Fabrication of a sensor for simultaneous determination of norepinephrine, acetaminophen and tryptophan using a modified carbon nanotube paste electrode
Mohammad Reza
Akhgar
a,
Hadi
Beitollahi
b,
Mohammad
Salari
a,
Hassan
Karimi-Maleh
*c and
Hassan
Zamani
d
aDepartment of Chemistry, Faculty of Science, Islamic Azad University, Kerman Branch, Kerman, Iran
bEnvironment Department, Research Institute of Environmental Sciences, International Center for Science, High Technology & Environmental Sciences, Kerman, Iran
cDepartment of Chemistry, Majlesi Branch, Islamic Azad University, Isfahan, Iran
dFaculty of Science, University of Applied Science and Technology, Isfahan, Iran
First published on 28th November 2011
Abstract
A carbon paste electrode (CPE) was modified by incorporation of carbon nanotubes and ferrocene (FC) and studied in pH 7.0 phosphate buffer solution (PBS) by cyclic voltammetry (CV). The modified electrode showed an excellent electrocatalytic effect on the oxidation of norepinephrine (NE). In PBS of pH 7.0, the oxidation current increased linearly with concentration of NE from 0.47 to 500.0 μmol L−1. The detection limit (3σ) obtained by differential pulse voltammetry (DPV) was 0.21 μmol L−1. Then the modified electrode was used to determine NE in an excess of acetaminophen (AC) and tryptophan (TRP) by DPV. Finally, this method was used for the determination of NE, AC and TRP in some real samples.
1. Introduction
Since the discovery of carbon nanotubes (CNTs) in 1991,1 they have received considerable attention in the fields of biotechnology and medicine due to their unique optical, magnetic, electronic and chemical properties, which differ greatly from those of the bulk material.2,3 There are four main advantages to the use of a nanotubes-modified electrode compared with a macroelectrode: high effective surface area, mass transfer, catalysis and control over the local microenvironment.4–7 The catalytic properties of some nanotubes can cause a decrease in the overpotential, producing a more reversible voltammetry than that displayed by the same material in a macroelectrode form. In addition, CNTs can effectively promote electron-transfer reactions. The better performance of the CNTs electrode compared to carbon electrodes may be due to the carbon nanotube dimensions, the electronic structure, and the topologic defects present on the tube surface.8–11NE is one of the derivatives of cathecholamines secreted in the adrenal medulla and plays important physiological roles in the central nervous system. It affects muscle and tissue control, stimulates arteriole contraction, decreases peripheral circulation and activates lipolysis in adipose tissue.12 It is also critical in mental disease, heart failure, DNA breaks in cardiac myoblast cells, and diabetes. Recent reports have indicated that NE enhances adhesion of human immunodeficiency virus-1 (HIV-1)-infected leukocytes to cardiac microvascular endothelial cells and also accelerates HIV replication via proteinkinase.13 Hence, it is very necessary to develop sensitive, selective and reliable methods for the direct determination of trace NE due to its physiological function and the diagnosis of some diseases in clinical medicine. Various methods including spectrophotometry, capillary electrophoresis and high-performance liquid chromatography (HPLC) have been employed to the determination of NE. Because NE is an electroactive compound, its electrochemical detection has been the focus of research for electroanalytical researchers and neurochemists and some modified electrodes have been used to determine NE.14,15
AC is a widely used anti-pyretic and analgesic drug with actions similar to aspirin. It is an effective and safe agent for the relief of mild to moderate pain associated with headache, arthritis and postoperative pain. Its ready access has resulted in its increased use in attempted suicide.16 Hence, it is very important to establish a rapid, sensitive and reliable method for the determination of AC. Current methods for the analysis of AC include spectrophotometric, chromatographic, mass spectrometry and electrochemical approaches.16–20
TRP is an essential amino acid for human and herbivores but is scarcely present in vegetable products. It is sometimes added to dietary and food products as a food fortifier and to pharmaceutical formulations in order to correct possible dietary deficiencies. The analysis of TRP is of great importance in the biochemical, pharmaceutical and dietetic fields as it is a precursor molecule of hormones, neurotransmitters and other relevant bimolecules.21 Methods for the determination of TRP are mainly based on HLPC and spectrophotometric procedures. Electroanalytical methods, with respect to their sensitivity, accuracy and simplicity, have been more of interest in recent years for TRP analysis.21–24
TRP is the essential amino acid that plays an integral role in the synthesis of the neurotransmitter serotonin (5-HT).25 AC administration is known to increase brain 5-HT levels because AC alters TRP metabolism by inhibiting tryptophan 2,3-dioxygenase (TDO) thus increasing the availability of TRP for the production of 5-HT26 and 5-HT is known to play a role in NE release in the brain.27,28 Therefore simultaneous determination of NE, AC and TRP is important.
In the present work, we described initially the preparation and suitability of a ferrocene modified carbon nanotube paste electrode (FCMCNPE) as a new electrode in the electrocatalysis and determination of NE in an aqueous buffer solution. Then we evaluated the analytical performance of the modified electrode in quantification of NE in the presence of AC and TRP. Finally, in order to demonstrate the catalytic ability of the modified electrode in the electrooxidation of NE, AC and TRP (Scheme 1) in real samples, we examined this method for the voltammetric determination of NE, AC and TRP in some real samples.
 | ||
| Scheme 1 Structures of norepinephrine, acetaminophen and tryptophan. | ||
2. Experimental
2.1. Apparatus and reagents
Voltammetric measurements were carried out using a computerized potentiostat/galvanostat Autolab (model PGSTAT 302 N, Eco Chemie B.V.A). The experiments were controlled with computer software of General Purpose Electrochemical System (GPES). All the electrochemical studies were performed at 25 ± 1 °C. An Ag/AgCl/KCl (3 mol L−1), a platinum wire, and a FCMCNPE were used as reference, auxiliary and working electrodes, respectively. A digital pH/mV meter (Metrohm model 710) was applied for pH measurements. NE, AC, TRP, graphite powder, paraffin oil and reagents were analytical grade from Merck. The buffer solutions were prepared from orthophosphoric acid and its salts in the pH range 2.0–12.0. Multiwalled carbon nanotubes (purity more than 95%) with o.d. between 10 and 20 nm, i.d. between 5 and 10 nm, and tube length from 10 to 30 μm were prepared from Nanostructured & Amorphous Materials, Inc.2.2. Preparation of the electrode
Modified carbon nanotube paste electrodes were prepared by dissolving 0.01 g of FC in diethyl ether and hand mixing with 89-times its weight of graphite powder and 10-times its weight of carbon nanotube with a mortar and pestle. The solvent was evaporated by stirring. A 70![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 30 (w/w) mixture of FC spiked carbon nanotube powder and paraffin oil was blended by hand mixing for 20 min until a uniformly-wetted paste was obtained. The paste was then packed into the end of a glass tube (ca. 3.4 mm i.d. and 8 cm long). Electrical contact was made by inserting a copper wire into the glass tube at the back of the mixture. When necessary, a new surface was obtained by pushing an excess of paste out of the tube and polishing it on a weighing paper.
30 (w/w) mixture of FC spiked carbon nanotube powder and paraffin oil was blended by hand mixing for 20 min until a uniformly-wetted paste was obtained. The paste was then packed into the end of a glass tube (ca. 3.4 mm i.d. and 8 cm long). Electrical contact was made by inserting a copper wire into the glass tube at the back of the mixture. When necessary, a new surface was obtained by pushing an excess of paste out of the tube and polishing it on a weighing paper.
For comparison, FC modified CPE (FCMCPE) without CNTs, CNTs paste electrode (CNPE) without FC, and unmodified CPE in the absence of both FC and CNTs were also prepared in the same way.
2.3. Procedure of real sample preparation
The NE injection was diluted 1000 times with water; then, a different volume of the diluted solution was transferred into a 10 mL volumetric flask and diluted to the mark with PBS (pH 7.0). The diluted sample was spiked with different amounts of NE, AC and TRP.Urine samples were stored in a refrigerator immediately after collection. Ten millilitres of the sample were centrifuged for 15 min at 2000 rpm. The supernatant was filtered out using a 0.45 μm filter. Then, a different volume of the solution was transferred into a 10 mL volumetric flask and diluted to the mark with PBS (pH 7.0). The diluted urine sample was spiked with different amounts of NE, AC and TRP.
3. Results and discussion
3.1. Electrochemistry of FMCMCNPE
Cyclic voltammetry was employed for investigation the electrochemical properties of FCMCNPE in a pure buffered aqueous solution (pH 7.0). The cyclic voltammogram exhibits an anodic and corresponding cathodic peaks with Epa = 0.37 V and Epc = 0.265 V vs.Ag/AgCl/KCl (3.0 mol L−1). The electrode capability for the generation of a reproducible surface was examined by cyclic voltammetric data obtained in optimum solution pH from five separately prepared FCMCNPEs. The calculated RSDs for various parameters were accepted as the criteria for a satisfactory surface reproducibility (1–4%). This degree of reproducibility is virtually the same as that expected for the renewal of an ordinary carbon paste surface.113.2. Electro-catalytic oxidation of NE
The electrochemical behavior of NE is dependent on the pH value of the aqueous solution, whereas the electrochemical properties of the Fc/Fc+ redox couple are independent of pH. Therefore, pH optimization of the solution seems to be necessary in order to obtain the electrocatalytic oxidation of NE. Thus the electrochemical behavior of NE was studied in 0.1 mol L−1PBS in different pH values (2.0 < pH < 12.0) at the surface of FCMCNPE by cyclic voltammetry. It was found that the electrocatalytic oxidation of NE at the surface of FCMCNPE was more favored under neutral conditions than in acidic or basic medium. This appears as a gradual growth in the anodic peak current and a simultaneous decrease in the cathodic peak current in the cyclic voltammograms drawn at the surface of FCMCNPE. The variation of Ipaversus the variation of pH was studied. The results showed that the anodic peak current value for electrooxidation of NE is high at a biological pH. Thus, the pH 7.0 was chosen as the optimum pH for electrocatalysis of NE oxidation at the surface of FCMCNPE.Fig. 1 depicts the CV responses for the electrochemical oxidation of 250.0 μmol L−1NE at unmodified CPE (curve b), CNPE (curve d), FCMCPE (curve e) and FCMCNPE (curve f). As it is seen, while the anodic peak potential for NE oxidation at the CNPE, and unmodified CPE are 380 and 450 mV, respectively, the corresponding potential at FCMCNPE and FCMCPE is ∼325 mV. These results indicate that the peak potential for NE oxidation at the FCMCNPE and FCMCPE electrodes shifts by ∼55 and 125 mV toward negative values compared to CNPE and unmodified CPE, respectively. However, FCMCNPE shows a much higher anodic peak current for the oxidation of NE compared to FCMCPE, indicating that the combination of CNTs and the mediator (FC) has significantly improved the performance of the electrode toward NE oxidation. In fact, FCMCNPE in the absence of NE exhibited a well-behaved redox reaction (Fig. 1, curve c) in 0.1 mol L−1PBS (pH 7.0). However, there was a drastic increase in the anodic peak current in the presence of 250.0 μmol L−1NE (curve f), which can be related to the strong electrocatalytic effect of the FCMCNPE towards this compound.29
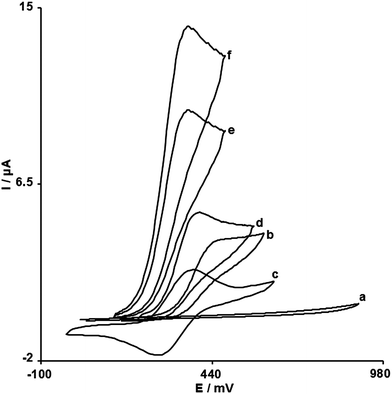 | ||
| Fig. 1 CVs of (a) unmodified CPE in 0.1 mol L−1PBS (pH 7.0) at scan rate of 10 mV s−1; (b) as (a) + 250.0 μmol L−1NE; (c) as (a) at the surface of FCMCNPE; (d) as (b) at the surface of CNPE; (e) as (b) at the surface of FCMCPE; (f) as (b) at the surface of FCMCNPE. | ||
The effect of the scan rate on the electrocatalytic oxidation of 250.0 μmol L−1NE at the modified electrode was investigated by linear sweep voltammetry (Fig. 2). The oxidation peak potential shifts with increasing scan rates toward a more positive potential, confirming the kinetic limitation of the electrochemical reaction. Also, a plot of peak height (Ip) against square root of scan rate (v1/2), in the range of 10–60 mV s−1, was constructed, which was found to be linear, suggesting that at sufficient overpotential the process is diffusion rather than surface-controlled (Fig. 2A). A plot of the scan rate-normalized current (Ip/ν1/2) vs. scan rate (Fig. 2B) exhibits the characteristic shape typical of an EC process.29
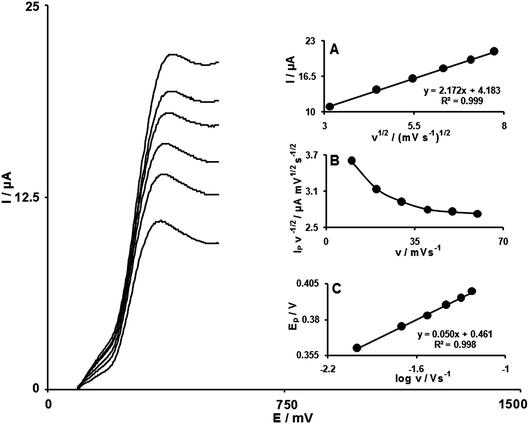 | ||
| Fig. 2 Linear sweep voltammograms of the FCMCNPE in the presence of 250 μmol L−1NE at various scan rates; from inner to outer correspond to: 10, 20, 30, 40, 50 and 60 mV s−1 scan rates, respectively. Variation of (A) anodic peak current vs. ν1/2; (B) normalized current (Ip/ν1/2) vs. ν; (C) anodic peak potential vs. log v. | ||
The Tafel slope (b) can be obtained from the slope of Epvs. log v using eqn (1):30
| Ep = b/2 log v + constant | (1) |
The Tafel slope was found to be 101.62 mV (Fig. 2, inset C), which indicates that a one-electron transfer process is the rate limiting step assuming a transfer coefficient (α) is about 0.42.
3.3. Chronoamperometric studies
Chronoamperometric measurements of NE at FCMCNPE were carried out by setting the working electrode potential at 0.4 V (at the first potential step) and at 0 V (at second potential step) vs.Ag/AgCl/KCl (3.0 mol L−1) for the various concentration of NE in buffered aqueous solutions (pH 7.0) (Fig. 3). For an electroactive material (NE in this case) with a diffusion coefficient of D, the current observed for the electrochemical reaction at the mass transport limited condition is described by the Cottrell equation.29 Experimental plots of I vs. t−1/2 were employed, with the best fits for different concentrations of NE (Fig. 3A). The slopes of the resulting straight lines were then plotted vs.NE concentration (Fig. 3B). From the resulting slope and Cottrell equation the mean value of D was found to be 5.4 × 10−6 cm2 s−1.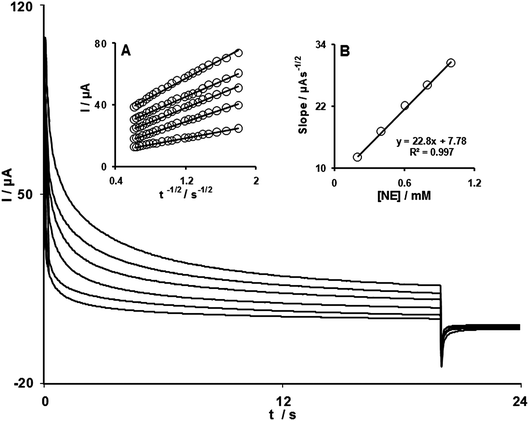 | ||
| Fig. 3 Chronoamperograms obtained at FCMCNPE in 0.1 mol L−1PBS (pH 7.0) for different concentrations of NE. From inner to outer corresponds to 0.0, 200.0, 400.0, 600.0, 800.0 and 1000.0 μmol L−1 of NE. Insets: (A) Plots of I vs. t−1/2 (B) Plot of the slope of the straight lines against NE concentration. | ||
Also, chronoamperometry can also be employed to evaluate the catalytic rate constant, k, for the reaction between NE and the FCMCNPE according to the method of Galus:31
| IC/IL = π1/2 γ1/2 = π1/2 (kCbt)1/2 | (2) |
3.4. Electrocatalytic determination of NE
The electrocatalytic peak current of NE oxidation at the surface of the modified electrode can be used for determination of NE in solution. Therefore, differential pulse voltammetry experiments were performed using the modified electrode in phosphate buffer solution containing various concentration of NE. The results show the electrocatalytic peak current of NE oxidation at the surface of the modified electrode was linearly dependent on the NE concentrations. The mediated oxidation peak currents of NE at the surface of a modified electrode were proportional to the concentration of the NE within the ranges 4.7 × 10−7 mol L−1– 5.0 × 10−4 mol L−1 (with a correlation coefficient of 0.9988) in differential pulse voltammetry (with modulation amplitude: 0.00195 V and scan rate of 10 mV s−1). The detection limit (3σ) was 2.1 × 10−7 mol L−1. These values are compared with values reported by other research groups for electrocatalytic oxidation of NE at the surface of chemically modified electrodes by other mediators (Table 1).| Electrode | Modifier | pH | Peak potential shift (mV) | Scan rate (mV s−1) | LOD (M) | LDR (M) | Ref. |
|---|---|---|---|---|---|---|---|
| Carbon paste | 3,4-dihydroxybenzaldehyde-2,4-dinitrophenylhydrazone | 7.0 | 215 | 10 | 7.7 × 10−8 | 1.0 × 10−7–8.0 × 10−4 | 32 |
| TiO2 nanoparticle -carbon paste | 2,2′-[1,2 buthanediylbis(nitriloethylidyne)]-bis-hydroquinone | 8.0 | 270 | 20 | 5.0 × 10−7 | 4.0 × 10−6–1.1 × 10−3 | 33 |
| Carbon paste | Chloranile | 7.0 | 340 | 20 | 1.12 × 10−8 | 3.0 × 10−8–5.0 × 10−4 | 34 |
| Carbon paste | Molybdenum(VI) complex | 7.0 | 125 | 10 | 4.3 × 10−8 | 8.0 × 10−8–7.0 × 10−4 | 35 |
| Carbon nanotube paste electrode | Ferrocene | 7.0 | 110 | 10 | 2.1 × 10−7 | 4.7 × 10−7–5.0 × 10−4 | This work |
3.5. Simultaneous determination of NE, AC and TRP at the surface of FCMCNPE
One of the main objectives of the present study was the development of a modified electrode capable of the electro-catalytic oxidation of NE and separation of the electrochemical responses of NE, AC and TRP. The utilization of the modified electrode for the simultaneous determination of NE, AC and TRP was demonstrated by simultaneously changing the concentrations of NE, AC and TRP. The differential pulse voltammetric results (with modulation amplitude: 0.00195 V and scan rate of 10 mV s−1), show three-well-defined anodic peaks (Fig. 4), while the bare carbon paste electrode only gave an overlapped and broad oxidation peak. Fig. 4 insets A, B and C show the dependence of differential pulse voltammetric peak currents on the concentration of NE, AC and TRP respectively.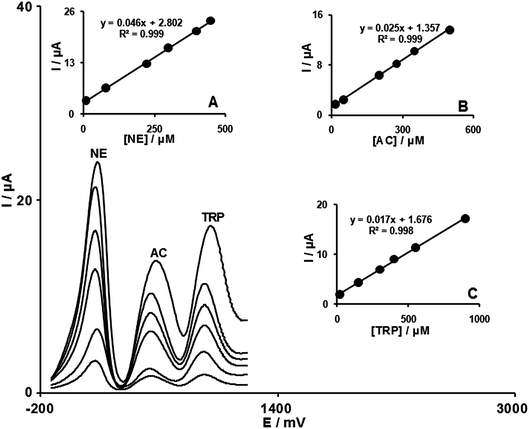 | ||
| Fig. 4 DPVs of FCMCNPE in 0.1 mol L−1PBS (pH 7.0) containing different concentrations of NE + AC + TRP in μmol L−1, from inner to outer: 8.0 + 15.0 + 20.0, 80.0 + 50.0 + 150.0, 225.0 + 200.0 + 300.0, 300.0 + 275.0 + 400.0, 400.0 + 350.0 + 550.0, and 450.0 + 500.0 + 900.0 respectively. Inset (A), plot of Ipvs.NE concentration. Also, (B) and (C) are plots of Ipvs. AC and TRP concentrations, respectively. | ||
The sensitivity of the modified electrode towards the oxidation of NE was found to be 0.046 μA μM−1. This is very close to the value obtained in the absence of AC and TRP (0.045 μA μM−1, see Section 3.4), indicating that the oxidation processes of these compounds at the FCMCNPE are independent and therefore, simultaneous determination of their mixtures is possible without significant interferences.
3.6. Real sample analysis
In order to evaluate the analytical applicability of the proposed method, it was applied to the determination of NE, AC and TRP in NE ampoule and urine samples. One millilitre of an NE ampoule was diluted to 10 mL with phosphate buffer solution (0.1 mol L−1, pH 7.0); then, a different capacity of the diluted solution was transferred into each of a series of 10 mL volumetric flasks and diluted to the mark with phosphate buffer. Each sample solution was transferred into the electrochemical cell and DPV was recorded between 0.0 and 0.6 V at a scan rate of 10 mV s−1. The Ipa was measured at the oxidation potential of NE and the concentration of this compound was obtained from the calibration plot. This procedure was repeated three times for each sample, and the average amount of NE in the injection was found to be 1.01 mg, a value in good agreement with the value on the ampoule label (1.0 mg). Also, to a series of 10 mL volumetric flasks, a different capacity of the diluted NE injection solution together with standard AC and TRP solutions were added and diluted to the mark with phosphate buffer. The DPVs were recorded and the anodic peak currents for each of NE, AC and TRP were measured at their own oxidation potentials. Also, the results for the determinations of NE, AC and TRP in urine samples are listed in Table 2. According to the results listed in Table 2, very good recoveries for the determinations of NE, AC and TRP were obtained with high reproducibility, which indicates that the sensor can be applied for the analysis of these compounds with no significant influence from each other.| Sample | Added (μmol L−1) | Found (μmol L−1) | Recovery (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| NE | AC | TRP | NE | AC | TRP | NE | AC | TRP | |
| a ND: Not detected. | |||||||||
| NE ampoule | 10.0 | 15.0 | 20.0 | 10.1 | 14.7 | 20.5 | 101.0 | 98.0 | 102.5 |
| 15.0 | 20.0 | 25.0 | 14.9 | 20.7 | 24.8 | 99.3 | 103.5 | 99.2 | |
| 20.0 | 25.0 | 30.0 | 20.6 | 24.9 | 29.6 | 103.0 | 99.6 | 98.7 | |
| Urine | 0 | 0 | 0 | NDa | ND | ND | — | — | — |
| 10.0 | 15.0 | 20.0 | 9.8 | 15.2 | 20.2 | 98.0 | 101.3 | 101.0 | |
| 15.0 | 20.0 | 25.0 | 15.5 | 19.9 | 24.6 | 103.3 | 99.5 | 98.4 | |
| 20.0 | 25.0 | 30.0 | 19.8 | 25.6 | 29.1 | 99.0 | 102.4 | 97.0 | |
4. Conclusion
In the present study, a carbon-paste electrode modified with carbon nanotubes and ferrocene (FC) was used for the determination of NE. The CV and DPV investigations showed effective electrocatalytic activity in lowering the anodic overpotential for NE. The high sensitivity and low detection limit (0.21 μmol L−1), together with the ease of preparation and surface regeneration of the modified electrode, are the advantages of the studied modified electrode. The modified electrode displays high selectivity in the voltammetric measurements of NE, AC and TRP in their mixture solutions.References
- S. Lijima, Nature, 1991, 354, 56 CrossRef.
- H. Pan, Y. W. Zhang, V. B. Shenoy and H. Gao, ACS Catal., 2011, 1, 99 CrossRef CAS.
- S. Komathi, A. I. Gopalan and K. P. Lee, Analyst, 2010, 135, 397 RSC.
- H. Beitollahi and I. Sheikhshoaie, Anal. Methods, 2011, 3, 1810 RSC.
- H. Beitollahi and I. Sheikhshoaie, Electrochim. Acta, 2011, 56, 10259 CrossRef CAS.
- S. M. Ghoreishi, M. Behpour, M. Khayatkashani and M. H. Motaghedifard, Anal. Methods, 2011, 3, 636 RSC.
- H. Beitollahi, J. B. Raoof and R. Hosseinzadeh, Talanta, 2011, 85, 2128 CrossRef CAS.
- W. F. Ribeiro, T. M. Guimarães Selva, I. C. Lopes, E. C. S. Coelho, S. G. Lemos, F. C. Abreu, V. B. Nascimento and M. C. U. Araújo, Anal. Methods, 2011, 3, 1202 RSC.
- H. Beitollahi, H. Karimi-Maleh and H. Khabazzadeh, Anal. Chem., 2008, 80, 9848 CrossRef CAS.
- S. H. Wu, F. H. Nie, Q. Z. Chen and J. J. Sun, Anal. Methods, 2010, 2, 1729 RSC.
- H. Beitollahi, J. B. Raoof and R. Hosseinzadeh, Electroanalysis, 2011, 23, 1934 CrossRef CAS.
- D. Voet and J. G. Voet, Biochemistry, 2nd ed.; Wiley: New York, 1995 Search PubMed.
- S. W. Cole, Y. D. Korin, J. L. Fahey and J. A. Zack, J. Immunol., 1998, 161, 610 CAS.
- H. Yaghoubian, V. Soltani-Nejad and S. Roodsaz, Int. J. Electrochem. Sci., 2010, 5, 1411 CAS.
- A. R. Taheri, A. Mohadesi, D. Afzali, H. Karimi-Maleh, H. Mahmoudi Moghaddam, H. Zamani and Z. rezayati zad, Int. J. Electrochem. Sci., 2011, 6, 171 CAS.
- M. Boopathi, M. S. Won and Y. B. Shim, Anal. Chim. Acta, 2004, 512, 191 CrossRef CAS.
- P. Fanjul-Bolado, P. J. Lamas-Ardisana, D. Hernández-Santos and A. Costa- García, Anal. Chim. Acta, 2009, 638, 133 CrossRef CAS.
- D. Sun and H. Zhang, Microchim. Acta, 2007, 158, 131 CrossRef CAS.
- S. F. Wang, F. Xie and R. F. Hu, Sens. Actuators, B, 2007, 123, 495 CrossRef.
- A. A. Ensafi, H. Karimi-Maleh, S. Mallakpour and M. Hatami, Sens. Actuators, B, 2011, 155, 464 CrossRef.
- G. Chen, J. S. Cheng, J. N. Ye and J. Fresenius, Fresenius J. Anal. Chem., 2001, 370, 930 CrossRef CAS.
- A. A. Ensafi, H. Karimi-Maleh and S. Mallakpour, Electroanalysis, 2011, 23, 1478 CrossRef CAS.
- S. Shahrokhian and L. Fotouhi, Sens. Actuators, B, 2007, 123, 942 CrossRef.
- A. A. Ensafi, S. Dadkhah-Tehrani and H. Karimi-Maleh, Anal. Sci., 2011, 27, 409 CrossRef CAS.
- Y. Mizano, K. Suzuki, N. Stone and T. Saitoh, Exp. Neurol., 2000, 166, 235 CrossRef.
- S. Daya and S. Anoopkumar-Dukie, Life Sci., 2000, 67, 235 CrossRef CAS.
- S. Xi-Ming Li, K. W. Perry and D. T. Wong, Neuropharmacology, 2002, 42, 181 CrossRef.
- H. Maharaj, D. S. Maharaj, K. S. Saravanan, K. P. Mohanakumar and S. Daya, Metab. Brain Dis., 2004, 19, 71 CrossRef CAS.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods: Fundamentals and Applications, 2nd ed., Wiley, New York, 2001 Search PubMed.
- J. A. Harrison and Z. A. Khan, J. Electroanal. Chem., 1970, 28, 131 CrossRef CAS.
- Z. Galus, Fundamentals of Electrochemical Analysis, Ellis Horwood, New York, 1976 Search PubMed.
- M. Mazloum-Ardakani, H. Beitollahi, M. K. Amini, F. Mirkhalaf and B. B. F. Mirjalili, Biosens. Bioelectron., 2011, 26, 2102 CrossRef CAS.
- M. Mazloum-Ardakania, H. Beitollahi, M. A. Sheikh-Mohseni, H. Naeimi and N. Taghavinia, Appl. Catal., A, 2010, 378, 195 CrossRef.
- H. Yaghoubian, V. Soltani-Nejad and S. Roodsaz, Int. J. Electrochem. Sci., 2010, 5, 1411 CAS.
- H. Beitollahi and I. Sheikhshoaie, J. Electroanal. Chem., 2011, 661, 336 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
