A facile transport assay for H+ coupled membrane transport using fluorescence probes†
Wanjun
Lan
a,
Hongliu
Ren
a,
Yu
Pang
a,
Chuseng
Huang
a,
Yufang
Xu
a,
Robert J.
Brooker
*b and
Jingyan
Zhang
*a
aState Key Laboratory of Bioreactor Engineering, School of Pharmacy, East China University of Science and Technology, Shanghai, 200237. E-mail: jyzhang@ecust.edu.cn; Fax: +86-021-64253846
bDepartment of Genetics, Cell Biology and Development, University of Minnesota, Minneapolis, MN 55455, USA. E-mail: brook005@umn.edu
First published on 14th November 2011
Abstract
A facile activity assay for an H+-coupled transporter using florescent probes was developed with an H+-coupled manganese transporter (MntH) as a model. Making use of coupled-proton transport, the transport activity (H+/Mn2+ cotransport) can be directly determined via fluorescence intensity changes of the probe, 5-(and-6)-carboxyfluorescein (5(6)-FAM). The approach of using highly sensitive fluorescence probes provides a more simple and convenient assay method for the determination of proton-coupled metal-ion uptake by transporters.
Nramp family members transport divalent metal cations, such as Fe2+ and Mn2+, into the cell coupled with the movement of protons.1–4 Such transporters exhibit essential functions in bacteria and humans,5 including dietary iron uptake,6,7immune response to pathogenic bacteria,3,4 and virulence of pathogenic bacteria.8,9 Because of their vital functions, much research concerning their structure and transport activity has been carried out.
The method of detecting metal ion uptake of these transporters is a critical aspect of transporters identification, purification, and function. A variety of biochemical and biophysical methods have been developed. Among them, radioisotope uptake assay is a commonly used method. For example, the activity of divalent cation transporter (DCT1), encoded by the gene Nramp2, was determined by radioactive uptake assays using 55Fe2+, 54Mn2+, 60Co2+, 65Zn2+, and 109Cd2+.5,10–13 The radioactive uptake method is also used to study many other metal ions transporters, but precautions and special protective procedures have to be taken because of radioactive emissions. In addition, the amount of metal ions transported across the membrane is relatively small compared with the metal ions that are absorbed or bound to the membrane surface. This can lead to a large error in measurement, which may undermine a precise determination of the metal ion transported.14
Fluorescence probes have been used to assay metal ion uptake for several transporters.15–17 For instance, the fluorescent dye calcein has been employed to assay the transport of Fe2+ and other divalent transition metal ions across the intestinal brush border membrane vesicles. The fluorescence of calcein is rapidly and stoichiometrically quenched by the formation of metal ion-calcein complexes, thus can be used to monitor the transport process.14,15 However, because calcein is a membrane impermeable fluorescent dye, it can be used only in the reconstituted vesicles in which it first is trapped inside the vesicles during reconstitution. pH-sensitive mutants of green fluorescent protein (‘pHluorins’) have also been employed to exploit the acidic pH inside secretory vesicles by linking it to a vesicle membrane protein.18 Similarly, a pH-sensitive derivative of the green fluorescent protein, designated ratiometric GFP, was used to measure intracellular fluorescence intensity of the probe in both gram-positive and gram-negative bacteria cells. The fluorescence intensity of the probe was then used to calculate the intracellular concentration of protons or the pH value based on a calibration curve.19 For some metal transporters, ICP-MS measurements have been employed to determine intracellular metal ion concentration.17
Herein, we describe a facile activity assay method for H+-coupled transport using an H+-coupled manganese transporter (MntH),20 which is a member of the Nramp family, as an example. MntH transports manganese ions as its preferred substrate. Because it is a proton-coupled transporter, fluorescent probes that are sensitive to pH changes can be used to indirectly measure the influx of Mn2+ by MntH. The high sensitivity of fluorescent probes and their structural diversities render the method applicable to many H+-coupled transport proteins.
E.coli. cells carrying an IPTG-inducible mntHgene on a plasmid were grown in YT media supplemented with chloramphenicol (25 μg mL−1), incubated for 3 h at 37 °C, and then induced with 0.25 M IPTG at mid-log phase for more than 2h.21 The cells were centrifuged at 7000 xg for 5 min and the cell pellet was washed with 50 mM, pH 6.5 potassium phosphate buffer and stored at −20 °C. Prior to the activity assay, the pH sensitivity of two fluorescence probes, 5(6)-FAM (probe I) and fluorescein derivative (probe II) (see structures in Figure S1†) was measured at their sensitive pH range of 5.5–7.5. Probe I contains a carboxylic acid that can be used to react with primary aminesviacarbodiimide activation of the carboxylic acid. The cell-impermeated Probe I can also be used as a nonfixable polar tracer to investigate fusion, lyses and gap-junctional communication and to detect changes in cell or liposome volume.22Fluorescein is the most common fluorescent derivatization reagent for labeling biomolecules due to its relatively high absorptivity, excellent fluorescence quantum yield, and good water solubility.23 The plots of pH versus fluorescence intensity of the two fluorescent probes (Probe I and Probe II) were used as standard curves. The linear standard curves of Probe I and Probe II indicate that both probes are pH sensitive. The higher slope of the plot for Probe I indicates that it is more sensitive than that of Probe II; hence only the results using the Probe I are presented. In addition, probe I is cell impermeable.24 Thus, the fluorescence intensity of probe I solely reflects the change of the pH of the extracellular environment. The maximal activity of Escherichia coli MntH is in the pH range of pH 5.5 to 6.5. Combining the property of the Probe I and the active pH range of MntH, we conducted all experiments at pH 6.5. The relative Fe2+ and Co2+ transport activity of Nramp2 and Mn2+ transport of MntH were all performed at pH 6.0.21,25,26
To examine the effects of cells on florescence intensity of the Probe I, Probe I was first incubated with the cell mixture in pH 6.5 potassium phosphate buffer for different times as shown in Figure S2a.† The fluorescence intensity of the samples first decreased and then increased suggesting that an interaction between probe I and the cell surfaces may occur. To avoid the interference of cell, the cells were removed by centrifugation, the fluorescence of the supernatant was measured. The fluorescence intensity of the supernatant remained the same in different incubation times as shown in Figure S2b.† Most of the measurements described below were done in this manner.
To verify that fluorescence changes induced by Mn2+ addition are due to H+ uptake into the cell, the activity of wild-type MntH was compared to the strain BL21, which lacks a functional mntHgene. In the experiment of Fig. 1a, the fluorescence intensity of the supernatants was determined for the wild-type strain in the presence and absence of Mn2+. Cells expressing wild-type MntH were resuspended in the phosphate buffer at OD600 of 1.5 and equilibrated with Probe I at 37 °C for 10 min before 0.4 μM Mn2+ was added. Fig. 1a indicates that the fluorescence intensity of the sample with Mn2+ is clearly higher than the sample without Mn2+. The higher fluorescence intensity indicates a higher extracellular pH, which is the result of the transport of protons by MntH into the cell. The results clearly suggest that the cells expressing wild-type MntH can transport Mn2+ with H+. As expected, no change is observed in the presence of Mn2+ ions for strain BL21 (Fig. 1b).
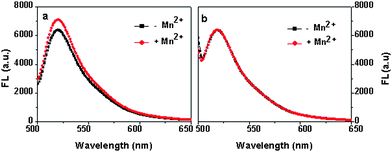 | ||
| Fig. 1 a) The uptake of Mn2+ by wild type (WT) and b) the strain without an mntHgene. The samples were incubated at 37 °C for 3 min. Mn2+ concnetration is 0.3 μM, the data was an average of triplits. | ||
The changes of fluorescence intensity of Probe I is also possibly related to the Mn2+ concentration in the cell suspension. Thus, the transport activity of MntH at different external Mn2+ concentrations (0–125 μM) was measured with the wild type strain. Fig. 2 depicts the fluorescence intensity of the cell suspension changes versus different Mn2+ concentrations. The control experiment of Mn2+ ions alone with Probe I was carried out too. The fluorescence intensity change of the control sample is negligible (black line in Fig. 2) compared with that of the MntH strain (red line in Fig. 2). The fluorescence intensity of the samples reached a plateau when the concentration of Mn2+ was 0.6 μM indicating that Mn2+ accumulation had reached an intracellular saturation level. According to this result, when the FL intensity reached the half of the maximum value, the corresponding Mn2+ concentration was 0.25 μM. The Km, roughly is 0.25 μM, which is similar to the reported data.21
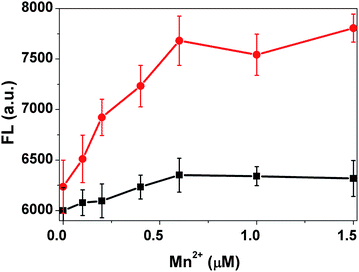 | ||
| Fig. 2 The transport activity of the MntH strain measured at different Mn2+ concentrations (red), and the buffer solution in the absence of the cells under the same condition as a control (black). | ||
The time course of the transport process was also investigated to further evaluate the current method. Fig. 3 shows the fluorescent intensity changes and pH changes induced by Mn2+ uptake of wild-type MntH and mutant D34N cells in the presence of Mn2+ ions and Probe I as a function of the incubation time. The fluorescence intensity of the WT cells increased with the incubation time, and reached equilibrium at ∼3 min under these conditions. In such a short time, Mn2+ efflux should not affect the assay.27 This is consistent with the literature, where the transport activity was measured using radioisotope uptake methods.21,27–31 While for mutant D34N that has been shown with very low activity,21 the fluorescent intensity is almost constant as function of time. These results indicate that the transport process can be reliably monitored over time using this method.
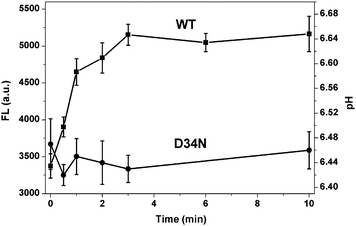 | ||
| Fig. 3 The fluorescent intensity changes and pH changes induced by Mn2+ uptake of WT strain and mutant D34N strain in the presence of Mn2+ ions and Probe I as a function of the incubation time. Mn2+ concnetration is 0.3 μM. | ||
Based on the secondary topology model of the MntH and site-directed mutagenesis experiments, five highly conserved acidic residues (Asp34, Asp109, Asp238, Glu102, and Glu112) were reported to be of importance for MntH function including Mn2+ binding, MntH conformational changes associated with metal binding, or maintaining the tertiary structure of MntH.21 Thus, as a validation of the current method, the activities of the above five mutant strains were determined using the current protocol. Figure S3 compares the activities of these mutants with the wild-type cells measured as a function of the incubation time. The wild-type strain exhibited the highest transport activity. The E154Q strain showed about half of the wild-type activity. The E102Q and E112Q strains exhibit a relatively low activity, while other mutants are inactive, and their fluorescence intensity was indistinguishable from the vector control strain. These results are in good agreement with the previous results obtained in radioisotope uptake studies.21 However, mutant E102Q, which was found to be inactive in a radioisotope uptake study, showed low activity using the current assay method.21
Although MntH is an H+/Mn2+ cotransporter, the stoichiometry between H+ and Mn2+ cotransport is not well-defined. For DCT1 transport, the uptake of protons and metal ions is “loosely coupled” because the transport process exhibits a variable stoichiometry at different pH values.5,32,33 It has been shown that at neutral pH DCT1 cotransports Fe2+ together with H+ with a stoichiometry of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1,5 while at a low pH, the number of H+ ions transported with one Fe2+ ion increased to ∼10 to 18.5,11,32 Thus it was suggested that metal transport was induced by the proton gradient, but proton slippage occurred at acidic pH.5,11,32,34,35 In the work described here, the fluorescence intensity changes in solution were used to determine proton concentration or pH value by comparison to a standard calibration curve. In principle, assuming the Mn2+ ions added are all transported, the Mn2+/H+ ratio for MntH can be determined. However, under a constant cell density, the determined concentration change of H+ resulted from MntH transport was much lower than that of Mn2+ concentration change determined by elemental analysis (as shown in Table S1†). This could be caused by the nonspecific binding of Mn2+ on the cell surface, and this binding also is related to the cell density concentration and pH of the solution.
1,5 while at a low pH, the number of H+ ions transported with one Fe2+ ion increased to ∼10 to 18.5,11,32 Thus it was suggested that metal transport was induced by the proton gradient, but proton slippage occurred at acidic pH.5,11,32,34,35 In the work described here, the fluorescence intensity changes in solution were used to determine proton concentration or pH value by comparison to a standard calibration curve. In principle, assuming the Mn2+ ions added are all transported, the Mn2+/H+ ratio for MntH can be determined. However, under a constant cell density, the determined concentration change of H+ resulted from MntH transport was much lower than that of Mn2+ concentration change determined by elemental analysis (as shown in Table S1†). This could be caused by the nonspecific binding of Mn2+ on the cell surface, and this binding also is related to the cell density concentration and pH of the solution.
In summary, we have developed a facile method of determining the activity of an H+ coupled metal transporter using fluorescence probes. The method is an effective tool to directly detect the Mn2+ transport activity in cells. The method should find applications in other H+ coupled transporters, and could provide a versatile tool for studying the transport of Mn2+ and other transition metals into cells.
Acknowledgements
This work was supported by National Science Foundation of China (No. 30870561, and 31070742), the State key laboratory of bioreactor engineering (No. 2060204), and 111 Project (No. B07023).References
- D. Radisky and J. Kaplan, J. Biol. Chem., 1999, 274, 4481 CrossRef CAS.
- Y. Nevo and N. Nelson, Biochim. Biophys. Acta, Mol. Cell Res., 2006, 1763, 609 CrossRef CAS.
- N. Nelson, EMBO J., 1999, 18, 4361 CrossRef CAS.
- G. Govoni and P. Gros, Inflammation Res., 1998, 47, 277 CrossRef CAS.
- H. Gunshin, B. Mackenzie, U. V. Berger, Y. Gunshin, M. F. Romero, W. F. Boron, S. Nussberger, J. L. Gollan and M. A. Hediger, Nature, 1997, 388, 482 CrossRef CAS.
- M. D. Fleming, C. C. Trenor, M. A. Su, D. Foernzler, D. R. Beier, W. F. Dietrich and N. C. Andrews, Nat Genet, 1997, 16, 383 CAS.
- J. C. Fleet, Nutr. Rev., 1998, 56, 88 CrossRef CAS.
- K. M. Papp-Wallace and M. E. Maguire, Annu. Rev. Microbiol., 2006, 60, 187 CrossRef CAS.
- E. S. Anderson, J. T. Paulley, J. M. Gaines, M. W. Valderas, D. W. Martin, E. Menscher, T. D. Brown, C. S. Burns and R. M. Roop, Infect. Immun., 2009, 77, 3466 CrossRef CAS.
- Y. Nevo and N. Nelson, J. Biol. Chem., 2004, 279, 53056 CrossRef CAS.
- A. Sacher, A. Cohen and N. Nelson, Journal of Experimental Biology, 2001, 204, 1053 CAS.
- P. Marciani, D. Trotti, M. A. Hediger and G. Monticelli, J. Membr. Biol., 2004, 197, 91 CrossRef CAS.
- M. Okubo, K. Yamada, M. Hosoyamada, T. Shibasaki and H. Endou, Toxicol. Appl. Pharmacol., 2003, 187, 162 CrossRef CAS.
- M. Knöpfel, G. Schulthess, F. Funk and H. Hauser, Biophys. J., 2000, 79, 874 CrossRef.
- W. Breuer, S. Epsztejn, P. Millgram and I. Z. Cabantchik, Am J Physiol Cell Physiol, 1995, 268, C1354 CAS.
- P. Courville, R. Chaloupka, F. Veyrier and M. F. M. Cellier, J. Biol. Chem., 2004, 279, 3318 CrossRef CAS.
- R. Chaloupka, P. Courville, F. Veyrier, B. Knudsen, T. A. Tompkins and M. F. M. Cellier, Biochemistry, 2004, 44, 726 CrossRef.
- G. Miesenbock, D. A. De Angelis and J. E. Rothman, Nature, 1998, 394, 192 CrossRef CAS.
- K. N. Olsen, B. B. Budde, H. Siegumfeldt, K. B. Rechinger, M. Jakobsen and H. Ingmer, Appl. Environ. Microbiol., 2002, 68, 4145 CrossRef CAS.
- V. V. Bartsevich and H. B. Pakrasi, J. Biol. Chem., 1996, 271, 26057 CrossRef CAS.
- H. A. H. Haemig and R. J. Brooker, J. Membr. Biol., 2004, 201, 97 CrossRef CAS.
- Ana J. Pérez-Berná, Jaime Guillén, Miguel R. Moreno, Angela Bernabeu, Georg Pabst, P. Laggner and J. Villalaín, J. Biol. Chem., 2006, 283, 8089 CrossRef.
- Lindsay E. Yandek, Antje Pokorny, Anders Florén, Kristina Knoelke, Ülo Langel and P. F. F. Almeida, Biophys. J., 2007, 92, 2434 CrossRef CAS.
- L. S. De Clerck, C. H. Bridts, A. M. Mertens, M. M. Moens and W. J. Stevens, J. Immunol. Methods, 1994, 172, 115 CrossRef CAS.
- S. Lam-Yuk-Tseung, G. Govoni, J. Forbes and P. Gros, Blood, 2003, 101, 3699 CrossRef CAS.
- D. Agranoff, I. M. Monahan, J. A. Mangan, P. D. Butcher and S. Krishna, J. Exp. Med., 1999, 190, 717 CrossRef CAS.
- D. G. Kehres, M. L. Zaharik, B. B. Finlay and M. E. Maguire, Mol. Microbiol., 2000, 36, 1085 CrossRef CAS.
- H. A. H. Haemig, P. J. Moen and R. J. Brooker, Biochemistry, 2010, 49, 4662 CrossRef CAS.
- H. Makui, E. Roig, S. T. Cole, J. D. Helmann, P. Gros and M. F. Cellier, Mol. Microbiol., 2000, 35, 1065 CrossRef CAS.
- S. Silver, P. Johnseine and K. King, J. Bacteriol, 1970, 104, 1299 CAS.
- I. Reeve, D. Hummell, N. Nelson and J. Voss, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 8608 CrossRef CAS.
- X.-Z. Chen, J.-B. Peng, A. Cohen, H. Nelson, N. Nelson and M. A. Hediger, J. Biol. Chem., 1999, 274, 35089 CrossRef CAS.
- B. Mackenzie, M. Ujwal, M.-H. Chang, M. Romero and M. Hediger, Pfluegers Arch., 2006, 451, 544 CrossRef CAS.
- D. I. Bannon, R. Abounader, P. S. J. Lees and J. P. Bressler, American Journal of Physiology-Cell Physiology, 2003, 284, C44 CAS.
- S. Tandy, M. Williams, A. Leggett, M. Lopez-Jimenez, M. Dedes, B. Ramesh, S. K. Srai and P. Sharp, J. Biol. Chem., 2000, 275, 1023 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1ay05549f |
| This journal is © The Royal Society of Chemistry 2012 |
