A biosensor fabricated by incorporation of a redox mediator into a carbon nanotube/nafion composite for tyrosinase immobilization: detection of matairesinol, an endocrine disruptor
Jahangir Ahmad
Rather
,
Sanaz
Pilehvar
and
Karolien
De Wael
*
University of Antwerp, Department of Chemistry, Groenenborgerlaan 171, B-2020 Antwerp, Belgium. E-mail: Karolien.DeWael@ua.ac.be; Tel: +32 32653424
First published on 26th October 2012
Abstract
An electrochemical matairesinol biosensor was fabricated by immobilizing tyrosinase on a poly(thionine)/nafion/multi-walled carbon nanotube composite film. A polymeric film of the redox dye thionine enables the stable immobilization of tyrosinase while acting as a mediator for the enzymatic process has been incorporated into the carbon nanotube/nafion composite film. The immobilization method is based on crosslinking of the tyrosinase layer with an electropolymerized film of poly(thionine). The good homogenization of the electron conductor CNTs in the integrated films provides the possibility of a three-dimensional electron conductive network. The biosensor was characterized by electrochemical impedance spectroscopy and electrochemical characterization. The composite electrode exhibits catalytic activity, high sensitivity, stability and is applicable over a wide range of concentrations from 180 nM to 4.33 μM with a detection limit (LOD) of 37 nM. The obtained results suggest that the developed sensor can be successfully used for the determination of phenolic endocrine disruptors over a concentration range covering their environmental levels.
1. Introduction
Global concern about the persistence of endocrine disrupting chemicals (EDCs) in the freshwater environment is more evident due to their possible disruptive effects in intact organisms, or their progenies, subsequent to endocrine function.1 Plant-derived phytoestrogens are a group of such chemicals which are stereochemically similar to the hormone 17-β-estradiol.2 They have other possible effects on some enzymes; inhibition of steroid metabolization, anti-proliferative and anti-angiogenic processes, protein tyrosine kinase inhibition and other biological effects have been described.3,4 Matairesinol (A) is a plant lignan found mainly in flax seeds and rye and has estrogen-like structure.5 In the gastrointestinal tract matairesinol is converted into the metabolite enterodiol which is known to have estrogenic properties.6 Therefore reliable data on the phytoestrogen matairesinol are necessary to assess the health implications on humans and other animals.The levels of concentrations of EDCs in different samples are low (1–72![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 ng L−1) and due to the complexity of the environmental matrices pre-concentration of the samples is required before LC-MS and ELISA analysis.7,8 Literature survey reveals the coulometric electrode array detection for determination of matairesinol in flax seeds with a detection limit of 5 μg g−1.9 All reported techniques for the detection of EDCs are reaching high accuracy with low detection limits, but are expensive, time-consuming and require the use of highly trained personnel. In response to these limitations, a large number of efforts have been directed towards the development of simple and effective methods for the determination of phenolic EDCs. In particular, the fabrication of biosensors has generated tremendous interest in this area. In recent years, electrochemical biosensors based on tyrosinase have attracted much interest for the advantages of good reliability, fast response, inexpensive instrument, low energy consumption, simple operation, time saving and high sensitivity. Thus, they are widely investigated for determining some phenolic environmental pollutants.10,11
000 ng L−1) and due to the complexity of the environmental matrices pre-concentration of the samples is required before LC-MS and ELISA analysis.7,8 Literature survey reveals the coulometric electrode array detection for determination of matairesinol in flax seeds with a detection limit of 5 μg g−1.9 All reported techniques for the detection of EDCs are reaching high accuracy with low detection limits, but are expensive, time-consuming and require the use of highly trained personnel. In response to these limitations, a large number of efforts have been directed towards the development of simple and effective methods for the determination of phenolic EDCs. In particular, the fabrication of biosensors has generated tremendous interest in this area. In recent years, electrochemical biosensors based on tyrosinase have attracted much interest for the advantages of good reliability, fast response, inexpensive instrument, low energy consumption, simple operation, time saving and high sensitivity. Thus, they are widely investigated for determining some phenolic environmental pollutants.10,11
Direct electron transfer between the electrode and the redox enzyme is very important for fundamental studies and construction of biosensors.12–14 However, the direct electron transfer between the enzyme and unmodified electrodes is usually prohibited due to shielding of redox active sites by the protein shells.15,16 Therefore, several studies have been made to enhance the electron transfer. Mediators are widely used to access the redox centre of the enzyme and thus act as charge carriers. These minimize the effects of interference, lower the operation potential of the electrodes and improve the linear response and sensitivity of the sensor.17 Thionine (phenothiazine, PTH) is a redox dye whose electroactivity lies not only in the heterocyclic nitrogen atoms and nitrogen bridges but also in its free amine groups and has been reported for fabrication of tyrosinase biosensors.18,19 On the other hand, nafion, as a perfluorosulfonated cation exchange polymer, is a perm-selective polymer known for its ability to incorporate positively charged ions and reject anionic species.20–22 It can be predicted that due to the presence of the anionic sites in the structure of nafion polymers, some analyte species with negative charge are repelled from the electrode surface.
Electrochemical biosensors incorporating enzymes with nanomaterials, which combine the recognition and catalytic properties of enzymes with the electronic properties of various nanomaterials, are new materials with synergistic properties originating from the components of the hybrid composites. Therefore, these systems have excellent prospects for interfacing biological recognition events with electronic signal transduction so as to design a new generation of bioelectronic devices with high sensitivity and stability. Carbon nanotubes (CNTs) are receiving considerable interest owing to their ability to enhance electron transportation of enzyme and biomolecules.23,24 The three-dimensional network of electronically conductive CNTs significantly increases the surface area for enzyme immobilization and provides an electronic circuit as a series of “nanowires” for the enzyme.25,26
In the present work, the deliberate combination of thionine, CNTs and nafion for tyrosinase immobilization is very interesting. It has been recently reported that CNTs showed a strong tendency to adsorb thionine molecules through donor–acceptor interaction and also π–π stacking force between these two kinds of conjugated frames.27,28 There is strong interaction between any of two among the three materials (ion-exchange process between thionine and nafion, strong adsorption of thionine by CNTs and wrapping and solubilizing of CNTs with nafion), which will result in the homogenization of the three materials that provides an efficient immobilization matrix for tyrosinase enzyme by increasing the electron transfer with the significant decrease of high overpotential. Our strategy is to immobilize the enzyme into an electrically conductive matrix of carbon nanotubes, thionine and nafion which potentially reduces the distance from the redox-centre of tyrosinase to the CNTs that promote faster electron transfer.
2. Experimental
2.1 Chemicals and reagents
All chemicals used were of analytical reagent grade quality and were employed without further purification. Tyrosinase from mushroom (E.C. 1.14.18.1, 3610 units per mg) was obtained from Sigma-Aldrich (Belgium) and was used as received. Multiwalled carbon nanotubes with 99% purity, i.d. = 2–15 nm and 1–10 μm tube length, were obtained from Aldrich. Matairesinol (5 mg) standard, Nafion® 117 solution (5%), glutaraldehyde (8%) solution and thionine blue were also obtained from Sigma Aldrich. KCl (0.1 M) solution was prepared in double distilled water and used as the supporting electrolyte. A stock solution of matairesinol (1.0 mM) was prepared in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio of dimethyl sulfoxide (DMSO) to phosphate buffer (PBS pH 7.0). Phosphate buffer (PBS) solutions of different pH were prepared by mixing appropriate volumes of KH2PO4 and Na2HPO4 neutralizing monosodium phosphate monohydrate and disodium phosphate heptahydrate.
4 ratio of dimethyl sulfoxide (DMSO) to phosphate buffer (PBS pH 7.0). Phosphate buffer (PBS) solutions of different pH were prepared by mixing appropriate volumes of KH2PO4 and Na2HPO4 neutralizing monosodium phosphate monohydrate and disodium phosphate heptahydrate.
2.2 Apparatus
Electrochemical measurements were performed using a μ-Autolab Potentiostat/Galvanostat PGSTAT from Metrohm (The Netherlands), fitted with a PC provided with the appropriate GPES 4.2 and FRA software. The utilized electrodes were Tyr/PTH/NAF/MWCNT/GCE as a working electrode, Ag/AgCl (3.0 M KCl) as a reference electrode and graphite as an auxiliary electrode. All the solutions examined by the electrochemical technique were purged for 10 min with purified nitrogen gas, after which a continuous stream of nitrogen was passed over the solutions during the measurements. All pH-metric measurements were made on a Decible DB-1011 digital pH meter fitted with a glass electrode and a saturated calomel electrode as the reference, which was previously standardized with buffers of known pH. Scanning electron microscopy (SEM) was carried out by using a Jeol 6300 electron microprobe system.2.3 Pretreatment of MWCNTs
Before modification of the GCE surface, the MWCNTs were pretreated as reported in the literature in order to remove the probable amorphous carbons and metallic catalyst impurities.31 For this treatment procedure 0.1 g of the purchased MWCNTs was refluxed in a mixture of concentrated H2SO4 and HNO3 for 4–5 h and then washed with doubly distilled water until the pH of the solution became neutral and then dried under an IR lamp.2.4 Fabrication of the biosensor
Prior to the modification, a GCE was polished with PK-4 polishing kit, BASi MF-2060 successively followed by rinsing thoroughly with redistilled deionized water until a mirror like finish was obtained. Then it was washed ultrasonically in double distilled water for 5 min and finally dried under a stream of nitrogen at room temperature. 1 mg of treated MWCNTs was dispersed in 5 mL of 0.5 wt% nafion (NAF) ethanol solution and allowed to sonicate for 30 min. Then 15 μL of the above solution was cast on the surface of the GCE, dried under an IR lamp and finally rinsed with double distilled water to remove loosely adsorbed MWCNTs. The PTH/NAF/MWCNT/GCE was prepared by electropolymerizing 0.1 mM thionine and 2.5% glutaraldehyde solution by cyclic voltammetry between −0.4 and +0.4 V at 50 mV s−1.29 The surface concentration of the electroactive moieties within the poly(thionine) films could be controlled by appropriately choosing the number of the cyclic scans. The immobilization of tyrosinase was achieved by dispersing 2 mg mL−1 (w/v ratio) tyrosinase in 0.1 M PBS (pH 7.0). Then 10 μL of the tyrosinase solution was dropped on the PTH/NAF/MWCNT/GCE. After being dried under ambient conditions for 2 h, the tyrosinase modified electrode (Tyr/PTH/NAF/MWCNT/GCE) was washed with the redistilled deionized water to remove the unimmobilized mixture. The finished biosensor was stored at 4 °C in a refrigerator before use. The procedure used for construction of the biosensor is shown in Scheme 1.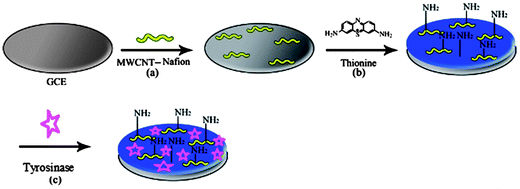 | ||
| Scheme 1 Fabrication of the biosensor. | ||
3. Results and discussion
3.1 Characterization of the biosensor
 | ||
| Fig. 1 Scanning electron micrographic image of NAF/MWCNT/GCE. | ||
![Cyclic voltammograms of 1.0 mM K3[Fe(CN)6] in 0.1 M KCl, scan rate 20 mV s−1 at different modified electrodes: GCE (), PTH/NAF/GCE (), PTH/NAF/MWCNT/GCE () and Tyr/PTH/NAF/MWCNT/GCE ().](/image/article/2013/AN/c2an35959f/c2an35959f-f2.gif) | ||
Fig. 2 Cyclic voltammograms of 1.0 mM K3[Fe(CN)6] in 0.1 M KCl, scan rate 20 mV s−1 at different modified electrodes: GCE ( ), PTH/NAF/GCE ( ), PTH/NAF/GCE ( ), PTH/NAF/MWCNT/GCE ( ), PTH/NAF/MWCNT/GCE ( ) and Tyr/PTH/NAF/MWCNT/GCE ( ) and Tyr/PTH/NAF/MWCNT/GCE ( ). ). | ||
![(A) Nyquist plot of 1.0 mM K3[Fe(CN)6] in 0.1 M KCl at different modified electrodes: GCE (), PTH/NAF/GCE (), PTH/NAF/MWCNT/GCE () and Tyr/PTH/NAF/MWCNT/GCE (). (B) Nyquist plot of 0.5 mM matairesinol in pH 7.0 at different modified electrodes: GCE (), PTH/NAF/GCE (), PTH/NAF/MWCNT/GCE () and Tyr/PTH/NAF/MWCNT/GCE ().](/image/article/2013/AN/c2an35959f/c2an35959f-f3.gif) | ||
Fig. 3 (A) Nyquist plot of 1.0 mM K3[Fe(CN)6] in 0.1 M KCl at different modified electrodes: GCE ( ), PTH/NAF/GCE ( ), PTH/NAF/GCE ( ), PTH/NAF/MWCNT/GCE ( ), PTH/NAF/MWCNT/GCE ( ) and Tyr/PTH/NAF/MWCNT/GCE ( ) and Tyr/PTH/NAF/MWCNT/GCE ( ). (B) Nyquist plot of 0.5 mM matairesinol in pH 7.0 at different modified electrodes: GCE ( ). (B) Nyquist plot of 0.5 mM matairesinol in pH 7.0 at different modified electrodes: GCE ( ), PTH/NAF/GCE ( ), PTH/NAF/GCE ( ), PTH/NAF/MWCNT/GCE ( ), PTH/NAF/MWCNT/GCE ( ) and Tyr/PTH/NAF/MWCNT/GCE ( ) and Tyr/PTH/NAF/MWCNT/GCE ( ). ). | ||
In addition, Fig. 3B shows the Nyquist plots obtained at GCE, PTH/NAF/GCE, PTH/NAF/MWCNT/GCE and Try/PTH/NAF/MWCNT/GCE in 0.5 mM matairesinol. After GCE was modified with PTH/NAF/MWCNTs, the surface resistance of the resulting electrode decreased to an even lower value than the bare GCE showing that the conductivity and surface area of the film have been increased due to the presence of carbon nanotubes. The diameter of the semicircle of the Try/PTH/NAF/MWCNT/GCE was found to be lower than all modified electrodes suggesting the catalytic activity of the tyrosinase at the surface of the PTH/NAF/MWCNT/GCE towards the oxidation of matairesinol.
3.2 Optimization of experimental conditions
3.3 Electrocatalytic oxidation of matairesinol
The cyclic voltammograms of 180 nM matairesinol in PBS (pH 7.0) at Tyr/PTH/NAF/MWCNT/GCE exhibit a single well defined anodic peak assigned to the oxidation of the –OH group which is not accompanied by the corresponding reduction indicating the irreversibility of the electrode process.Fig. 4 illustrates the voltammograms, CV (Fig. 4A) and SWV (Fig. 4B) of matairesinol at a bare GCE and Tyr/PTH/NAF/MWCNT/GCE in pH 7.0. On the basis of these observations, it is clear that the biosensor has a significant catalytic effect on the matairesinol oxidation leading to a decrease of the overpotential and an enhancement of the peak current.
 | ||
| Fig. 4 (A) Cyclic voltammetric behaviour: (a) blank, (b) 180 nM matairesinol at GCE, and (c) 180 nM matairesinol at Tyr/PTH/NAF/MWCNT/GCE; scan rate: 20 mV s−1. (B) Square wave voltammetric behaviour: (a) blank, (b) 180 nM matairesinol at GCE, and (c) 180 nM matairesinol at Tyr/PTH/NAF/MWCNT/GCE. | ||
3.4 Scan rate studies and mechanism of electrode reaction
Useful information about the mechanism of the electrode reaction can be acquired from the relationship between the scan rate and peak current. As the scan rate was increased from 10 to 50 mV s−1, the peak potential shifted towards a more positive value with increase in current confirming the irreversible nature of the oxidation process (Fig. 5).32 | ||
| Fig. 5 Cyclic voltammograms of 180 nM matairesinol at Tyr/PTH/NAF/MWCNT/GCE in pH 7.0 at different scan rates: 10, 20, 30, 40 and 50 mV s−1. Inset: Ipvs. υ1/2. | ||
A linear Randles–Sevcik plot (plot of Ipvs. υ1/2) was obtained indicating that the diffusion was the means of mass transport: Ip/μA = 0.0713υ1/2 (mV s−1) − 0.1399; r2 = 0.9698. The finding was further confirmed by plotting log Ipvs. log υ corresponding to the equation: log Ip/μA = 0.4876 log υ (mV s−1) − 1.957; r2 = 0.996. The obtained slope of 0.48 is close to 0.5 which confirms diffusion controlled nature of the electrode process.
For a typical diffusion-controlled irreversible electrode process, the relationship of Epvs. υ obeys the following equation.33
| Ep = E0′ + (RT/αnaF)[0.78 + ln(D01/2/k0) + ln(αnaFν/RT)1/2] | (1) |
In the present study Ep linearly depends on the logarithm of the scan rate according to the following equation:
| Ep = 11.7 + 0.0533 (ln υ); r2 = 0.9686 | (2) |
| (RT/αnaF) = 0.0533 | (3) |
The value of αna calculated from eqn (3) is 1.372, as for a totally irreversible electrode process, α is assumed to be 0.5, so the na was calculated to be 2.14, which shows that two electrons are involved in the oxidation of matairesinol.
It is well known that tyrosinase is a copper protein, which shows two catalytic functions: ortho-hydroxylation of monophenols to o-diphenols in the presence of molecular oxygen (cresolase activity) and a two-electron oxidation of o-diphenols into o-quinones (catecholase activity).34,35 Matairesinol is a monophenol compound, which could bind to the axial position of one of the coppers of the oxy tyrosinase site during the cresolase cycle to generate o-diphenolate. When tyrosinase was immobilized on the Tyr/PTH/NAF/MWCNT/GCE surface, the oxidation peak current of matairesinol was much higher than that at GCE, which might be attributed to the electrooxidation of o-diphenolate to o-quinone after the enzyme catalytic oxidation of matairesinol to generate o-diphenolate. In the light of our results electrochemical reaction mechanisms of matairesinol could be expressed as in Scheme 2.
 | ||
| Scheme 2 Mechanism of the electrode reaction. | ||
3.5 Linearity range and detection limit
Square wave voltammograms of matairesinol at Tyr/PTH/NAF/MWCNT/GCE showed that the peak current increased linearly with increasing concentration (Fig. 6). The linearity evaluated by linear regression analysis was calculated by the least square regression method.36,37 The linearity was obtained over the concentration range of 180 nM to 4.33 μM and the linear regression equation is Ip/μA = 0.1713 (μM) + (0.1813); r2 = 0.9829 (n = 3). The detection limit (LOD) estimated as 3s/m is found to be 37 nM with s representing the standard deviation of the peak currents (n = 3) and m is the slope of the calibration curve. | ||
| Fig. 6 Linearity of square wave voltammetric peak current of matairesinol at Tyr/PTH/NAF/MWCNT/GCE (pH 7.0) at different concentrations: (a) blank, (b) 180 nM, (c) 653 nM, (d) 984 nM, (e) 1.68 μM, (f) 2.65 μM, and (g) 4.33 μM. The error bars represent the standard deviation from three separate experiments. | ||
3.6 Accuracy, precision and recovery
The intra-day and inter-day accuracy and precision of the proposed procedure were estimated by analyzing 2.0 to 4.0 μM matairesinol solutions three times in three successive days (Table 1). The relative error of −0.10 to 0.01 and %RSD of 0.54 to 1.65 indicate the high accuracy and precision of the proposed method.The recovery of matairesinol was performed in the same concentration range 2.0 to 4.0 μM. The results show that the recoveries vary in the range of 96.0% to 100.1% and the relative standard deviation was ±1.3%.
3.7 Stability, reproducibility and interferences of the biosensor
The stability of Tyr/PTH/NAF/MWCNT/GCE and its effect on the oxidation of 180 nM matairesinol were investigated by recording cyclic voltammograms. It is observed that after 10 days the biosensor current response maintained about 87% of the initial current response, which shows that the biosensor has a good long-term stability.The intra-day reproducibility of Tyr/PTH/NAF/MWCNT/GCE was evaluated by repeating four measurements of cyclic voltammogram on the same day and in the same solution of (180 nM) matairesinol. An RSD value of 0.84% was obtained. Inter-day variation of the same concentration of matairesinol was analyzed for four consecutive days by performing four measurements on each day. The RSD value is 1.25% which demonstrates good reproducibility of the biosensor.
The tolerance limit was defined as the maximum concentration of the interfering substance that caused an error less than ±5% for the determination of matairesinol. Under optimal conditions, the interference test was performed in the presence of 100 fold concentration of phenol, ethanol, Cu2+, Ca2+, Fe3+, Mg2+, Al3+, Zn2+, SO42− and NO3−. The results indicate that they have no influence on the signals of 180 nM matairesinol with deviation below 5.0%. Due to the excellent reproducibility, stability and the result of interference tests, Tyr/PTH/NAF/MWCNT/GCE can be used in practical analytical applications.
4. Conclusion
In the present work, through a simple route by incorporating a redox mediator thionine into the CNT/nafion composite matrix for immobilization of tyrosinase, a new electrochemical sensing platform is designed, due to the ion-exchange ability of nafion and the strong adsorption of CNTs. Benefiting from the coupling of the antifouling/discriminative properties of nafion with the three-dimensional electronic conductivity of CNTs and highly intimate contact with thionine, the integrated thionine and CNT/nafion composite film shows a stable matrix which enhances the electrocatalytic response of tyrosinase toward the oxidation of matairesinol at a much lower potential. The composite electrode showed an excellent electrocatalytic activity in lowering the anodic overpotential and remarkable enhancement of the anodic current of matairesinol compared with the electrochemical performances obtained at a GCE. The fabricated biosensor has been successfully used for detection of matairesinol with remarkable properties such as fast response, broad linear range, good reproducibility, acceptable stability and low detection limit.Acknowledgements
The authors are highly thankful for the mobility grant (Non-Europe Postdoc Fellowship) for one of the authors (Jahangir Ahmad Rather) supported by the Belgian Federal Science Policy (Belspo) co-funded by the Marie Curie Actions from the European Commission.References
- J. Arts, G. G. J. M. Kuiper, J. M. M. F. Janssen, J. Ar. Gustafsson, C. W. G. M. Lower, H. A. P. Pols and J. P. T. M. Van Leeuwen, Endocrinology, 1997, 138, 5067–5070 Search PubMed.
- Y. Zhang and J. L. Zhou, Water Res., 2005, 39, 3991–4003 CrossRef CAS.
- M. Fitzpatrick, J. Agric. Food Chem., 1998, 46, 3396–3397 Search PubMed.
- D. Ibarreta, A. Daxenberger and H. H. D. Meyer, APMIS, 2001, 10, 9161–9184 Search PubMed.
- H. Adlercreutz and W. Mazur, Ann. Med., 1997, 29, 95–120 Search PubMed.
- S. Heinonen, T. Nurmi, K. Liukkonen, K. Poutanen, K. Wa ha la, T. Deyama, S. Nishibe and H. Adlercreutz, J. Agric. Food Chem., 2001, 49, 3178–3186 CrossRef CAS.
- P. Lopez-Roldan, M. J. L. de Alda and D. Barcelo, Anal. Bioanal. Chem., 2004, 378, 599–609 CrossRef CAS.
- M. Petrovic, M. J. Lopez de Alda and D. Barcelo, J. Chromatogr., B: Biomed. Sci. Appl., 2001, 20, 637–648 Search PubMed.
- T. Kraushofer and G. Sontag, J. Chromatogr., B: Anal. Technol. Biomed. Life Sci., 2002, 777, 61–66 CrossRef CAS.
- L. Chen, B. Gu, G. Zhu, Y. Wu, S. Liu and C. Xu, J. Electroanal. Chem., 2008, 61, 77–83 Search PubMed.
- Z. Liu, Y. Liu, H. Yang, Y. Yang, G. Shen and R. Yu, Anal. Chim. Acta, 2005, 533, 3–9 CrossRef CAS.
- Q. Xu, C. Mao, N. Liu, J. Zhu and J. Sheng, Biosens. Bioelectron., 2006, 22, 768–773 CrossRef CAS.
- J. Wang and M. Musameh, Anal. Chem., 2003, 75, 2075–2079 CrossRef CAS.
- J. Wang and P. Pamidi, Anal. Chem., 1998, 70, 1171–1175 CrossRef CAS.
- D. Tang, R. Yuan and Y. Chai, Anal. Chem., 2008, 80, 1582–1588 CrossRef CAS.
- E. Katz and I. Willner, ChemPhysChem, 2004, 5, 1084–1104 CrossRef CAS.
- D. Tang, R. Yuan and Y. Chai, Electroanalysis, 2006, 18, 259–266 Search PubMed.
- C. Hajizadeh, H. T. Tang, B. Halsall and W. R. Heineman, Anal. Lett., 1991, 24, 1453–1469 CAS.
- E. Dempsey, D. Diamond and A. Collier, Biosens. Bioelectron., 2004, 20, 367–377 CrossRef CAS.
- W. J. Vining and T. J. Meyer, J. Electroanal. Chem., 1987, 237, 191–208 Search PubMed.
- C. R. Martin, I. Rubinstein and A. J. Bard, J. Am. Chem. Soc., 1982, 104, 4817–4824 CrossRef CAS.
- Perfluorinated Ionomer Membranes, ed. A. Eisenberg and H. L. Yeager, ACS Symposium Series 180, American Chemical Society, Washington, DC, 1982 Search PubMed.
- S. Guo and E. Wang, Anal. Chim. Acta, 2007, 598, 181–192 CrossRef CAS.
- J. Wang, Analyst, 2005, 130, 421–426 RSC.
- E. Katz, A. N. Shipway and I. Willner, in Nanoparticles – From Theory to Applications, ed. G. Schmid, Wiley-VCH, Weinheim, Germany, 2004 Search PubMed.
- M. H. Huang, H. Q. Jiang, X. H. Qu, Z. A. Xu, Y. L. Wang and S. J. Dong, Chem. Commun., 2005, 5560–5562 RSC.
- Q. W. Li, J. Zhang, H. Yan, M. S. He and Z. F. Liu, Carbon, 2004, 42, 287–291 CrossRef CAS.
- R. Yang, C. Ruan, W. Dai, J. Deng and J. Kong, Electrochim. Acta, 1999, 44, 1585–1596 Search PubMed.
- H. Liu, G. Wang, D. Chen, W. Zhang, C. Li and B. Fang, Sens. Actuators, B, 2008, 128, 414–421 CrossRef.
- M. E. Orazem and B. Tribollet, Electrochemical Impedance Spectroscopy, John Wiley & Sons, 2008 Search PubMed.
- R. Scully, D. C. Silverman and M. W. Kendig, Electrochemical Impedance: Analysis and Interpretation, ASTM, 1993 Search PubMed.
- A. J. Fry, Synthetic Organic Electrochemistry, Marcel Dekker, New York, 1975 Search PubMed.
- A. J. Bard and L. R. Faulkner, Electrochemical Methods Fundamentals and Applications, Wiley, New York, 1980, p. 222 Search PubMed.
- A. C. Pereira, A. S. Santos and L. T. Kubota, J. Colloid Interface Sci., 2003, 265, 351–358 CrossRef CAS.
- N. Duran, M. Rosa, A. D'Annibale and L. Gianfreda, Enzyme Microb. Technol., 2002, 31, 907–931 CrossRef CAS.
- J. C. Miller and J. N. Miller, Statistics for Analytical Chemistry, Ellis Horwood, New York, 4th edn, 1993 Search PubMed.
- M. E. Swatz and I. S. Krull, Analytical Method Development and Validation, Marcel Dekker, New York, 1997 Search PubMed.
| This journal is © The Royal Society of Chemistry 2013 |

