Regular undulation and polarization modulation on the film surface of a planarly aligned SmC* polymer†
Chunying
Zhang
,
Ryohei
Ishige
,
Ryou
Yasumatsu
,
Sungmin
Kang
*,
Masatoshi
Tokita
and
Junji
Watanabe
Department of Organic and Polymeric Materials, Tokyo Institute of Technology, 2-12-1 O-okayama, Tokyo, Japan. E-mail: skang@polymer.titech.ac.jp; Fax: +81-3-5734-2888; Tel: +81-3-5734-2633
First published on 13th October 2010
Abstract
Chiral smectic C (SmC*) in a main-chain type of polymer exhibits undulations on its glassy film surface; these undulations are produced by the helical assembly of polymer conforming to the helical field of SmC*. In this study, we have found a polarization modulation superimposed on the undulation from the combined observations of Kelvin force microscopy (KFM) and atomic force microscopy (AFM) images. This modulation is caused by the spontaneous polarization of each smectic layer; its direction is parallel to the layer and perpendicular to the tilt direction of molecules within a layer, and rotates around the helical axis. The polarization modulation length corresponds perfectly to the undulation length, i.e., helical pitch of SmC*. By clarifying the relative direction of the spontaneous polarization to the tilt direction of mesogens, we described how the polymers are accommodated to produce such a regular undulation and polarization modulation.
1. Introduction
Chiral smectic C (SmC*) liquid crystal (LC) is interesting because it exhibits a ferroelectric response and forms a helical structure.1 The helical structure has attracted attention because of its characteristic optical properties, such as large optical rotation and selective reflection of the circularly polarized light, which can be applied to optical materials that manipulate the light.Further interest in the SmC* system of main-chain type of polymers2,3 is because of the polymers themselves, which assume a helical conformation consistent with the helical LC field. This structural aspect, which is completely different from that in the low-molecular-weight system, produces the interesting surface morphology on the SmC* solid sample, as reported in a previous paper.4 The most typical example is observed on the fracture surface. Through scanning electron microscopy (SEM) and atomic force microscopy (AFM) observations, very clear undulations with regular repeating can be detected (see Fig. S1†). The peak-to-peak distance corresponds to the helical pitch of the SmC* phase (150–600 nm depending on the chiral content in the polymers) and the top-to-bottom depth is around 20–70 nm. Such an undulation mode in the fracture surface is due to the helical arrangement of polymers to form the helical SmC* structure because the cleavage easily occurs along the helical polymer chain. According to this preferential fracture model, the ratio of the depth to pitch simply depends on the tilt angle of the mesogens to the layer and can be calculated to be about 12% in a previous system with a tilt angle of 20°. In fact, this value coincides with the observed values.
The undulation morphology is also observed on the film surface of planarly aligned samples. Although the depth is relatively smaller than that observed on the fracture surface because of interfacial tension, the easy preparation of wide-area monodomain SmC* films provides a powerful method for fabricating regular surface undulation, which can be applied in processes such as diffraction gratings.5,6
In this paper, we will show another interesting structural aspect, a polarization modulation superimposed on the undulation film surface of the SmC* solid. This polarization modulation results from the spontaneous polarization of each layer, the direction of which rotates around the helical axis.
2. Experimental section
2.1. Materials
The polymers were synthesized by melt transesterification from dimethyl 4,4′-biphenyldicarboxylate as a mesogenic moiety and corresponding alkane diols as a spacer with isopropyl titanate as a catalyst.2 The number average molecular weight and molecular weight distribution were determined from the GPC curve, which was calibrated with polystyrene standards. The (R)-(+)-2-methyl butanediol ([α]D = +12.5° in neat) and (S)-(−)-2-methyl butanediol ([α]D = −12.5° in neat) were used as purchased from TCI, Japan. (R)-(+)-2-methoxy butanediol ([α]D = +7.2° in CHCl3) and (S)-(−)-2-methoxyl butanediol ([α]D = −7.0° in CHCl3) were synthesized from dimethyl D-(+)-malate and dimethyl L-(−)-malate, respectively, both purchased from TCI. This was preformed under the standard conditions for Williamson's ether synthesis and LAH reduction (refer to Scheme S1†).2.2. Method
The textures were observed under crossed polarizers using an Olympus BX50 polarizing optical microscope (POM) equipped with a temperature-controlled Mettler FP 90 hot stage. Transition temperatures were determined by differential scanning calorimetry (DSC) using a Perkin–Elmer DSCPyris1 calorimeter. The reflection CD spectra were measured with JASCO J-20. Electro-optic response and polarization reversal current were observed using a high-speed voltage amplifier (FLC Electronics, F20A) connected to a function generator (NF Electronic Instruments, WF1945A). AFM and Kelvin force microscopy (KFM) measurements were conducted using a MAC mode III 5500 (Agilent Technology, Inc., Santa Clara, CA) in the AC mode at room temperature. Imaging was conducted in the tapping mode using a silicon cantilever with a resonance frequency of 50–100 kHz.3. Results and discussion
3.1. Polymers forming the SmC*
Homopolymer BB-6 with hexamethylene (HM) spacer forms an SmA phase,7 whereas homopolymer BB-4(2-Me) with 2-methyl butylene (MB) spacer forms nematic and SmC phases, although the SmC phase is monotropic.7 The incorporation of a BB-4(2-Me) unit into a BB-6 polymer results in an alteration of the SmA to an SmC phase,2,3 which is due to the packing difficulty of the methyl-substituted spacer into the SmA type of association. Thus, the copolymers with intermediate content form the SmC phase in a wide temperature region, show polymorphism with a sequence of SmA and SmC phases for decreasing temperature. Note that the use of chiral MB alters the SmC phase to the SmC* phase.For the present study, copolymerization offers the additional advantage of easy solidification of liquid crystals. This is possible because copolymerization does not interrupt the liquid crystallization, but the crystallization.2,3 Perfect solidification of the SmC* can thus be achieved in copolymers with 30%–60% fractions of the MB unit. Such glassy materials are convenient for the examination of surface morphology.
We prepared two main-chain types of BB-n copolymers forming the SmC* phase as shown in Scheme 1; one type of copolymer consists of HM and chiral MB spacers, and the other one consists of HM and chiral 2-methoxy butylene (MOB) spacers. In both copolymers, the ratio of HM to chiral butylene spacer is selected to be 40/60. The copolymers are named here as P-40(HM)/60(X), where X shows the type of the chiral butylene spacer. Because each chiral butylene unit has two optical enantiomers, (S)-2-methy butylene (S-MB) and (R)-2-methy butylene (R-MB) units for the chiral MB, and (S)-2-methoxyl butylene (S-MOB) and (R)-2-methoxyl butylene (R-MOB) units for the chiral MOB, four copolymers have been prepared. Their molecular weights and transition temperatures are listed in Table 1.
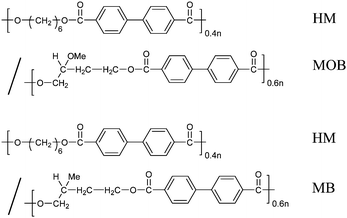 | ||
| Scheme 1 Chemical structure of two BB-n copolymers | ||
| Polymer | Mw × 103 | Mw/Mn | Tiso-Aa | TA-Ca | Tga | ΔHiso-Aa | ΔHA-Ca |
|---|---|---|---|---|---|---|---|
| °C | kJ mol−1 | ||||||
| a Transition temperatures and enthalpies are based on cooling DSC data. | |||||||
| P-40(HM)/60(S-MOB) | 5.5 | 1.8 | 210 | 152 | 25 | 7.2 | ∼0.1 |
| P-40(HM)/60(R-MOB) | 4.5 | 1.7 | 205 | 152 | 23 | 7.2 | ∼0.1 |
| P-40(HM)/60(S-MB) | 8.6 | 2.0 | 224 | 172 | 24 | 7.0 | ∼0.1 |
| P-40(HM)/60(R-MB) | 9.7 | 1.9 | 219 | 169 | 23 | 6.7 | ∼0.1 |
All the copolymers exhibit similar transition with a sequence of Iso–SmA*–SmC*–SmC* glass on cooling. As observed in Table 1, the SmC* phase transformed from the SmA* exists in a wide temperature region, below 150 and 170 °C for MOB- and MB-based polymers, respectively. The glass-transition temperature of the SmC* is around 25 °C.
3.2. Helical structure of SmC*
The helical pitch and sense of SmC* phase were determined from the reflection CD band, which is well observed in the planarly aligned sample with the helical axis perpendicular to the film. The homeotropic orientation was achieved by shear deformation between two glasses,8 and the typical reflection spectra measured with a light irradiation parallel to the helical axis are shown for four polymers in Fig. 1. The negative CD peak for the S-enantiomers in both copolymers indicates the right-handed helical structure, while the positive CD peak for the R-enantiomers exhibits the left-handed helical structure. The helical pitches, P, were determined from the peak wavelength, λm, using the equation λm = n P (n: average refractive index of 1.6). As listed in Table 2, they are around 200 and 390 nm for the MOB- and MB-based polymers, respectively. Thus, MOB-based polymers have a twisting power which is two times larger than that of MB-based polymers. | ||
| Fig. 1 Reflection CD bands observed for the homeotropically aligned SmC* solids of four polymers with the light irradiation parallel to the helical axis. Here, the homeotropically aligned SmC* phase was prepared by shearing between two glasses and quenching to ambient temperature. | ||
3.3. Surface morphology of SmC* film by AFM and KFM methods
For observing surface topography, phase, and potential images, the SmC* film was prepared planarly with the helical axis parallel to the film surface. Before observation, a thin film was spin coated with chloroform solution onto polyimide-coated glass substrate, annealed at a SmA* temperature of 180 °C for 10 min, and then cooled to ambient temperature at a rate of 1 °C min−1. The well-developed fan-shaped texture exhibits the planar orientation of molecules in the SmC* phase as well as the SmA* phase. Parts labelled i) in Fig. 2(a)–(d) show the topography images on the film surface of the solid SmC* phase. As reported in a previous paper,4 the undulation morphology can be detected for all the polymers. The distances between two neighboring tops are around 220 and 420 nm for MOB- and MB-based polymers, respectively. As listed in Table 2, these values coincide with the helical pitches determined from reflection CD spectra.4 | ||
| Fig. 2 AFM topography images (Part i) and KFM surface potential images (Part ii), observed on the film surface of the planarly aligned solid SmC* phase. The cross-sectional profiles of AFM and KFM images along the direction indicated by arrows (Parts i and ii in (a)) are shown at the top and bottom of Part iii, respectively. | ||
The electric KFM potential image, which exhibits several levels of contrast with respect to the electric potential,9–11 is most informative for the SmC* structure. All the samples show clear KFM images in Part ii) of Fig. 2, indicating distinct polarization modulation with the following features. First, the modulated contrast appears with the repeating length corresponding perfectly to the surface undulation length. Second, the surface potential is more negative (up to −0.8 V) on the peak of the surface undulation than that of the valley for MOB-based polymers, while the reverse trend is observed for MB-based polymers. This is best seen in Part iii) of Fig. 2, which shows the cross-sectional profiles obtained along the directions indicated by arrows on the AFM and KFM images. Note that the potential difference between the top and bottom in MOB-based polymers is twice as large as that in MB-based polymers.
To clarify whether or not the KFM image is artificial, we observed the AFM and KFM images for the PVA film imprinting the surface undulation of SmC*. Here, the PVA solution in water was cast onto the SmC* film and the PVA film was removed carefully from the surface after the water evaporated. Successful imprinting of the undulation mode can be realized from the AFM image; however, no definite modulation mode is detected in the KFM image (see Fig. S2a†).
3.4. Electro-optic switching behavior of SmC* phase
The surface potential measured by the KFM method arises from the ferroelectric structure of the SmC*, and its reverse trend between the MOB- and MB-based polymers suggests the opposite direction of the spontaneous polarization related to the tilt direction of molecules. To clarify these points, we measured the electro-optic switching behavior against an external electric field. Electro-optic switching was observed for a sample sandwiched between two glass plates with an indium tin oxide (ITO) electrode. The substrate surface was polyimide coated. Rubbing directions in the top and bottom plates were set in an antiparallel way with a cell gap of 6 μm.First, the unswitched cell is heated to form the SmA*. After confirming the planarly aligned SmA*, the sample is cooled down to form the SmC* phase at 120 °C. Using this method, we can obtain a perfectly aligned SmC* phase with the helical axis parallel to the rubbing direction, i.e., with the layer perpendicular to the rubbing direction. The extinction of this sample is then observed when the polarizer axis corresponds to the helical axis, as was found from the optical microscopic textures in Part A of Fig. 3. The sample is subjected to a DC voltage (100 V) of known polarity; the plus voltage indicates that the top plate is positively charged. The helix unwinds and the material becomes poled in a uniform direction with its spontaneous polarization being coupled to the applied field. As a result, the material becomes bright (see Part B of Fig. 3), and hence the extinction direction is now at an angle relative to the reference (rubbing) direction. The sample is then rotated through the minimum angle required to return it to extinction (Part C of Fig. 3). The extinction direction from polarizer axis is estimated to be around 15°, which corresponds to the tilt angle of molecules to the layer as elucidated from the X-ray spacing data.2,3
 | ||
| Fig. 3 Optical microscopic textures of planarly aligned samples observed on electric switching. Here, samples are sandwiched between two glass plates with an indium tin oxide (ITO) electrode. The substrate surface was polyimide coated. The rubbing directions in the top and bottom plates are set in an antiparallel way and the cell gap is around 6 μm. Part A shows the textures of unswitched SmC* phases with the helical axis (the reference axis) set parallel to one of the cross polarizers. Part B shows the textures observed on applying a plus DC voltage (+100 V) with the top plate positively charged. The helix unwinds and the material poles in a uniform direction with its spontaneous polarization being coupled to the applied field. As a result, the material becomes bright; hence, the extinction direction is now at an angle relative to the reference direction. Part C shows the textures observed after the sample is rotated through the minimum angle required to return it to extinction. The extinction direction from polarizer axis is estimated as a tilt angle. In Part B, the relationship between the polarization direction and the tilted direction within a layer is illustrated according to the definition in Fig. 4. | ||
The rotational sense of the sample defines the direction that the molecules are tilted with respect to the layer normal, and thus the direction of the spontaneous polarization. The relationship between the tilt and polarization directions is illustrated in Fig. 4. Here, there are two possible directions of the spontaneous polarization to the tilt one (see Fig. 4a). When the dipoles that produce the spontaneous polarization act along the positive x direction, and when an object molecule is tilted back into the page in the zy-plane, positive polarization (PS(+)) occurs; the reverse situation gives rise to negative polarization (PS(−)).1Fig. 4b shows a schematic illustration of the spatial relationship between PS(+) and PS(−) with respect to the tilt axis of the molecule (the repeating unit in this polymeric case). When the results of Fig. 3 are connected with this spatial relationship, we obtain PS(+) for P-40(HM)/60(S-MOB) and PS(−) for P-40(HM)/60(R-MOB). The opposite trend is clearly observed for MB-based polymers: PS(−) for P-40(HM)/60(S-MB) and PS(+) for P-40(HM)/60(R-MB). The spatial alignments of molecules according to the expression of Fig. 4b are illustrated in Part B of Fig. 3.
 | ||
| Fig. 4 (a) The relationship between the tilt direction and the polarization direction in the ferroelectric SmC* phase and (b) an illustration of the repeating unit with PS(+) and PS(−) projected along the polarization direction. | ||
The opposite direction of spontaneous polarization between MOB- and MB-based polymers is interesting. The dipole moment in an MB-based polymer mainly arises from the ester linkage groups in the backbone, while that in an MOB-based polymer contains an additional contribution from the methoxy group in the butylene spacer. Thus, the average direction of the dipole moment in the methoxy group is suggested to be opposite to that of the backbone ester group.
Fig. 5a shows the typical switching behavior observed for the SmC* of P-40(HM)/60(S-MOB) under a triangular wave field at 25.0 Hz, which is a well-known way to determine its ferroelectricity and magnitude of PS.12 A single switching current peak on one cycle of the field confirms its ferroelectricity. In Fig. 5b, the values (magnitudes) of spontaneous polarization (PS) are plotted against the temperature. A general trend can be observed with PS increasing with decreasing temperature. Note that the maximum magnitude of PS for the MOB-based polymer is around 100 nC cm−2, significantly larger than that of the MB-based polymer, 20 nC cm−2. Considering the opposite sign of the PS between two types of polymers, the difference of PS is about 120 nC cm−2, which is attributable to a contribution from the dipole moment in the methoxy group.
 | ||
| Fig. 5 (a) Polarization reversal current in the SmC* phase of P-40(HM)/60(S-MOB) observed under the application of a triangular wave (temperature: 135 °C, voltage: 340 Vpp, frequency: 25 Hz, cell gap: 4.5 μm). Ferroelectric switching is ensured by a single switching current peak. (b) Plotting of the spontaneous polarization collected in P-40(HM)/60(S-MOB) and P-40(HM)/60(S-MB) against the temperature. | ||
3.5. Structural model producing undulation and polarization modulation on film surface
From the overall results, we obtain the key information on1. the helical conformation of polymer,
2. the relative direction of spontaneous polarization to the tilt direction of mesogen, and
3. the relationship between the undulation and polarization surface structures,
and thus we can describe how the helical polymer is accommodated to produce the undulation and polarization surface structure. In P-40(HM)/60(S-MOB), for instance, the peak of the undulation is negatively charged and the valley is positively charged from the combined observations of AFM and KFM images. By coupling this with the PS(+) elucidated from the electro-optic measurement, the repeating units are set as illustrated in Fig. 6. Because the continuous linking of repeating units makes a polymer, P-40(HM)/60(S-MOB) assumes the right-handed helix.13 By a similar argument, P-40(HM)/60(R-MOB) with PS(−) assumes the left-handed helix. On the other hand, MB-based polymers show the positive charge on the peak and the negative charge in the valley, leading to the right-handed helix with PS(−) for P-40(HM)/60(S-MB) and the left-handed helix with PS(+) for P-40(HM)/60(R-MB). The overall structural features are schematically represented in Fig. 7. Note that the helical senses thus determined correspond to those elucidated from the reflection CD band.
 | ||
| Fig. 6 Illustration of the polarization on the undulated surface in P-40(HM)/60(S-MOB). The peak of the undulation is negatively charged and the valley is positively charged. By coupling this with the PS(+), the repeating units are set as illustrated. When the repeating units are continuously linked, the polymer shown by dashed curve assumes the right-handed helix. | ||
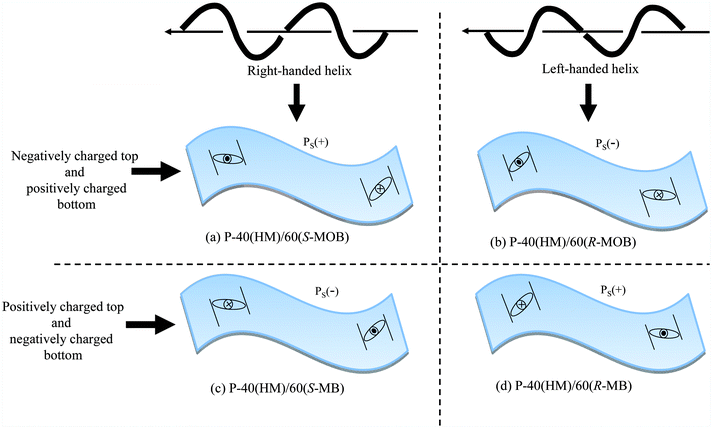 | ||
| Fig. 7 Illustration of the surface undulation and polarization modulation with the PS(+) or PS(−) produced by the helical polymers in four types of polymer. | ||
4. Concluding remarks
In conclusion, SmC* shows undulation on its solid film. Such an undulation is caused by helical conformation of the constituent polymer, which conforms to the helical structure of SmC*. The combined observations of KFM with AFM represent the clear surface image where the polarization modulation is superimposed on the undulation. This is due to the ferroelectric structure of the SmC*; each smectic layer has spontaneous polarization, the direction of which is parallel to the layer and perpendicular to the tilt direction of molecules within a layer and rotates around the normal to the layer to form the helical structure. Thus, we can produce the functional solid surface with regular undulation and polarization modulation using the monodomain state of the SmC* LC.The present studies also reveal that there is a remarkable difference in the SmC* structural properties, helical twisting power, and spontaneous polarization, between MOB- and MB-based polymers. MOB-based polymers exhibit twisting power and spontaneous polarization larger than MB-based polymers. This indicates the significant contribution of the dipole moment in the methoxy group. The magnitude of spontaneous polarization is extremely large in the MOB-based polymer; maximum values are around 100 nC cm−2 for the MOB-based polymer and 20 nC cm−2 for the MB-based polymer. Considering the common backbone and the opposite sign of PS in these two polymers, the methoxy group contributes to create the spontaneous polarization of about 120 nC cm−2. In future, this study will be further extended to understand these effects of the methoxy group through the conformational analysis of the chiral spacer group.
Acknowledgements
This research was supported by the Grant-in-Aid for Creative Scientific Research from Ministry of Education, Science, Sports and Culture.References
- J. W. Goodby, R. Blinc, N. A. Clark, S. T. Lagerwall, M. A. Osipov, S. A. Pikin, T. Sakurai, K. Yoshino, B. Zeks, Ferroelectric Liquid Crystals; Principles, Properties and Applications, Gordon and Breach Science Publishers: Paris, 1991, vol. 7, pp. 99–241 Search PubMed.
- J. Watanabe, M. Hayashi, A. Morita and M. Tokita, Macromolecules, 1995, 28, 8073 CrossRef CAS.
- J. Watanabe, M. Hayashi, Y. Nakata, T. Niori and M. Tokita, Prog. Polym. Sci., 1990, 22, 1053.
- C. Zhang, S. Edo, R. Ishige, M. Tokita and J. Watanabe, Macromolecules, 2008, 41, 5361 CrossRef CAS.
- L. J. Guo, J. Phys. D: Appl. Phys., 2004, 37, R123 CrossRef CAS.
- W. Lee, M.-K. Jin, W.-C. Yoo and J.-K. Lee, Langmuir, 2007, 20, 7665.
- J. Watanabe and M. Hayashi, Macromolecules, 1989, 22, 4083 CrossRef CAS.
- M. Tokita, K. Tokunaga, S. Funaoka, K. Osada and J. Watanabe, Macromolecules, 2004, 37, 2527 CrossRef CAS.
- K. P. Puntambekar, P. V. Pesavento and C. D. Frisbie, Appl. Phys. Lett., 2003, 83, 5539 CrossRef CAS.
- M. Chiesa, L. Burgi, J.-S. Kim, R. Shikler, R. H. Friend and H. Sirringhaus, Nano Lett., 2005, 5, 559 CrossRef CAS.
- M. Luna, D. F. Ogletree and M. Salmeron, Nanotechnology, 2006, 17, S178 CrossRef CAS.
- K. Miyasato, S. Abe, H. Takezoe, A. Fukuda and E. Kuze, Jpn. J. Appl. Phys., 1983, 22, L661.
- R. S. Cahn, C. Ingold and V. Prelog, Angew. Chem., Int. Ed. Engl., 1966, 5, 385 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Scheme S1, Fig. S1 and S2. See DOI: 10.1039/c0sm00668h |
| This journal is © The Royal Society of Chemistry 2011 |
