In vitro gene expression and enzyme catalysis in bio-inorganic protocells†
Mei
Li
a,
David C.
Green
a,
J. L. Ross
Anderson
b,
Bernard P.
Binks
c and
Stephen
Mann
*a
aCentre for Organized Matter Chemistry, School of Chemistry, University of Bristol, Bristol, BS8 1TS, UK
bSchool of Biochemistry, University of Bristol, Bristol, BS8 1TD, UK
cSurfactant & Colloid Group, Department of Chemistry, University of Hull, Hull, HU6 7RX, UK
First published on 7th July 2011
Abstract
Silica nanoparticles with a balance of hydrophilic and hydrophobic surface properties exhibit surfactant-like behaviour, and as a consequence can strongly adsorb at oil/water interfaces to stabilize the formation of water micro-droplets. Here we exploit this strategy to construct a model of a primitive bio-inorganic protocell, which unlike conventional paradigms based on self-assembled vesicles, is structurally delineated by a porous inorganic membrane rather than a lipid-based bilayer. As proof-of-principle we show that the nanoparticle-stabilized droplets (colloidosomes) can support a range of functionally active biomolecules and bio-machinery related to metabolic and informational processing. Specifically, we demonstrate that the rate of cell-free in vitrogene expression of enhanced green fluorescent protein (eGFP) is essentially the same within the colloidosome interior as in bulk aqueous solution. In addition, we report considerable enhancements in the specific activity of enzymes such as lipoprotein lipase, chymotrypsin or alkaline phosphatase when entrapped within the nanoparticle-stabilized water droplets. Our results suggest that artificial protocells based on the construction of biological/inorganic nanoscale components could have considerable potential in areas such as synthetic biology and bionanotechnology. In a wider perspective, studies on bio-inorganic protocells could provide alternative models for evaluating potential prebiotic pathways prior to the emergence of lipid-based compartmentalization on the early Earth.
Introduction
Engineering artificial cellular systems with life-like properties is of major concern and importance in fields such as synthetic biology, bionanotechnology and origins of life research.1 The ability to design microscale compartments that mimic aspects of primitive metabolism and/or replication should provide a plethora of novel developments in living technologies of the future,2 and contribute profoundly to our understanding and elucidation of plausible prebiotic pathways responsible for the emergence of life on the early Earth.3 Current studies on synthetic protocell models have focused on minimal systems that confine and support biological reactions in localized volumes of aqueous space delineated by organic boundaries. For the most part, this has been achieved by entrapping biomolecules and biological machinery within the aqueous interior of phospholipid bilayer vesicles. In so doing, key processes such as enzyme catalysis,4 enzymatic-mediated RNA synthesis5 and replication,6 polymerase chain reaction (PCR)-induced DNA amplification,7 and gene expression of single components8 or cascading networks9,10 have been successfully demonstrated. Such processes are often limited by the high impermeability of the lipid bilayer that prevents continuous activity within the vesicles due to mass transfer and osmotic pressure restrictions. In contrast, artificial protocells based on the layer-by-layer fabrication of polymer hydrogel capsules11 exhibit high levels of permeability, and have been exploited in a wide range of enzymatic reactions,12–16 as well as in PCR-mediated DNA amplification,17 and RNA synthesisviaDNA transcription.18Given the complexity of lipid synthesis and polymer hydrogel shell production, it is conceivable that more simple representations of compartmentalization were generated on the early Earth prior to the emergence of supramolecular containers based on organic macromolecules and long chain amphiphiles. In this regard, the deposition of mineral sulphides around hydrothermal vents in the form of chambered black-smoker chimneys has been suggested as a plausible scenario for the confinement and catalysis of primitive metabolic cycles.19 However, to the best of our knowledge, the possibility of discrete, free-floating protocells that are structurally delineated by a semi-permeable inorganic membrane has not been considered, even though the use of inorganic colloids in emulsion stabilization (Pickering emulsions) has been known for many years.20,21 These so-called “colloidosomes” have selective permeabilities22,23 and can be prepared for example from functional nanoparticles with magnetic23 or semiconducting24 properties. Moreover, recent studies have shown that the self-assembly of inorganic nanoparticles at various interfaces can be explicitly controlled by designing the appropriate hydrophobic/hydrophilic balance at the particle surface.25 As a consequence, silica nanoparticles with different levels of silanol (–O3SiOH) and dimethylsilane (–O2Si(CH3)2) surface groups have been used to prepare various types of dispersed micro-compartments such as water-in-oil, oil-in-water, air-in-water, and water-in-air (so-called “dry water”) droplets.25
In this paper, we use 20–30 nm-sized hydrophobic/hydrophilic silica nanoparticles to stabilize the formation of microscale water droplets in oil, and exploit these capsules as a model of a protocell that is delineated specifically by a semi-permeable inorganic membrane. We show that a range of biomolecules can be entrapped without denaturation within the inorganic compartments, and that the colloidosomes can be surface-modified to facilitate phase transfer as intact structures into a continuous water phase. Significantly, we demonstrate that a large ensemble of biomolecules in the form of a cell-free gene expression system can be encapsulated, and subsequently used in the presence of entrapped plasmid DNA for in vitroprotein synthesis. We also show three examples of enzyme catalysis that can be undertaken and sustained within the nanoparticle-enclosed water droplets; in each case, considerable enhancements in specific enzyme activity are achieved due to phase transfer through the inorganic membrane. Taken together, our results demonstrate the potential of colloidosomes as microscale bio-reactors, and suggest that studies on bio-inorganic protocells could provide an alternative paradigm of pre-biotic organization that is not based on lipid compartmentalization.
Results and discussion
Addition of a dry powder of surface-modified 20–30 nm-sized silica nanoparticles to a water/dodecane mixture at an aqueous/oil volume fraction (φw) of 0.033 resulted in the sedimentation of a turbid lower phase comprising a water-in-oil emulsion below a transparent upper oil layer. Similar results were obtained using aqueous solutions of various enzymes (lipoprotein lipase, α-chymotrypsin, alkaline phosphatase), proteins (cytochrome c, ferritin, myoglobin), metal ion salts (Cu(NO3)2), or gold nanoparticles (Fig. 1a). In each case, the lower layer was shown to comprise a Pickering emulsion of nanoparticle-stabilized water micro-droplets in oil (Fig. 1b). As a consequence, the colloidosomes were structurally stable and did not coalesce at room temperature over several months. However, direct transfer of the nanoparticle-stabilized droplets from the emulsion phase into pure water resulted in disassembly of the silica nanoparticle membrane and release of the entrapped solutes. This could be circumvented by stabilizing the inorganic shell with respect to aqueous phase transfer by addition of small amounts of tetramethoxysilane to the emulsion oil phase after assembly of the colloidosomes. This procedure resulted in the deposition of a secondary shell of amorphous silica that blocked the interstices between the nanoparticles of the primary layer to produce an impermeable inorganic membrane that prevented leakage of the entrapped biomolecules from the aqueous droplets into the continuous water phase (Fig. 2a). Corresponding SEM investigations of the colloidosomes after transfer into water confirmed the presence of a thin continuous membrane of occluded silica nanoparticles (Fig. 2b).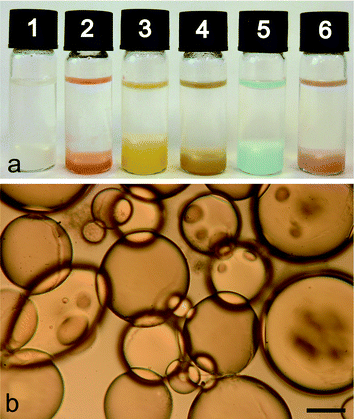 | ||
| Fig. 1 (a) Photograph of sample tubes showing phase separation into a colourless dodecane upper layer and turbid water-in-oil emulsion lower layer comprising silica nanoparticle-stabilized micro-droplets of (1) pure water, or aqueous solutions of (2) cytochrome c, (3) ferritin, (4) myoglobin, (5) Cu(NO3)2 and (6) Au nanoparticles. (b) Optical microscopy image of silica nanoparticle-stabilized water droplets in dodecane produced at φw = 0.033 and a loading of 0.3 mg nanoparticles per μL of aqueous phase. Scale bar = 100 μm. | ||
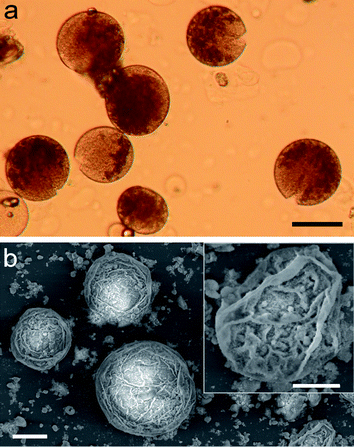 | ||
| Fig. 2 (a) Optical micrograph showing intact silica-stabilized microdroplets of the aqueous protein, ferritin, after shell reconstruction and transfer into water. The red-brown colouration of ferritin is associated specifically with the droplet interior, consistent with a sealed bio-inorganic compartment. Scale bar = 100 μm. (b) SEM micrograph of silica-stabilized colloidosomes after shell reconstruction and transfer into water; scale bar = 100 μm. Inset, higher magnification image showing detail of partially collapsed inorganic membrane; scale bar = 50 μm. | ||
The size, and to a lesser extent, shape of the silica nanoparticle-stabilized water microdroplets were dependent on the amount of particles added per unit volume of the aqueous phase at a constant φw value of 0.033. Most experiments were carried out using a loading of 0.3 mg silica nanoparticles per μL of the aqueous phase; under these conditions a polydisperse population of well defined spherical droplets with a mean diameter of 256 (σ = 159) μm was observed (Fig. S1†). Lowering the amount of silica nanoparticles to values of 0.1 or 0.2 mg μL−1 generated larger, irregularly shaped colloidosomes up to 800 μm in size, whereas increased levels of the inorganic component (0.4 and 0.5 mg μL−1) produced well defined spherical droplets that were often less than 100 μm in diameter (Fig. 3). The irregularity in shape observed at low nanoparticle concentrations appeared to often originate from the fusion of preformed droplets that became immobilized after coalescence. These observations were consistent with a stabilization mechanism that was dependent on the surface area/volume-dependent coverage of the droplets with an adsorbed monolayer of the hydrophilic/hydrophobic silica nanoparticles.25
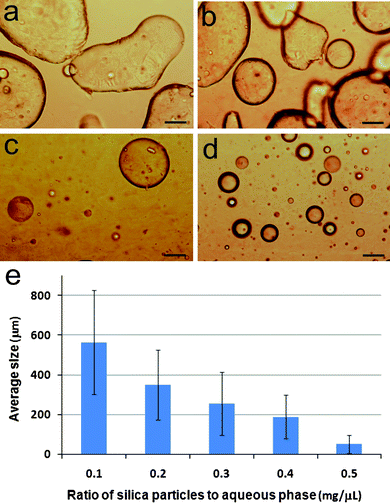 | ||
| Fig. 3 (a–d) Optical microscopy images of silica nanoparticle-stabilized water droplets in dodecane produced at φw = 0.033 and nanoparticle loading per μL of aqueous phase of (a) 0.1, (b) 0.2, (c) 0.4 and (d) 0.5 mg. Scale bars = 100 μm. (e) Plot showing mean size (bars) and standard deviations (lines on bars) of colloidosomes prepared at silica nanoparticle loadings between 0.1–0.5 mg μL−1 at φw = 0.033. | ||
To demonstrate the scope of nanoparticle-stabilized micro-droplets as plausible bio-inorganic protocells, we prepared silica-based colloidosomes as above but in toluene and in the presence of a cell-free in vitrogene expression aqueous solution that contained at least two hundred functional components, including ribosomes, tRNA, transcription and translation co-factors, reaction buffer, nucleotides for mRNA synthesis, amino acids for translation, and an ATP regeneration system to prolong and sustain gene expression. In vitro expression of enhanced green fluorescent protein (eGFP) was undertaken by constructing a plasmid DNA vector, pEXP5-NT/eGFP, using polymerase chain reaction procedures, and adding the plasmid to the cell-free gene expression solution prior to formation of the colloidosomes. Optical microscopy images showed well defined silica nanoparticle-stabilized spherical aqueous droplets, 20–150 μm in diameter. Significantly, in vitro synthesis of eGFP within the droplets over a period of 24 h was confirmed by fluorescence microscopy, which indicated that the expressed eGFP was confined specifically to the water droplets (Fig. 4). In contrast, only a weak background autofluorescence was observed at 540 nm in the absence of GFP synthesis. Expression of eGFP within the bio-inorganic compartments or in free aqueous solution was monitored over time by measuring the increase in fluorescence intensity at 509 nm using an excitation wavelength of 470 nm (Fig. 5a and Fig. S2†). In both cases, the reaction profiles showed a similar quasi-exponential decrease in the rates of gene expression (Fig. 5b), which was attributed to the depletion of amino acids and ATP, as well as accumulation of by-products such as inorganic phosphate, in the reaction mixture.26Protein synthesis was essentially complete within 6 h in both systems. When normalized, the initial rates were similar, with a marginal ×1.5 increase observed for eGFP synthesis in the silica nanoparticle-stabilized droplets, possibly due to the increased local concentration of the gene expression components within the colloidosome compartments.
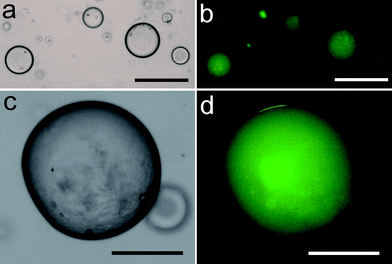 | ||
| Fig. 4 (a,c) Low and high magnification optical microscopy images showing silica nanoparticle-stabilized water droplets (toluene, φw = 0.033, 0.3 mg μL−1) containing plasmid pEXP5-NT/eGFP and a cell-free gene expression solution. (b,d) Corresponding fluorescence micrographs of droplets after 24 h incubation at 37 °C showing characteristic green fluorescence associated with in vitrogene expression of eGFP. Samples were excited in blue light. Scale bars; (a,b) 50 μm, (c,d) 100 μm. | ||
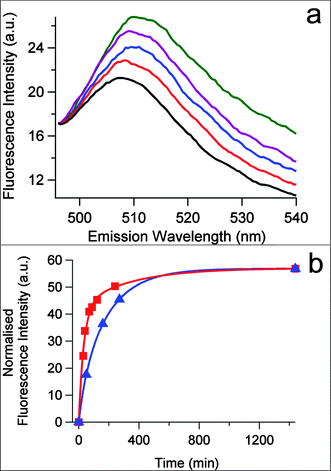 | ||
| Fig. 5 (a) Time dependent fluorescence spectra of silica nanoparticle-stabilized water droplets (toluene, φw = 0.033, 0.3 mg μL−1) containing the plasmid pEXP5-NT/eGFP and a cell-free gene expression solution recorded at 37 °C after 0 (black), 10 (red), 60 (blue), 210 (purple) and 1440 (green) min. (b) Plots showing time dependent change in fluorescence intensity at 509 nm corresponding to rate of expression of eGFP in silica nanoparticle-stabilized water droplets (red) or in a bulk water phase (blue). Arbitrary units (a.u) in the red curve were re-calibrated by normalization of the completed reaction values obtained at 1440 min for both red and blue plots in order to facilitate comparison of the rates. | ||
The above observations indicated that transcription of the pEXP5-NT/eGFP plasmid into mRNA followed by translation to the protein product via the ribosome machinery could be successfully undertaken within silica nanoparticle-stabilized aqueous compartments. Under the conditions employed, no inhibition in cell-free gene expression was observed even though putative interactions between the inorganic membrane and encapsulated biomolecules were expected to produce some level of interference in the expression pathway. However, the expression process was critically dependent on establishing a 30 min incubation period at 37 °C prior to addition of the feed buffer and encapsulation within the colloidosomes. This suggested that exposure of the aqueous reaction mixture to the toluene continuous phase during the very early stages of the transcriptional process severely inhibited in vitrogene expression. Thus, whilst we clearly demonstrated that in vitrotranslation occurred within the colloidosomes, we could not rule out the possibility that some transcriptional steps took place prior to encapsulation. As more advanced systems of in vitrogene expression have been successfully undertaken in surfactant-stabilized water-in-oil emulsion droplets,27 it seems likely that increasingly sophisticated procedures, such as the directed evolution of proteins and RNA, should also be possible using colloidosome-based compartmentalization. Thus, the construction of artificial protocells based on lipid-free bio-inorganic components could have significant potential in fields such as synthetic biology and bio-reactor technology.
As colloidosomes are known to exhibit semi-permeable properties,22,23 we envisaged that protocells fabricated from nanoparticle-based membranes could have several advantages over constructs produced for example from phospholipid bilayers, which are often compromised by their high solute impermeability. As proof-of-principle, we therefore tested whether the interfacial reaction of an oil-soluble substrate with an aqueous enzyme, followed by subsequent phase transfer of a water-soluble product into the droplet interior, could be undertaken and sustained by entrapment of enzymes specifically within the nanoparticle-enclosed compartments. We demonstrated this principle by preparing silica nanoparticle-stabilized droplets of an aqueous solution of the enzyme lipoprotein lipase (LPL), followed by addition of the substrate 4-nitrophenyl butyrate (PNPB, water solubility (s) at 25 °C = 145 mg L−1) to the continuous oil phase (dodecane). The colour of the emulsion phase became yellow within 2 min (Fig. 6a), indicating that the water-soluble product (p-nitrophenol, s = 7507 mg L−1) from the LPL-catalysed hydrolysis reaction was located specifically within the aqueous compartments. This confirmed that the enzyme molecules were encapsulated within the water micro-droplets without denaturation, and that the lipase was able to catalyze the organic-soluble substrate by reaction and phase transfer at regions of the oil/water interface that were exposed by the interstices in the surrounding inorganic membrane.
![(a) Sample tubes showing phase separation into a colourless dodecane upper layer and turbid water-in-dodecane emulsion lower layer comprising silica nanoparticle-stabilized micro-droplets of an aqueous LPL enzyme solution (φw = 0.033, 0.3 mg μL−1); (1) before and (2) 2 mins after addition of the PNPB substrate showing yellow colouration due to presence of water-soluble p-nitrophenol product in the colloidosome interiors. (b) Corresponding Lineweaver–Burk plot of 1/v (v = initial rate) against 1/[S] (S = substrate concentration) confirming Michaelis–Menten enzyme kinetics in the presence of the colloidosomes. (c) Plots showing inverse correlation between colloidosome mean diameter (blue circles) and enzyme initial reaction rate (red squares) for LPL-containing droplets prepared with different silica nanoparticle/aqueous phase volume ratios (mg μL−1).](/image/article/2011/SC/c1sc00183c/c1sc00183c-f6.gif) | ||
| Fig. 6 (a) Sample tubes showing phase separation into a colourless dodecane upper layer and turbid water-in-dodecane emulsion lower layer comprising silica nanoparticle-stabilized micro-droplets of an aqueous LPL enzyme solution (φw = 0.033, 0.3 mg μL−1); (1) before and (2) 2 mins after addition of the PNPB substrate showing yellow colouration due to presence of water-soluble p-nitrophenol product in the colloidosome interiors. (b) Corresponding Lineweaver–Burk plot of 1/v (v = initial rate) against 1/[S] (S = substrate concentration) confirming Michaelis–Menten enzyme kinetics in the presence of the colloidosomes. (c) Plots showing inverse correlation between colloidosome mean diameter (blue circles) and enzyme initial reaction rate (red squares) for LPL-containing droplets prepared with different silica nanoparticle/aqueous phase volume ratios (mg μL−1). | ||
Spectroscopic monitoring of the time-dependent change in PNPB concentration in the oil phase was used to construct a Lineweaver–Burk plot at 37 °C, and determine the maximum reaction rate (Vmax), Michaelis constant (KM) and specific activity associated with the LPL biomolecules confined within the water droplets (Fig. 6b). As for the enzyme reaction in bulk aqueous medium, Michaelis–Menten kinetics were observed in the presence of the colloidosomes. The values of KM and Vmax were 5.1 mM and 1250 μM min−1, respectively, compared with corresponding values of 2.5 mM and 1.46 μM min−1 for the enzyme in bulk aqueous solution. The large increase in Vmax indicated a much greater turnover number (kcat) under conditions of substrate saturation for the colloidosome system (302 s−1) compared with bulk aqueous solution (0.35 s−1). Significantly, the specific activity of LPL within the droplets was 250 μmol min−1 mg−1, which was 858 times higher than that of the same reaction carried out in aqueous solution at the same concentration of enzyme and substrate (specific activity = 0.29 μmol min−1 mg−1). We attributed the large increase in specific activity of the colloidosome-encapsulated LPL to removal of the p-nitrophenol product from the oil/water interface into the water phase of the droplets, and to significant expansion in interfacial area associated with compartmentalization. The latter mechanism was confirmed by performing the enzymatic reaction in colloidosomes of different mean size produced by varying the ratio of silica nanoparticles to the volume of the dispersed aqueous phase. Increasing the silica loading from 0.1 to 0.3 mg μL−1 decreased the droplet mean diameter from 564 (σ = 263) to 256 (σ =159) μm, and increased the initial reaction rate from 0.17 μM min−1 to 0.62 μM min−1 (Fig. 6c). We also confirmed that partitioning of the substrate and product between the oil and water phases, respectively, was also responsible for a small but significant increase in enzymatic behaviour. For this, control experiments were undertaken under identical conditions but in which an aqueous solution of LPL was directly added to dodecane in the absence of silica nanoparticles. Although the specific activity (0.4 μmol min−1 mg−1) was much reduced compared with the colloidosome-entrapped enzyme reaction, the activity was 1.4 times higher than that observed in bulk aqueous solution.
The above results suggested that the stabilization of enzyme-containing water droplets through interfacial assembly of a porous membrane of silica nanoparticles could provide a lipid-free route to the construction of microscale bio-reactors operating in oil media. To test the generality of this approach, and to develop the scope of such systems as potential bio-inorganic protocells with enhanced enzyme activity, we prepared water-in-dodecane emulsions of silica nanoparticle-stabilized aqueous droplets of the enzymes chymotrypsin (ChTRP) or alkaline phosphatase (ALP) with their corresponding substrates, N-benzoyl-L-tyrosine ethyl ester (BTEE, s = 135 mg L−1) or p-nitrophenyl phosphate (PNPP, s = 2556 mg L−1), respectively, added initially to the continuous oil phase. Whereas BTEE had high oil solubility, and therefore mirrored conditions described above for the LPL/PNPB system, PNPP was water soluble and rapidly transferred from the oil into the interior of the colloidosomes. In both cases, Lineweaver–Burk plots were observed, consistent with Michaelis–Menten kinetics for the production of the corresponding water soluble products, N-benzyl-L-tyrosine (s = 907 mg mL−1) and p-nitrophenol (s = 7507 mg L−1) (Fig. S3 and S4†). The values for KM, Vmax, kcat and specific activity of ChTRP within the colloidosomes were 7.8 mM, 10235 μM min−1, 1264 s−1 and 3033 μmol min−1 mg−1, respectively; the latter was 30 times higher than that of the same reaction carried out under identical concentrations but in aqueous solution (specific activity = 101 μmol min−1mg−1, KM = 37 μM, Vmax = 337 μM min−1, kcat = 42 s−1). Similar observations were made for silica nanoparticle-stabilized droplets containing ALP, which exhibited a specific activity of 70422 μmol min−1 mg−1 (KM = 510 mM, Vmax = 35211 μM min−1, kcat = 1.89 × 105 s−1) that was 424 times higher than the same reaction in bulk aqueous solution (specific activity = 166 μmol min−1mg−1, KM = 12.6 mM, Vmax = 83 μM min−1, kcat = 446 s−1).
Conclusions
In this paper we have successfully demonstrated that complex biological processes such as in vitrogene expression and enhanced enzymatic transformations can be undertaken within micro-droplets of water stabilized by the interfacial self-assembly of silica nanoparticles comprising an appropriate balance of hydrophilic and hydrophobic surface groups. In a wider context, this compartmentalized micro-system can be considered as a novel type of bio-inorganic protocell, in which a chemically inert, semi-permeable inorganic nanostructured membrane provides structural stability, confinement, and material exchange and interaction with the external environment. As more conventional approaches involving surfactant-stabilized enzyme-containing emulsion water droplets28 often lead to denaturation of the encapsulated biomolecules,29 microscale bioreactors based on colloidosome self-assembly could have significant potential in the development of new procedures in synthetic biology and bionanotechnology. From a wider perspective, given the ability of nanoparticle-stabilized droplets to support both informational and metabolic types of biological activity, it seems feasible that studies on bio-inorganic protocells could provide a model system for evaluating plausible prebiotic pathways to the emergence of lipid-free compartmentalization on the early Earth.Experimental procedures
Preparation of silica nanoparticles and water-in-oil colloidosomes
Hydrophilic/hydrophobic silica nanoparticles with spherical morphology and primary mean diameter of 20–30 nm were prepared as described previously.25,30 In brief, hydrophilic silica nanoparticles were silylated to various extents by reaction with dichlorodimethylsilane in the presence of water followed by drying at 300 °C for 2 h. The resulting hydrophilic/hydrophobic particles were surface functionalized with ca. 50% silanol (–O3SiOH) and 50% dimethylsilane (–O2Si(CH3)2) groups.Colloidosomes consisting of silica nanoparticle-stabilized micro-droplets of pure water or aqueous solutions of enzymes (see below), proteins (cytochrome c, ferritin, myoglobin), metal ion salts (Cu(NO3)2), citrate-capped 13 nm-sized gold nanoparticles, or an in vitrogene expression kit (see below) were produced in oil as follows. Typically, 100 μL of an aqueous solution were added to 30 mg of the partially silylated silica nanoparticles and mixed by gentle rotation. 3 mL of anhydrous dodecane (or toluene for gene expression experiments) was then added, and the mixture hand-shaken vigorously for 1 min to facilitate colloidosome formation. The volume fraction of aqueous phase relative to oil (φw) was fixed at 0.033, and the amount of silica nanoparticles used was between 0.1–0.5 mg per μL of the aqueous phase.
Silica nanoparticle-stabilized aqueous droplets in oil were transferred into a continuous water phase as follows. 50 μL (321 μmoles) of tetramethoxysilane (TMOS) was added to 3 mL of the emulsion and the mixture left standing at room temperature overnight, after which the top clear oil phase was discarded and 1 mL of ethanol added to the emulsion and gently shaken. The dispersion was then centrifuged at 3000 rpm for 10 s and the top clear solution discarded. Another 1 mL of ethanol was added and the dispersion re-centrifuged. This was then repeated, first with 1 mL of a 1: 1 ethanol/water mixture, and then finally with 1 mL of distilled water to complete the phase transfer process.
Construction of pEXP5-NT/eGFP plasmid
The egfpgene for encoding the folding- and fluorescence-enhanced mutant of Green Fluorescent Protein (eGFP) was amplified from pET45b(+)-eGFP (Novagen®) and cloned into pEXP5-NT/CALML3 (Invitrogen™) using ligase-independent cloning to yield the plasmid pEXP5-NT/eGFP.31 Primers were designed to facilitate replacement of calml3 in pEXP5-NT/CALML3 while retaining the TEV-cleavable N-terminal hexahistidine tag: vecfwd 5′-CCCGAAAGGAAGCTGAGTTGGC-3′; lvecfwd 5′-AGGGTGA-TCCGGCTGCTAACAAAGCCCGAAAGGAAGCTGAGTTGGC-3′; vecrvs 5′-GCTGC-TACCATGATGATGATGATGATG-3′; lvecrvs 5′-AAGGGACTGAAAATACAG-GTTTTCGCCGCTGCTACCATGATGATGAT-GATGATG-3′; insfwd 5′-GTGGG-TACCGGTTCGAATGATGAC-3′; linsfwd 5′-GGCGAAAACCTGTATTTTCAGTCCCT-TGTGGGTACCGGTTCGAATG-ATGAC-3′; insrvs 5′-TTACCAGACTCGAGATC-TGAGTCCG-3′; linsrvs 5′-CTTTGTTAGCAGCCGGATCACCCTTTACCAGACTCGA-GATCTGAGTCCG-3′.In vitro gene expression
Cell-free gene expression of eGFP from pEXP5-NT/eGFP was undertaken using an in vitrogene expression system (Expressway™ Cell-Free E. coli Expression System, Invitrogen™) comprising S30 E coli extract, reaction buffer, proprietary T7 enzyme mix, amino acid (-Met) mixture and methionine solution. The kit was used according to the manufacturer's instructions unless otherwise stated. Typically, 20 μL of S30 E coli extract, 20 μL of the reaction and feed buffers, 1 μL proprietary T7 enzyme mix, 1.25 μL of a 50 mM amino acid (-Met) mixture, 1 μL 75 mM methionine solution and 909 ng of pEXP5-NT/eGFP were mixed together, made up to a final volume of 50 μL with DNAase/RNAse-free water, and incubated in a 1.5 mL PCR-ready, sterile centrifuge tube at 37 °C for 30 min. After incubation, a feed solution containing 25 μL feed buffer, 2.5 μL of 50 mM amino acid (-Met) mixture, 2 μL 75 mM methionine solution and 20.5 μL of DNAase/RNAse-free water was added to the extract/reaction mixture, which was gently homogenised by tapping the side of the vial to produce an in vitrogene expression solution with a final volume of 100 μL.Water-in-oil colloidosomes were prepared as described above using 50 μL of the aqueous in vitrogene expression solution and 1.5 mL of toluene as the oil phase (φw = 0.033, 0.3 mg silica nanoparticles/μL aqueous phase). (Dodecane was not used as the continuous oil phase due to quenching of eGFP fluoresecence). The aqueous cell-free gene expression solution was incubated for an initial period of 30 mins at 37 °C before encapsulation in the silica-stabilized water droplets in order to initiate the in vitro expression system. Minimal protein synthesis was observed during this incubation time. The resulting emulsion was then incubated at 37 °C for 24 h. In vitrogene expression of eGFP was monitored on individual droplets by using bright-field optical and fluorescence (excitation 450–490 nm) microscopies. Protein synthesis was also monitored by measuring the time-dependent change in fluorescence intensity at 509 nm using an excitation wavelength of 470 nm (Jasco FP-6500 spectrofluorometer). Control studies on cell-free gene expression in water in the absence of colloidosomes were also undertaken. Plots of the time-dependent change in eGFP concentration for the control or colloidosome systems were normalised to take into account the large difference in total concentrations and scattering effects between the two systems. For this, the fluorescence intensity associated with completion of eGFP synthesis in the colloidosomes after 24 h was set to the same intensity value determined after 24 h for gene expression in the control. Other intensity values on the colloidosome profile were then re-calibrated accordingly.
Evaluation of enzyme activity in silica nanoparticle-stabilized water micro-droplets
Studies on enzyme-mediated reactions in colloidosomes were carried out using the following biomolecules and reagents: Lipoprotein lipase (Pseudomonas sp. lyophilized powder, 1493 units mg−1), α-chymotrypsin (bovine pancreas, Type VII, essentially salt-free and TLCK-treated to inactivate residual tryspin activity, lyophilized powder, 40 units mg−1), 4-nitrophenyl butyrate, N-benzoyl-L-tyrosine ethyl ester, p-nitrophenyl phosphate, and anhydrous dodecane and toluene were purchased from Sigma-Aldrich. Alkaline phosphatase (2000 units mg−1) was purchased from CALBIOCHEM.14.93 units of lipoprotein lipase (LPL) in tris buffer (0.1 M, pH 7.2) were encapsulated within silica-stabilized water droplets prepared at 37 °C in 2 mL of dodecane at φw = 0.033 and 0.3 mg silica nanoparticles per μL LPL. Various amounts of substrate (4-nitrophenyl butyrate, PNPB, 50 mM stock solution in dodecane) were added to the oil phase to give final concentrations between 25 μM and 125 μM, and the reaction rates within the unstirred colloidosomes determined by monitoring the time-dependent change in PNPB concentration (optical density at 265 nm) in the oil phase using a UV-vis spectrometer (Perkin Elmer Lambda II UV-VIS) equipped with a PTP-6 Peltier temperature controller. Experiments were also undertaken at φw = 0.033 and 0.1 or 0.2 mg silica nanoparticles per μL LPL.
The maximum enzymatic rate (Vmax) and Michaelis constant (KM) were determined from the dependence of the initial reaction rate (υ0) on substrate concentration [S], using a standard Lineweaver–Burk plot of 1/[S] against 1/υ0, according to the relationship32
| 1/υ0 = (KM + [S])/(Vmax[S]) = {(KM/Vmax) × 1/[S]} + 1/Vmax |
The specific activity of LPL was calculated from the value of Vmax, according to the relationship; specific activity = (Vmax × total volume of oil and aqueous phases)/(mass of enzyme). The turnover number kcat. which is the maximum number of molecules of substrate that an enzyme can convert to product per catalytic site per unit of time, was determined from kcat = Vmax/[Eo], where [Eo] was the initial enzyme concentration.
Experiments were also undertaken at φw = 0.033 and 0.3 mg silica particles per μL using chymotrpsin (ChTRP, 0.26 units, tris buffer (80 mM, pH 7.8, 25 °C, containing 53 mM CaCl2)), and various amount of substrate (N-benzoyl-L-tyrosine ethyl ester, BTEE). Stock solutions of BTEE (40 mM) were prepared in a 3:1 ethanol:dodecane mixed solvent, and aliquots added to 2 mL of dodecane to give final concentrations ranging from 0.125 mM to 1 mM. Reaction rates within the unstirred colloidosomes were obtained by spectroscopically monitoring the change with time in BTEE concentration in the oil phase using the change in optical density at 279 nm. A Lineweaver–Burk plot of 1/[S] against 1/υ0 was used to determine values for Vmax, KM and the specific activity.
Similar studies were performed at φw = 0.033 and 0.3 mg silica particles per μL using alkaline phosphatase (ALP, 0.013 units, glycine buffer (0.1 M, pH 8.8, 37 °C, containing 1 mM MgCl2). Various amounts of substrate (p-nitrophenyl phosphate, PNPP, 0.69 M in a mixed solvent of 1:1.7:0.8 water:ethanol:chloroform) were added to dodecane to give final concentrations from 3.45 mM to 10.35 mM. The rates of enzymatic activity were obtained by monitoring the change in optical density at 310 nm associated with time-dependent decrease in the concentration of PNPP.
In each case, values of VmaxKM, kcat and specific activity determined for the above colloidosome-entrapped enzymes were compared with kinetic parameters obtained from identical reactions undertaken in unstirred bulk aqueous solutions at constant temperature. PNPB and BTEE were dissolved in acetonitrile or methanol, respectively, and then added to the corresponding aqueous solutions of LPL or ChTRP. PNPP was dissolved directly into the aqueous solution of ALP.
Acknowledgements
We thank EPSRC (Grant EP/F023626/1) for financial support, and the Centre for Nanoscience and Quantum Information, University of Bristol, for use of optical and fluorescence microscopes. We also thank Wacker-Chemie for the supply of fumed silica particles.References
- Protocells: Bridging Nonliving and Living Matter. ed. S. Rasmussen, M. A. Bedau, L. Chen, D. Deamer, D. C. Krakauer, N. H. Packard, and P. F. Stadler, MIT Press, 2009 Search PubMed.
- A. Porhorille and D. Deamer, Trends Biotechnol., 2002, 20, 123–128 CrossRef.
- J. P. Schrum, T. F. Zhu and J. W. Szostak, Cold Spring Harbor Perspect. Biol., 2010, 2(9) CAS , a002212, 1–15.
- P. Walde and S. Ichikawa, Biomol. Eng., 2001, 18, 143–177 CrossRef CAS.
- P. Walde, A. Goto, P. A. Monnard, M. Wessicken and P. L. Luisi, J. Am. Chem. Soc., 1994, 116, 7541–7547 CrossRef CAS.
- T. Oberholzer, R. Wick, P. L. Luisi and C. K. Biebricher, Biochem. Biophys. Res. Commun., 1995, 207, 250–257 CrossRef CAS.
- T. Oberholzer, M. Albrizio and P. L. Luisi, Chem. Biol., 1995, 2, 677–682 CrossRef CAS.
- S. M. Nomura, K. Tsumoto, T. Hamada, K. Akiyoshi, Y. Nakatani and K. Yoshikawa, ChemBioChem, 2003, 4, 1172–1175 CrossRef CAS.
- K. Ishikawa, K. Sato, Y. Shima, I. Urabe and T. Yomo, FEBS Lett., 2004, 576, 387–390 CrossRef CAS.
- V. Noireaux and A. Libchaber, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 17669–17674 CrossRef CAS.
- B. Städler, A. D. Price, R. Chandrawati, L. Hosta-Rigau, A. N. Zelikin and F. Caruso, Nanoscale, 2009, 1, 68–73 RSC.
- Y. Wang and F. Caruso, Chem. Commun., 2004, 1528–1529 RSC.
- R. Srivastava, J. Q. Brown, H. Zhu and M. J. McShane, Macromol. Biosci., 2005, 5, 717–727 CrossRef CAS.
- A. Yu, I. Gentle, G. Lu and F. Caruso, Chem. Commun., 2006, 2150–2152 RSC.
- T. Borodina, E. Markvicheva, S. Kunizhev, H. Moehwald, G. B. Sukhorukov and O. Kreft, Macromol. Rapid Commun., 2007, 28, 1894–1899 CrossRef CAS.
- O. Kreft, M. Prevot, H. Moehwald and G. B. Sukhorukov, Angew. Chem., Int. Ed., 2007, 46, 5605–5608 CrossRef CAS.
- W. C. Mak, K. Y. Cheung and D. Trau, Adv. Funct. Mater., 2008, 18, 2930–2937 CrossRef CAS.
- A. D. Price, A. N. Zelikin, K. L. Wark and F. Caruso, Adv. Mater., 2010, 22, 720–723 CrossRef CAS.
- W. Martin and M. J. Russell, Philos. Trans. R. Soc. London, Ser. B, 2003, 358, 59–85 CrossRef CAS.
- S. U. Pickering, J. Chem. Soc. Trans., 1907, 91, 2001–2021 RSC.
- B. P. Binks, Curr. Opin. Colloid Interface Sci., 2002, 7, 21–41 CrossRef CAS.
- A. D. Dinsmore, M. F. Hsu, M. G. Nikolaides, M. Marquez, A. R. Bausch and D. A. Weitz, Science, 2002, 298, 1006–1009 CrossRef CAS.
- H. Duan, D. Wang, N. S. Sobal, M. Giersig, D. G. Kurth and H. Möhwald, Nano Lett., 2005, 5, 949–952 CrossRef CAS.
- Y. Lin, H. Skaff, A. Boker, A. D. Dinsmore, T. Emrick and T. P. Russell, Science, 2003, 299, 226–229 CrossRef CAS.
- (a) B. P. Binks and R. Murakami, Nat. Mater., 2006, 5, 865–869 CrossRef CAS; (b) S. Arditty, C. P. Whitby, B. P. Binks, V. Schmitt and F. Leal-Calderon, Eur. Phys. J. E, 2003, 11, 273–281 CrossRef CAS.
- V. Noireaux, Y. T. Maeda and A. Libchaber, Proc. Natl. Acad. Sci. U. S. A., 2011, 108, 3473–3480 CrossRef CAS.
- B. T. Kelly, J.-C. Baret, V. Taly and A. D. Griffiths, Chem. Commun., 2007, 1773–1788 RSC.
- R. Sheldon, Adv. Synth. Catal., 2007, 349, 1289–1307 CrossRef CAS.
- Y. L. Khmelnitsky, R. Hilhorst, A. Visser and C. Veeger, Eur. J. Biochem., 1993, 211, 73–77 CrossRef CAS.
- B. P. Binks and S. O. Lumsdon, Langmuir, 2000, 16, 8622–8631 CrossRef CAS.
- (a) D. Tillett and B. A. Neilan, Nucleic Acids Res., 1999, 27, e26 CrossRef CAS; (b) H. Chiu, P. E. March, R. Lee and D. Tillett, Nucleic Acids Res., 2004, 32, e174 CrossRef.
- L. A. Segel and M. Slemrod, SIAM Rev., 1989, 31, 446–477 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c1sc00183c |
| This journal is © The Royal Society of Chemistry 2011 |
