Detection of native chondroitin sulfate impurities in heparin sodium with a colorimetric micro-plate based assay†‡
Abstract
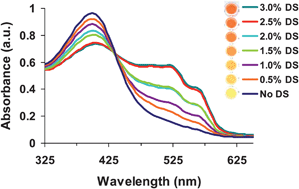
We have recently described a 96-well plate format assay for visually detecting oversulfated chondroitin sulfate A (OSCS) in heparin that uses a water soluble cationic polythiophene polymer (3-(2-(N-(N′-methylimidazole))ethoxy)-4-methylthiophene (LPTP)) and heparinase digestion. Because dermatan sulfate (DS, a.k.a. chondroitin sulfate B (CSB)) and, to a lesser degree, chondroitin sulfate A (CSA) are the most common native impurities of heparin active pharmaceutical ingredients (APIs) we adapted the previously established micro-plate assay for the visual detection of DS and CSA in heparin sodium. We describe a naked-eye detection test of these common heparin impurities with sensitivity appropriate for the United States Pharmacopeia (USP) 1% percent galactosamine in total hexosamine specification. For example, the test detects DS ≥ 1.0% w/w visually and 0.5% using a plate reader, or CSA ≥ 1.0 to 2.0% w/w visually and at ≥1.0% with a plate reader. In contrast to the test developed for oversulfated glycosaminoglycans such as OSCS, the LPTP–chondroitinase test developed here utilizes chondroitinase ABC instead of heparinases and requires a centrifugal filtration step to remove un-digested heparin. The test takes advantage of the sensitivity of the LPTP chemosensor to low molecular weight glycosaminoglycan (GAG) fragments formed by digesting DS or CSA. Importantly, the LPTP–chondroitinase method detects DS and CSA in heparin sodium in a format potentially amenable to high throughput screening.
- This article is part of the themed collection: Pharmaceutical Analysis

 Please wait while we load your content...
Please wait while we load your content...