 29, 782 Search PubMed.
- P. Taboada, Y. Fernández and V. Mosquera, Biomacromolecules, 2004, 5, 2201 Search PubMed.
- Y. Teng, H. Zhang and R. T. Liu, Mol. BioSyst., 2011, 7, 3157 RSC.
- F. Ding, G. Y. Zhao, J. L. Huang, Y. Sun and L. Zhang, Eur. J. Med. Chem., 2009, 44, 4083 CrossRef CAS.
- W. A. Schroeder, J. R. Shelton, J. B. Shelton, B. Robberson, G. Apell, R. S. Fang and J. Bonaventura, Arch. Biochem. Biophys., 1982, 214, 397 Search PubMed.
- M. R. N. Murthy, T. J. Reid, A. Sicignano, N. Tanaka and M. G. Rossmann, J. Mol. Biol., 1981, 152, 465 CAS.
- T. J. Reid III, M. R. N. Murthy, A. Sicignano, N. Tanaka, W. D. L. Musick and M. G. Rossmann, Proc. Natl. Acad. Sci. U. S. A., 1981, 78, 4767 Search PubMed.
- J. Durner and D. F. Klessig, Proc. Natl. Acad. Sci. U. S. A., 1995, 92, 11312 Search PubMed.
- R. J. Feuers, F. M. Pattillo, C. K. Osborn, K. L. Adams, D. de Luca and W. G. Smith, Free Radical Biol. Med., 1993, 15, 223 Search PubMed.
- N. Purwar, J. M. McGarry, J. Kostera, A. A. Pacheco and M. Schmidt, Biochemistry, 2011, 50, 4491 Search PubMed.
- D. Lia, B. Jia and J. Jin, J. Lumin., 2008, 128, 1399 CrossRef CAS.
- Z. M. Wang and Z. H. Song, Analyst, 2010, 135, 2546 RSC.
- S. R. El-Shaboury, G. A. Saleh, F. A. Mohamed and A. H. Rageh, J. Pharm. Biomed. Anal., 2007, 45, 1 CrossRef CAS.
- C. Lu, N. Zhang, J. G. Li and Q. Q. Li, Talanta, 2010, 81, 698 CrossRef CAS.
- V. Ródenas, M. S. García, C. Sànchez-Pedreño and M. I. Albero, J. Pharm. Biomed. Anal., 1997, 15, 1687 CrossRef CAS.
- M. A. Omar, O. H. Abdelmageed and T. Z. Attia, Talanta, 2009, 77, 1394 CrossRef CAS.
- X. M. Chong, C. Q. Hu, Y. C. Feng and H. H. Pang, Vib. Spectrosc., 2009, 49, 196 CrossRef CAS.
- V. M. Johnson, J. P. Allanson and R. C. Causon, J. Chromatogr., B: Biomed. Sci. Appl., 2000, 740, 71 CrossRef CAS.
- S. Choi, J. Ryu, H. Lee, M. Lee, J. Seo, S. Tak and K. Lee, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2009, 877, 4059 CrossRef CAS.
- A. R. Solangi, S. Q. Memon, M. Y. Khuhawar and M. I. Bhanger, Acta Chromatogr., 2007, 19, 81 CAS.
- W. Liu, Z. J. Zhang and Z. Q. Liu, Anal. Chim. Acta, 2007, 592, 187 CrossRef CAS.
- J. R. Lakowicz and G. Weber, Biochemistry, 1973, 12, 4161 CrossRef CAS.
- T. M. Speight, R. N. Brogden and G. S. Avery, Drugs, 1972, 3, 9 Search PubMed.
- Z. M. Wang, X. J. Tan, D. H. Chen, Q. L. Yue and Z. H. Song, J. Fluoresc., 2009, 19, 801 CrossRef CAS.
- P. D. Ross and S. Subramanian, Biochemistry, 1981, 20, 3096 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Details of experimental information and determination results. See DOI: 10.1039/c2ay05642a |
| ‡ This article is part of a themed issue on Pharmaceutical Analysis. |
| This journal is © The Royal Society of Chemistry 2012 |
