Polymer-capped fiber-optic Raman probe for non-invasive Raman spectroscopy
Paul I.
Okagbare
and
Michael D.
Morris
*
Department of Chemistry, University of Michigan, Ann Arbor, MI 48109. E-mail: mdmorris@umich.edu
First published on 4th November 2011
Abstract
Advances in fiber optic probe design are moving Raman spectroscopy into the clinic, although there remain important practical problems. While much effort has been devoted to minimizing Raman and fluorescence background from fibers, less attention has been given to the need to generate reference Raman signals that can correct for variations in tissue albedo, which is important in quantifying changes in tissue composition. To address this shortcoming, we have developed a fiber optic probe that incorporates a fluorinated ethylene-propylene copolymer (FEP) cap at the end of each excitation fiber. Transmission of laser light through the transparent cap generates a 732 cm−1 Raman band whose intensity scales linearly with the laser power delivered to the tissue of interest. In our first design, the FEP cap functions as a waveguide with only a small insertion loss (∼5%). Laser transmission through 1 mm of the polymer is sufficient to generate a usable reference Raman signal. We show the application of the probe to quantitative non-invasive Raman spectroscopy of animal tissues using rat leg phantoms as models. Ex-vivo Raman spectroscopy of excised rat tibia supports the use of the probe for spectroscopy of various tissues. These results provide proof of principle that the Raman probe can be used in multiple spectroscopic applications.
Advances in fiber optic Raman probe design have enabled practical spectroscopy in turbid media in a wide range of application areas.1–8 The development of spatially offset Raman spectroscopy9–12 and transmission Raman spectroscopy13 has allowed depth resolved probing,14 and rapid spectroscopy.15 However, many systems often have variable albedo.16–19 Non-invasive spectroscopy of human subjects or live animals is an important case.20,21 There is a wide range of pigmentation, of course, but skin hydration also varies in an individual depending upon activity level and what materials the subject has recently touched.22 Albedo variation makes quantitative spectroscopy in turbid media difficult.23 The problem is further complicated because the details of light propagation parameters of turbid media are rarely known in detail because light absorption and scattering depends on surface and subsurface composition and morphology.23 Collection efficiency depends on the details of collection fiber design and placement.24 The situation is different in spectroscopy of excised tissue specimens, where light scattering can be neglected and normalization to a tissue component band is often feasible. In subsurface spectroscopy and especially in imaging, this strategy often fails.
It is possible to correct for these problems by normalizing to collected exciting laser intensity or a signal proportional to it, as measured at each point at which Raman scatter is collected.25 In principle, it is possible to use collected laser light itself, with spectrograph prefilters attenuating the light to a level similar to collected Raman scatter. Because the laser light signal is several orders of magnitude more intense than the Raman scatter, and the remitted Raman signal varies dramatically with depth, it would be difficult to keep the laser excitation intensity within the dynamic range of the detector systems without tuning filter characteristics to each use.
A simpler approach is to use a less intense signal proportional to the laser intensity as the light exits the excitation fiber or fibers. While the silica bands of commonly employed fibers might serve this function, in practice they are sufficiently broad that their effect is to create broad backgrounds that, together with specimen fluorescence become difficult to remove accurately.1 An alternative is to generate a narrow reference Raman signal from the fiber itself or from a material attached to it. We report here the use of fluorinated ethylene propylene copolymer (FEP) to generate a reference Raman signal proportional to delivered laser power. FEP is transparent, slightly flexible and machinable. It has a Raman spectrum similar to that of Teflon, with wide spectral windows of featureless flat background. Because it is a fluorocarbon polymer, the spectral bands occur at wavenumber shifts that are not coincident with important bands of many other materials. FEP and other transparent fluorocarbon polymers are generally biocompatible, making them well-suited to biomedical applications.
In this communication, we describe a fiber optic probe that incorporates an FEP cap at the end of each excitation fiber. Transmission of laser light through the cap generates a strong Raman band at 732 cm−1, and weaker bands at 290, 385, 597, 1216, 1298 and 1379 cm−1 (see Fig. 1A). The intensities of the Raman bands scale linearly with the laser power delivered to the object or specimen under test and correct for light diffusion through the specimen to the collection fibers.
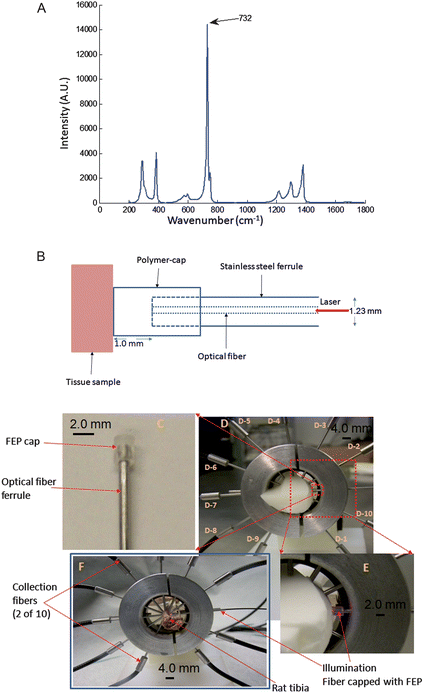 | ||
| Fig. 1 (A) Raman spectrum of FEP. The most intense band is at 732 cm-1, weaker bands are at 290, 385, 1216, 1298 and 1379 cm−1. (B) Schematic representation of the polymer-capped fiber-optic Raman probe showing the polymer cap fastened to the tip of the stainless steel ferrule that houses the optical fiber. The laser light is transported from the optical fiber through the polymer cap to the tissue sample. The polymer cap makes contact with the tissue and also guides the laser light to the tissue with transmission efficiency >90%. (C) Photo image of the FEP-capped excitation probe showing the orientation of FEP cap on a stainless steel ferrule that house a 300 μm core optical fiber; the integrated optical system serves as the laser illumination source for Raman spectroscopy. (D) Experiment set-up for Raman spectroscopy on rat leg phantom using the FEP-capped illumination probe. Collection fibers (probes) are labeled D-1 to D-10. (E) Expanded view of a section of the apparatus in (D) showing the orientation of the illumination probe and the wave-guiding capability of the FEP cap. (F) Experiment configuration for Raman spectroscopy on an excised rat tibia. | ||
Our initial application is to in vivo non-invasive Raman spectroscopy of animal bone tissue and to phantoms that model light propagation through animal tissue to generate Raman spectra of bone mineral and matrix. We report results on excised rat tibiae from animals treated and sacrificed according to a protocol approved by the University of Michigan committee on use and care of animals, and on rat tibia phantoms. These objects are highly turbid and their albedo varies widely. Thus, a reference Raman signal is important for correcting for the variation in optical properties and obtaining quantitative information about bone mineral and matrix composition. In our first design herein reported, an FEP cap is placed on the ferrule at the distal (delivery) end of each excitation fiber (see Fig. 1B). The polymer (FEP) functions as a waveguide with only about a 5% loss of delivered power. We have found that laser transmission through 1 mm of FEP is sufficient to generate a usable reference signal. However, the length can be varied as needed to enhance the intensity of the reference signal.
The FEP cap consists of a 2.0 mm diameter sleeve that fits tightly over the fiber-terminating ferrule (Fig. 1B and 1C). The ferrule is 1.23 mm diameter stainless steel hypodermic tubing. The waveguiding portion of the polymer cap extends 1.0 mm from the ferrule and is placed in contact with the specimen (see Fig. 1C). FEP has a lower refractive index than the fused silica of the optical fibers. The effect is to reduce the numerical aperture (NA) as seen by the specimen. However, elastic light scattering in the specimen diffuses the laser light, so that the smaller NA has no practical consequence on spatial resolution. The inner diameter of the FEP cap's sleeve (see Fig. 1B) matches the outer diameter of the fiber ferrule that the ferrule can be pushed into the sleeve and is not displaced during use (see Fig. 1C). The design of the Raman collection fiber array has been previously described.26 Briefly, the Raman collection probe consists of 50- collection fibers arranged into 10 branches, with each branch housing 5-fibers in a 1.23 mm (outside diameter) stainless steel ferrule. The fibers were arranged in a linear array for coupling to the Raman spectrograph.
The experiment apparatus with the FEP-capped illumination probe and the Raman collection probes for non-invasive Raman spectroscopy with a tissue phantom24 mounted is shown in Fig. 1D. The design of the phantom has been previously described.24 Briefly, the phantom uses materials that closely mimic the optical properties and spectroscopy of a rat leg including the location and morphology of the tibia, fibula and overlying soft tissue. The probe holder for Raman spectroscopy on a rat tibia is shown in Fig. 1F. All fibers are in contact with the specimen surface to minimize losses in transmitted laser light and the generated Raman signals. The Raman signals were collected by the ten collection bundles and directed to a Raman spectrograph (Rxn1, Kaiser Optical Systems Inc., Ann Arbor, MI, USA).
Laser excitation with the FEP-capped illumination probe generates a reference FEP Raman band which spreads by elastic scattering in the same manner as the delivered laser light through the tissue phantoms. Raman scatter is detected at each collection position (see Fig. 1D and 1E). The collected Raman spectra from each of the collection bundles are shown in Fig. 2A. It is clear that, the intensity of the collected FEP reference varies with the position of the detector fibers, because the distance of the detection bundle from the excitation fiber (FEP-capped illumination fiber) varies. This distance relationship is vital in achieving accurate tomographic reconstruction of subsurface tissues from transcutaneous Raman data acquired from any tissue specimen.27 The mean spectrum from all the collection fibers is shown in Fig. 2B. The 732 cm−1FEP polymer band is clearly visible. The entire spectrum is normalized to it after background subtraction. The advantage is that the 732 cm−1 FEP band serves as internal reference to facilitate generation of quantitative spectral information from the specimen under investigation. This is important in non-invasive optical spectroscopy especially sub-surface spectroscopy and tomography for quantifying changes in biochemical composition of diseased tissue.28 Tissue components bands often cannot be used for this purpose especially if they are common to any constituent of the overlying tissue.
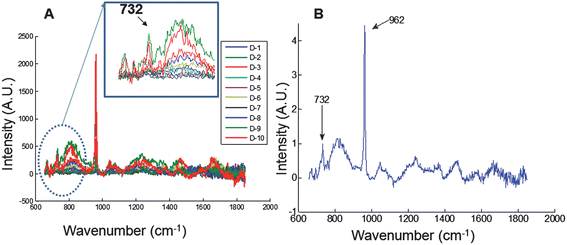 | ||
| Fig. 2 Transcutaneous Raman spectroscopy of rat leg phantom using the FEP-capped illumination probe. (A) Raman spectra from all 10 collection bundles (labeled D-1 to D-10); Raman intensity of the FEP band at 732 cm−1 varies and depends on the position of each collection bundle. (B) Mean spectrum of all collection bundles normalized to the FEP reference band at 732 cm−1. | ||
To investigate the use of the Raman probe for quantitative Raman measurement, transcutaneous Raman spectra were acquired from rat leg phantoms with different weight fractions of hydroxyappatite (HAp) in the bone layer, ranging from 0% to 20%. The resulting Raman data is presented in Fig. 3. The data were normalized to the mean FEP band at 732 cm−1. The observed intensity for the HAp at 962 cm−1 scaled linearly with the fractions of HAp. This was further confirmed in the calibration plot presented in Fig. 3B. This linear relationship supports the potential use of the FEP reference band as an internal reference standard for quantitative measurement and to correct for variations in elastic light scattering as laser light propagates through layers of tissues. Indeed a similar externally generated signal has been used for this purpose in diffuse fluorescence tomography.28
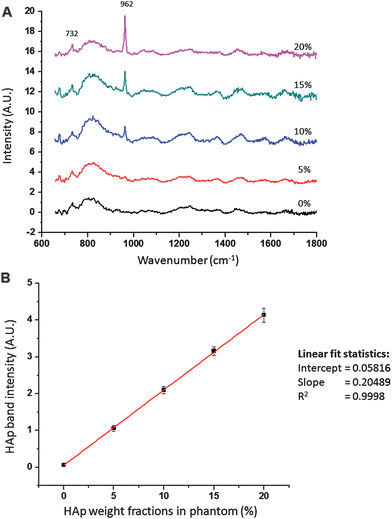 | ||
| Fig. 3 (A). Raman spectra of rat leg phantom with different weight fractions of HAp in the bone simulating region. Spectra were normalized to the polymer band at 732 cm−1. Raman intensity of HAp at 962 cm−1 is linear with weight fractions of HAp. (B). Plot of HAp band intensity with HAp weight fractions. Data were normalized to FEP band at 732 cm−1. Error bars represent standard deviation of intensities from five repeated measurements (n = 5). | ||
We also investigated the potential ability of the FEP-capped illumination probe to generate information that correlates with the laser intensity injected into the tissue. Transcutaneous Raman measurements on the phantoms were carried out at different laser intensities and intensity of the generated reference signal measured at different laser powers. As expected, the FEP signal intensity scaled linearly (R2 = 0.9994) with laser power (data not shown), thus indicating the utility of the reference signal to normalize for changes in laser power. Because tissues vary considerably in their albedo, this reference signal corrects for variation in laser power distributed through the tissue, and can minimize or eliminate the variations in the specimen's Raman signal caused by varying optical properties of the tissues.
The FEP-capped illumination probe can easily be modified to enhance the intensity of the reference signal as needed in different applications. For example, we have used an FEP-cap with length of 2.0 mm from the tip of the fiber ferrule (see Fig. 1B) and compared the resulting Raman spectrum to the FEP-cap with length of 1.0 mm from the tip of the fiber ferrule. The intensity of the FEP reference band for the 2.0 mm FEP-cap is almost double that for the 1.0 mm FEP-cap (data not shown). This proportionality makes this approach adaptable to different applications. It is also important to note that other transparent materials can be employed in the probe design. Fabrication is simpler than that required for glasses or ceramics that require high temperature working. Other amorphous fluorocarbon polymers, chlorinated hydrocarbons or even polystyrene could be used.
The Raman probe was also used for Raman data acquisition from excised rat tibiae (see Fig. 1F). The fiber probes were placed at the mid-diaphyses of a tibia. The Raman spectra are shown in Fig. 4. In Fig. 4A, the Raman spectrum (mean of all detection fibers) is normalized to the background subtracted FEP reference signal at 732 cm−1.
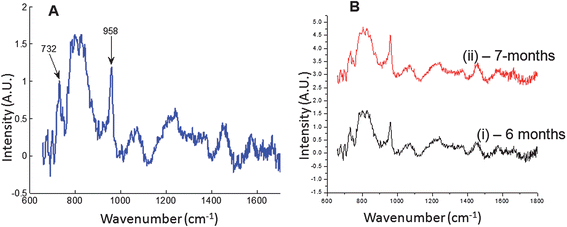 | ||
| Fig. 4 Raman data from excised rat tibia. (A) Mean spectrum of all collection probes normalized to the FEP reference band at 732 cm−1. (B) Raman spectra of rat tibia from two rats, (i) 6-month old rat, (ii) 7-month old rat. The 7-month old rat showed increased mineral composition relative to the 6-month old rat when data were normalized to the FEP band at 732 cm−1. | ||
To demonstrate the utility in quantifying small differences, measurements were made on rat tibiae from a 6-month old rat and a 7-month old rat. The results are presented in Fig. 4B. The intensity of the bone mineral band at 958 cm−1 is greater for the older rat (7-month old) than for the younger rat as expected. The mineral/matrix ratio (phosphate/proline or 958 cm−1/920 cm−1) and the carbonate substitution (carbonate/phosphtate or 1070 cm−1/958 cm−1), which are Raman markers for bone mineralization29 were extracted from the spectra. The mineral/matrix ratio increased from 2.61 for the 6-month old rat to 2.94 for the 7-month old rat, about 10%. The carbonate component decreased from 0.326 for the 6-month old rat to 0.291 for the 7-month old rat. The phosphate/CH2-wag-collagen increased from 3.03 for the 6-month old rat to 3.62 for the 7-month old rat. These observations all confirm increased mineralization.29 These 10–20% differences may be obscured in the absence of a suitable reference band for normalization. These results provide proof of principle that this Raman probe can be used for Raman spectroscopy of biological specimens.
In conclusion, we have developed a fiber optic probe that incorporates a transparent polymer cap (FEP-cap) at the end of each excitation fiber. The polymer cap functions as a waveguide with only about a 5% loss of delivered power. Transmission of laser light through this cap yields Raman bands whose intensity directly measures the laser power actually delivered to the object or specimen under test. We have demonstrated the potential for non-invasive in vivoRaman spectroscopy of animals. The calibration signal can potentially resolve a major challenge in spatially offset9,30 Raman spectroscopy, where a reference signal is needed for tomographic reconstruction and also for quantification of subsurface species.27
We have used a flat-tipped waveguide. However, reference signals could also be generated with materials terminating in a curved surface or incorporating a refractive index gradient so that they also functioned as lenses. We believe that a similar approach could be useful in the more common fluorescence and light absorption versions of diffuse optical tomography, and possibly for endoscopic Raman and/or fluorescence spectroscopies. For example, fluorescent material could be incorporated into the cap to generate a fluorescence reference signal for spectral normalization.
Although, we have used a design with a sleeve that fits over the ferrule to hold the cap in place, the cap could also be inserted into the ferrule or otherwise attached to the distal end of the fiber. Alternatively, the reference signal could be generated at the launch end of the fiber. The simplicity and flexibility of this approach remains unchanged.
Acknowledgements
The authors acknowledge support of this work through National Institutes of Health grants R01AR055222 and R01AR056646.References
- H. Sato; H. Shinzawa; Y. Komachi. In Emerging Raman Applications and Techniques in Biomedical and Pharmaceutical Fields; Matousek, P., Morris, M. D., ed.; Springer Berlin Heidelberg: 2010, p 25 Search PubMed.
- H. P. Buschman, E. T. Marple, M. L. Wach, B. Bennett, T. C. B. Schut, H. A. Bruining, A. V. Bruschke, A. van der Laarse and G. J. Puppels, Anal. Chem., 2000, 72, 3771 CrossRef CAS.
- Y. Komachi, H. Sato and H. Tashiro, Appl. Opt., 2006, 45, 7938 CrossRef CAS.
- Y. Komachi, H. Sato, K. Aizawa and H. Tashiro, Appl. Opt., 2005, 44, 4722 CrossRef.
- J. T. Motz, M. Hunter, L. H. Galindo, J. A. Gardecki, J. R. Kramer, R. R. Dasari and M. S. Feld, Appl. Opt., 2004, 43, 542 CrossRef.
- Y. Hattori, Y. Komachi, T. Asakura, T. Shimosegawa, G. Kanai, H. Tashiro and H. Sato, Appl. Spectrosc., 2007, 61, 579 CrossRef CAS.
- P. Chen, A. G. Shen, X. D. Zhou and J. M. Hu, Anal. Methods, 2011, 3, 1257 RSC.
- U. Utzinger and R. R. Richards-Kortum, J. Biomed. Opt., 2003, 8, 121 CrossRef.
- P. Matousek, Appl. Spectrosc., 2006, 60, 1341 CrossRef CAS.
- P. Matousek, Chem. Soc. Rev., 2007, 36, 1292 RSC.
- N. S. N. Stone, M. Kerssens, G. R. Lloyd, K. Faulds, D. Graham and P. Matousek, Chem. Sci., 2011, 2, 776 RSC.
- J. M. Yuen, N. C. Shah, J. T. Walsh, M. R. Glucksberg and R. P. Van Duyne, Anal. Chem., 2010, 82, 8382 CrossRef CAS.
- K. Buckley and P. Matousek, J. Pharm. Biomed. Anal., 2011, 55, 645 CrossRef CAS.
- M. V. Schulmerich, K. A. Dooley, T. M. Vanasse, S. A. Goldstein and M. D. Morris, Appl. Spectrosc., 2007, 61, 671 CrossRef CAS.
- P. Matousek, F. Thorley, P. Chen, M. Hargreaves, C. Tombling, P. Loeffen, M. Bloomfield and D. Andrews, Spectroscopy, 2011, 26, 44 CAS.
- H. Ramachandran, Curr. Sci., 1999, 76, 1334 Search PubMed.
- K. Michielsen, H. De Raedt, J. Przeslawski and N. Garcia, Phys. Rep.-Rev. Sec. Phys. Lett., 1998, 304, 90 Search PubMed.
- G. Zonios and A. Dimou, Opt. Express, 2009, 17, 1256 CrossRef CAS.
- W. F. Cheong, S. A. Prahl and A. J. Welch, IEEE J. Quantum Electron., 1990, 26, 2166 CrossRef.
- R. H. Wilson and M. A. Mycek, Technol. Cancer Res. Treat., 2011, 10, 121 CAS.
- S. Kumari and A. K. Nirala, Optik, 2011, 122, 807 Search PubMed.
- R. K. Hari, T. R. Patel and A. M. Martin, Food Rev. Int., 1994, 10, 49 CrossRef CAS.
- S. L. Jacques and B. W. Pogue, J. Biomed. Opt., 2008, 13 CrossRef.
- P. I. Okagbare, F. W. L. Esmonde-White, S. A. Goldstein and M. D. Morris, Analyst, 2010, 135, 3142 RSC.
- H. Meresman, A. J. Hudson and J. P. Reid, Analyst, 2011 Search PubMed.
- P. I. Okagbare, F. W. L. Esmonde-White, S. A. Goldstein and M. D. Morris, Proc. of SPIE, 2011, 7883 CAS.
- J. L. D. J. L. Demers; B. Pogue; F. Leblond; F. Esmonde-White; P. Okagbare; M. Morris. In Multimodal Biomedical Imaging Vi; Azar, F. S., Intes, X., ed.; Spie-Int Soc Optical Engineering: Bellingham, 2011; Vol. 7892 Search PubMed.
- S. C. Davis, K. S. Samkoe, J. A. O'Hara, S. L. Gibbs-Strauss, K. D. Paulsen and B. W. Pogue, J. Biomed. Opt., 2010, 15 CrossRef.
- Morris, M.; Matousek, P., Morris, M. D., ed.; Springer Berlin Heidelberg: 2010, p 347.
- P. Matousek, E. R. C. Draper, A. E. Goodship, I. P. Clark, K. L. Ronayne and A. W. Parker, Appl. Spectrosc., 2006, 60, 758 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2012 |
