Symmetry related [DyIII6MnIII12] cores with different magnetic anisotropies†
Jun-Liang
Liu
a,
Fu-Sheng
Guo
a,
Zhao-Sha
Meng
a,
Yan-Zhen
Zheng
d,
Ji-Dong
Leng
a,
Ming-Liang
Tong
*a,
Liviu
Ungur
b,
Liviu F.
Chibotaru
*b,
Katie J.
Heroux
c and
David N.
Hendrickson
*c
aKey Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, State Key Laboratory of Optoelectronic Materials and Technologies, School of Chemistry & Chemical Engineering, Sun Yat-Sen University, Guangzhou, 510275, P. R. China. E-mail: tongml@mail.sysu.edu.cn; Fax: (+)86 20 8411-2245
bDivision of Quantum and Physical Chemistry and INPAC – Institute of Nanoscale Physics and Chemistry, Katholieke Universiteit Leuven, Celestijnenlaan 200F, B-3001, Belgium. E-mail: Liviu.Chibotaru@chem.kuleuven.be
cDivision of Quantum and Physical Chemistry and INPAC – Institute of Department of Chemistry and Biochemistry-0358, University of California at San Diego, La Jolla, California 92093-0358, U.S.A. E-mail: dhendrickson@ucsd.edu
dApplied Chemistry Research Center, Frontier Institute of Science and Technology, Xi'an JiaoTong University, Xi'an, 710049, China
First published on 19th April 2011
Abstract
Two heterometallic [DyIII6MnIII12] clusters comprising of the same [MnIII8O13] fragment, four isolated MnIII ions and two linear [DyIII3] units have been synthesised. Except for the same composition, the main difference of these two cores lies in the coordination environment and the orientations of the linear [DyIII3] units. This difference leads to an alternation in the symmetry of the two cores that significantly modulates their magnetic properties including ground spin state and slow relaxation behavior.
Since the first [Mn12] cluster that exhibits slow relaxation of the magnetization was discovered in the 1990's,1–4 considerable effort has been focused on exploring single-molecule magnets (SMMs).5,6 These unique complexes require both a high spin ground-state (ST) and an appreciable uniaxial magnetic anisotropy (D < 0), leading to an energy barrier between the bistable states that slows down magnetization reversal.4,7,8 Although numerous SMMs have been reported, finding an SMM with both high ground spin-state and large magnetic anisotropy is still a challenge for synthetic chemists.
The importance of both factors for an SMM can be well illustrated by the example of the [Mn19] complex,9 which holds a record ground spin state of S = 83/2, but fails to be an SMM because of the nearly absent magnetic anisotropy.10,11 By deliberately replacing the central eight-coordinate MnII ion with the highly anisotropic DyIII ion, slow relaxation of the magnetization was observed.12 Another example of the significance of the magnetic anisotropy on the energy barrier of the SMMs is the large-spin (S = 37) molecule with an energy barrier of only 13 K due to the very small magnetic anisotropy.13
Inspired by the large magnetic anisotropy of the heavier lanthanides, great synthetic efforts have been devoted to, not only the pure 4f,14–17 but also 3d–4f12,18–23clusters in recent years. Although the magnetic interactions between the contracted 4f electrons are usually weak, the recently reported linear [DyIII3] and [TbIII3] complexes showed promising ferromagnetic coupling (up to J = +7.5 K) and thus, a high ground spin state.15 Besides the ground state the control of the magnetic anisotropy is also very important as can be seen in a triangular [DyIII3] molecule with non-collinearity of the principle axes.16f,16g However, the intrinsic correlation between the spin-state and the magnetic anisotropy in these systems haven't been completely elucidated, which requires further exploration.
Herein we demonstrated two 3d–4f complexes by employing an extended in situ synthetic route we have isolated three new 3d–4fclusters (structures shown in Fig. 1), [Dy6Mn12O7(OH)10(OAc)14(mpea)8]·20H2O·4MeOH (1) and [Ln6Mn12O9(OH)8(OAc)10(mpea)8(mp)2(MeOH)2(H2O)2]·solv (2. While Ln = Dy, solv = 26H2O·2MeOH 2-Dy; Ln = Y, solv = 15H2O·4MeOH 2-Y), where Hmp = 2-hydroxy-5-methyl-isophthalaldehyde and H2mpea = 2-hydroxy-3-((2-hydroxy-ethylimino)methyl)-5-methylbenzaldehyde), with symmetry related [LnIII6MnIII12] cores. Interestingly, the two complexes 1-Dy and 2-Dy show different ground-state spins and slow magnetization behaviors, which may be influenced by the different symmetries of their cores (vide infra).
 | ||
| Fig. 1 a) Molecule structures of 1-Dy (left) and 2-Dy (right). b) The cluster cores of 1-Dy (left) and 2-Dy (right). Each LnIII is highlighted as a green polyhedron and the Jahn–Teller axes of MnIII ions are highlighted as blue sticks. Hydrogen atoms and lattice solvent molecules have been omitted for clarity. | ||
After the Schiff-base was synthesized in situ, it was added to LnCl3·6H2O, Mn(OAc)2·4H2O, and triethylamine in different solvents. Complex 1 crystallizes in the orthorhombic space groupFddd while 2 is of lower symmetry and crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (see ESI†). As can be seen in Fig. 1, though isomeric, the [LnIII6MnIII12] cores possess D2 site symmetry in 1 and C1 symmetry in 2. All of the Mn and Ln ions in both compounds are trivalent, which are confirmed by bond-valence-sum (BVS) calculations (Table S1†),24 charge considerations, and the observation of Jahn–Teller distortion around the MnIII centers.
space group (see ESI†). As can be seen in Fig. 1, though isomeric, the [LnIII6MnIII12] cores possess D2 site symmetry in 1 and C1 symmetry in 2. All of the Mn and Ln ions in both compounds are trivalent, which are confirmed by bond-valence-sum (BVS) calculations (Table S1†),24 charge considerations, and the observation of Jahn–Teller distortion around the MnIII centers.
The cores can be described as being composed of five parts; ABCBA for 1 and AB′CB′′A for 2 (Figures S1 and S2†). Two isolated MnIII ions capping a linear [LnIII3] trimer form fragment AB, while fragment C comprises a [MnIII8O13] unit that contains a twisted [MnIII4O5] tetragon that is capped by four MnIII ions alternating above and below the tetragon with oxo-bridges. It should be mentioned that the central oxygen atom of [MnIII4O5] acts as μ4-oxo bridge binding the [MnIII4] unit in 1 and a μ5-oxo bridge binding [MnIII4] and the central LnIII ion of a [LnIII3] trimer in 2. The main symmetry differences between the two cores arise from the orientation of the [LnIII3] units which leads to differing orientations of the Jahn–Teller axes in the central twisted [MnIII4O5] tetragon in 1 and 2.
The coordination environments and orientations of the two linear [LnIII3] units of 1 and 2 differ significantly. The coordination sphere of each LnIII ion comprises only O atoms, presenting various types of coordination environments: (i) For 1, the central LnIII of fragment B, [LnIII3O6], is 8-coordinate, whereas both end-positions are 9-coordinate. The LnIII polyhedra are all face-sharing within fragment B while the end polyhedra are vertex-sharing with the second B fragment in 1; (ii) For 2, the LnIII ions in fragment B′, [LnIII3O4], are all 8-coordinate edge-sharing polyhedra whereas fragment B′′, [LnIII3O6], comprises three face-sharing 9-coordinate LnIII polyhedra. In other words, two identical [LnIII3O6] trimers link together with oxo-bridges to form a [LnIII6O14] unit as a twisted hexagon in 1, while differing [LnIII3O4] and [LnIII3O6] timers remain magnetically separated from one another in 2. It is worth mentioning that the two [LnIII3] units are nearly parallel (∼11°) and perpendicular (∼83°) to one another in 1 and 2, respectively.
Dc magnetic susceptibility data were collected in the temperature range 1.8–300 K at 500 Oe (Fig. 2). At 300 K, the χMT values are 123.6 and 120.3 cm3 K mol−1 for 1-Dy and 2-Dy, respectively, which are close to the expected value of 121.0 cm3 K mol−1 for an uncoupled [DyIII6MnIII12] core (DyIII: S = 5/2, L = 5, 6H15/2, g = 4/3; MnIII: S = 2, g = 2).18,25 Upon lowering the temperature, the χMT value of 1-Dy remains roughly constant and then sharply increases to a maximum value of 409.5 cm3 K mol−1 at 3.5 K, suggesting a high-spin ground state. The χMT value of 2-Dy first slightly decreases to 107.0 cm3 K mol−1 at 23 K, then increases to a maximum of 117.9 cm3 K mol−1 at 5 K and then lowers again sharply to 100.88 cm3 K mol−1 at 2 K, which is likely attributed to competition between ferromagnetic and antiferromagnetic coupling and/or crystal field effects of DyIII.14a,26,27
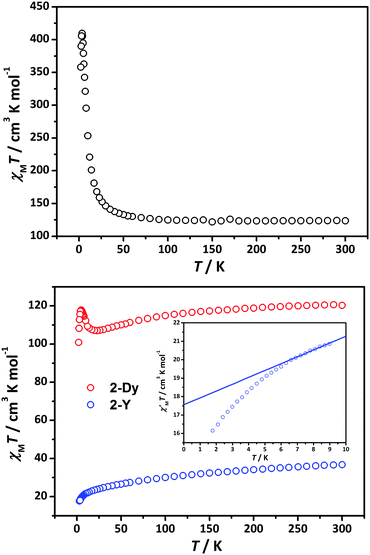 | ||
| Fig. 2 Temperature dependence of the χMT products at 500 Oe for 1-Dy (up), 2-Dy (bottom, red) and 2-Y (bottom, blue). Inset: The low-frequency χM′T data were extrapolated to 0 K for 2-Y. | ||
Ac susceptibility measurements were carried out in a 5 Oe ac field oscillating at 1–1488 Hz, and with a zero dc field (Fig. 4, S3, S4†) in order to investigate the presence of SMM-like behavior.
An intriguing question is why the magnetic behaviour of 1-Dy and 2-Dy is so different (Fig. 2) despite common structural blocks. In order to further investigate the interactions of the spin carriers in 2, YIII without spins are the candidate ions to replace the DyIII ions, so we make a try to have a look at 2-Y. The decay of χMT data suggests the presence of an antiferromagnetic interaction between Mn⋯Mn. We can subtract the plot 2-Y from the plot 2-Dy (Figure S6†) to investigate the interactions between Ln⋯Ln and Ln⋯Mn. The resulting ΔχMT values increase on low temperature, confirming the ferromagnetic interactions between Ln⋯Ln and/or Ln⋯Mn in 2-Dy. Going to the low-frequency (1 Hz) χM′T data extrapolated to 0 K for 2-Y (Fig. 2), the values of 17.7 cm3 K mol−1 corresponds to an S = 3 [MnIII8O13] unit (fragment C) and four uncoupled MnIII (S = 2) ions as fragment A and C are magnetically separated by two [YIII3] units. Indeed, the S = 3 for the fragment [MnIII8O13] in 2-Y is also apparently supported by the field-dependent magnetization measurement (Figure S5†), approaching a value of 22 μB at 7 T for 2-Y, which would be true in the case of four isotropic marginal MnIII (S = 2) and an isotropic S = 3 for the central [MnIII8O13].
However, the ab initio calculations of the four MnIII fragments (see ESI† for details) reveal a zero-field splitting of the ground S = 2 of ca. 12 cm−1. This relatively strong ionic anisotropy will induce the zero-field splitting of the ground term. Despite a much lower value of the supposed ground state spin (S = 3) compared to the maximal possible (S = 16) in this unit, the zero-field splitting of both these manifolds is expected to be of similar extent, as it was also the case in Mn12 acetate complex.28 Given their structural similarity, it is reasonable to suppose that the amplitude of zero-field splitting of the ground spin (S = 3) should be comparable to the latter compound, i.e. it cannot be ignored. As a result, the total molar magnetization at low temperature will be much lower than in the case of an isotropic spin. In conclusion, the total spin of the central fragment [MnIII8O13] in 2-Yshould be larger than 3 but still lower than 8, in order to keep an overall antiferromagnetic coupling seen in susceptibility measurements.
This conclusion is also confirmed by the molar magnetization of 2-Dy which approaches 57 μB at fields of 7 T, a value higher than 52 μB corresponding to four isotropic MnIII ions (4 × 4 = 16 μB), six Ising Dy(III) ions (6 × 5 = 30 μB) and an isotropic S = 3 (6 μB) of the central [MnIII8O13] core. The difference is even larger if one takes into account the lowering of the magnetization due to zero-field splitting on marginal MnIII ions and of the spin of the central [MnIII8O13] core.
For the complex 1-Dy, the difference between the measured magnetization (71 μB) and the predicted one in the case of four MnIII (4 × 4 = 16 μB), six Dy ions (6 × 5 = 30 μB) leads to a value of 25 μB, which clearly can only be expected in the case of predominantly ferromagnetic coupling (S > 8 total spin) of the [MnIII8O13] core. This difference is, again, larger if one takes into account the lowering of the total magnetization due to the zero-field splitting, as discussed above.
The difference in the magnetic behavior between complexes 1-Dy and 2-Dy is therefore due to several factors: 1) the value of the spin of the [MnIII8O13] central core is larger in 1-Dy compared to 2-Dy; 2) the orientations of the main anisotropy axes on Dy ions are different due to the structural arrangements of the [DyIII3] triades (see Tables S5 and S9†); 3) the exchange coupling between the [DyIII3] triades and the spin of the central [MnIII8O13] core is ferromagnetic in 1-Dy and antiferromagnetic in 2-Dy. Note that whatever the sign of the exchange interaction between [DyIII3] triades and the central core, the arrangements of the total magnetic moments within each of [DyIII3] triades is ferromagnetic in both complexes.
The ab initio calculated29 main anisotropy axes of the ground Kramers doublets on Dy sites in 1-Dy and 2-Dy are shown in Fig. 3 (see ESI† for computational details, energies local g tensors of the ground Kramers doublets on Dy sites and relative angles between main anisotropy axes in 1-Dy and 2-Dy).
 | ||
| Fig. 3 Local magnetic moments in the ground state on Dy ions of a) 1-Dy; b) 2-Dy. Dashed lines represent the local anisotropy axes, while the arrows show the orientation of the local magnetization. | ||
Frequency-dependent out-of-phase (χM′′) signals in the ac susceptibility show that both complexes behave as SMMs but exhibit different slow relaxation of magnetization. For 1-Dy, one peak is observable at 3.7 K for 1488 Hz, while at 2.0 K for 2-Dy for 1488 Hz. A higher spin and the more colinear local anisotropy axes of 1-Dy are likely to enhance the blocking temperature (TB) in contrast to 2-Dy. The ac susceptibilities, along with Cole-Cole plots showing quasi-semicircles and fitting Debye functions30 for 1-Dy and 2-Dy (Figure S7, α1 = 0.30–0.36, α2 = 0.48–0.51†), indicate the presence of different distribution processes of slow relaxation. According to φ = (ΔTp/Tp)/Δ(log f), the value of φ is, respectively, 0.14 for 1-Dy and 0.23 for 2-Dy (Figure S8†), suggesting a superparamagnetic-like behavior rather than spin glass behavior.31 Hence, the effective energy barriers (and characteristic relaxation time) can be obtained by fitting to the Arrhenius law giving Δeff/kB = 35.1(5) K (τ0 = 1.00 × 10−8 s) for 1-Dy and Δeff/kB = 18.2(5) K (τ0 = 3.02 × 10−8 s) for 2-Dy, respectively (insets of Fig. 4).
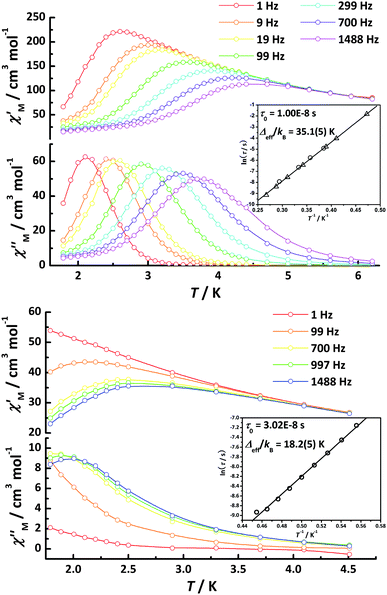 | ||
| Fig. 4 Plots of ac susceptibility vs. temperature for 1-Dy (left) and 2-Dy (right) oscillating at 1–1488 Hz at Hac = 5 G and Hdc = 0. Inset: The ln(τ)versus T−1 plots obtained from χM′′(T) (△) and χM′′(ν) (○); The solid lines correspond to the Arrhenius law for 1-Dy (left) and 2-Dy (right). | ||
However, no out-of-phase signals were observed under the resolution limit of the susceptometer above 1.8 K for 2-Y (Figure S4†). In terms of ac susceptibility, strong frequency-dependent signals were observed for 2-Dy while absent for 2-Y. We inferred it mainly originate from the [DyIII3] units. It is clear that introducing the highly anisotropic lanthanide ions into [LnIII6MnIII12] do play an important role, making it towards SMM.
In summary, we have generated two giant 3d–4fclusters with symmetry related [DyIII6MnIII12] cores which possess different molecular symmetry, resulting in different magnetic behaviors and energy barriers. For the high-symmetry one, the existing DyIII ions do not only introduce large anisotropy but also enhances the ground-state simultaneously in this system. For the low-symmetry one, we successfully replace the anisotropic DyIII with diamagnetic YIII, confirming the ferromagnetic interaction of Dy⋯Dy and/or Dy⋯Mn and demonstrating the anisotropic lanthanide ions make it toward an SMM.
Acknowledgements
This work was supported by the NSFC (Grant No. 50872157, 90922009 and 20821001), the “973 Project” (2007CB815305), the Research Fund for the Doctoral Program of Higher Education (20100171110015) and the US NSF.Notes and references
- T. Lis, Acta Crystallogr., Sect. B: Struct. Crystallogr. Cryst. Chem., 1980, 36, 2042 CrossRef.
- P. D. W. Boyd, Q. Li, J. B. Vincent, K. Folting, H. R. Chang, W. E. Streib, J. C. Huffman, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1988, 110, 8537 CrossRef CAS.
- (a) A. Caneschi, D. Gatteschi, R. Sessoli, A. L. Barra, L. C. Brunel and M. Guillot, J. Am. Chem. Soc., 1991, 113, 5873 CrossRef CAS; (b) R. Sessoli, D. Gatteschi, A. Caneschi and M. A. Novak, Nature, 1993, 365, 141 CrossRef CAS.
- (a) R. Sessoli, H. L. Tsai, A. R. Schake, S. Wang, J. B. Vincent, K. Folting, D. Gatteschi, G. Christou and D. N. Hendrickson, J. Am. Chem. Soc., 1993, 115, 1804 CrossRef CAS; (b) S. M. J. Aubin, Z. Sun, L. Pardi, J. Krzystek, K. Folting, L.-C. Brunel, A. L. Rheingold, G. Christou and D. N. Hendrickson, Inorg. Chem., 1999, 38, 5329 CrossRef CAS.
- W. Wernsdorfer, Nat. Nanotechnol., 2009, 4, 145 CrossRef CAS.
- (a) K. Katoh, Y. Yoshida, M. Yamashita, H. Miyasaka, B. K. Breedlove, T. Kajiwara, S. Takaishi, N. Ishikawa, H. Isshiki, Y. F. Zhang, T. Komeda, M. Yamagishi and J. Takeya, J. Am. Chem. Soc., 2009, 131, 9967 CrossRef CAS; (b) L. Margheriti, D. Chiappe, M. Mannini, P.-E. Car, P. Sainctavit, M.-A. Arrio, F. B. de Mongeot, J. C. Cezar, F. M. Piras, A. Magnani, E. Otero, A. Caneschi and R. Sessoli, Adv. Mater., 2010, 22, 5488 CrossRef CAS; (c) W.-G. Wang, A.-J. Zhou, W.-X. Zhang, M.-L. Tong, X.-M. Chen, M. Nakano, C. C. Beedle and D. N. Hendrickson, J. Am. Chem. Soc., 2007, 129, 1014 CrossRef CAS.
- M. N. Leuenberger and D. Loss, Nature, 2001, 410, 789 CrossRef CAS.
- D. Gatteschi and R. Sessoli, Angew. Chem., Int. Ed., 2003, 42, 268 CrossRef CAS.
- A. M. Ako, I. J. Hewitt, V. Mereacre, R. Clérac, W. Wernsdorfer, C. E. Anson and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 4926 CrossRef CAS.
- O. Waldmann, A. M. Ako, H. U. Güdel and A. K. Powell, Inorg. Chem., 2008, 47, 3486 CrossRef CAS.
- T. Glasera, I. Liratzis, A. M. Ako and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2296 CrossRef CAS.
- A. M. Ako, V. Mereacre, R. Clerac, W. Wernsdorfer, I. J. Hewitt, C. E. Anson and A. K. Powell, Chem. Commun., 2009, 544 RSC.
- (a) E. E. Moushi, T. C. Stamatatos, W. Wernsdorfer, V. Nastopoulos, G. Christou and A. J. Tasiopoulos, Inorg. Chem., 2009, 48, 5049 CrossRef CAS; (b) M. Atanasov, P. Comba and C. A. Daul, Inorg. Chem., 2008, 47, 2449 CrossRef CAS; (c) S. A. Stoian, C. Paraschiv, N. Kiritsakas, F. Lloret, E. Münck, E. L. Bominaar and M. Andruh, Inorg. Chem., 2010, 49, 3387 CrossRef CAS.
- (a) P.-H. Lin, T. J. Burchell, R. Clérac and M. Murugesu, Angew. Chem., Int. Ed., 2008, 47, 8848 CrossRef CAS; (b) M. T. Gamer, Y. Lan, P. W. Roesky, A. K. Powell and R. Clérac, Inorg. Chem., 2008, 47, 6581 CrossRef CAS; (c) Y.-Z. Zheng, Y. Lan, C. E. Anson and A. K. Powell, Inorg. Chem., 2008, 47, 10813 CrossRef CAS; (d) Y. Gao, G.-F. Xu, L. Zhao, J. Tang and Z. Liu, Inorg. Chem., 2009, 48, 11495 CrossRef CAS.
- (a) I. J. Hewitt, Y. Lan, C. E. Anson, J. Luzon, R. Sessoli and A. K. Powell, Chem. Commun., 2009, 6765 RSC; (b) F.-S. Guo, J.-L. Liu, J.-D. Leng, Z.-S. Meng, Z.-J. Lin, M.-L. Tong, S. Gao, L. Ungur and L. F. Chibotaru, Chem.–Eur. J., 2011, 17, 2458 CAS.
- (a) B. Hussain, D. Savard, T. J. Burchell, W. Wernsdorferb and M. Murugesu, Chem. Commun., 2009, 1100 RSC; (b) P.-H. Lin, T. J. Burchell, L. Ungur, L. F. Chibotaru, W. Wernsdorfer and M. Murugesu, Angew. Chem., Int. Ed., 2009, 48, 9489 CrossRef CAS; (c) H. Ke, G.-F. Xu, L. Zhao, J. Tang, X.-Y. Zhang and H.-J. Zhang, Chem.–Eur. J., 2009, 15, 10335 CrossRef CAS; (d) Y.-N. Guo, G.-F. Xu, P. Gamez, L. Zhao, S.-Y. Lin, R. Deng, J. Tang and H.-J. Zhang, J. Am. Chem. Soc., 2010, 132, 8538 CrossRef CAS; (e) I. J. Hewitt, J. Tang, N. T. Madhu, C. E. Anson, Y. Lan, J. Luzon, M. Etienne, R. Sessoli and A. K. Powell, Angew. Chem., Int. Ed., 2010, 49, 6352 CrossRef CAS; (f) J. Tang, I. Hewitt, N. T. Madhu, G. Chastanet, W. Wernsdorfer, C. E. Anson, C. Benelli, R. Sessoli and A. K. Powell, Angew. Chem., Int. Ed., 2006, 45, 1729 CrossRef CAS; (g) J. Luzon, K. Bernot, I. J. Hewitt, C. E. Anson, A. K. Powell and R. Sessoli, Phys. Rev. Lett., 2008, 100, 247205 CrossRef.
- R. Sessoli and A. K. Powell, Coord. Chem. Rev., 2009, 253, 2328 CrossRef CAS.
- C. Benelli and D. Gatteschi, Chem. Rev., 2002, 102, 2369 CrossRef CAS.
- (a) A. Mishra, W. Wernsdorfer, K. A. Abboud and G. Christou, J. Am. Chem. Soc., 2004, 126, 15648 CrossRef CAS; (b) C. M. Zaleski, E. C. Depperman, J. W. Kampf, M. L. Kirk and V. L. Pecoraro, Angew. Chem., Int. Ed., 2004, 43, 3912 CrossRef CAS; (c) A. Mishra, W. Wernsdorfer, S. Parsons, G. Christoua and E. K. Brechin, Chem. Commun., 2005, 2086 RSC; (d) V. Mereacre, A. M. Ako, R. Clérac, W. Wernsdorfer, G. Filoti, J. Bartolomé, C. E. Anson and A. K. Powell, J. Am. Chem. Soc., 2007, 129, 9248 CrossRef CAS.
- (a) V. Mereacre, A. M. Ako, R. Clérac, W. Wernsdorfer, I. J. Hewitt, C. E. Anson and A. K. Powell, Chem.–Eur. J., 2008, 14, 3577 CrossRef CAS; (b) C. Papatriantafyllopoulou, W. Wernsdorfer, K. A. Abboud and G. Christou, Inorg. Chem., 2011, 50, 421 CrossRef CAS.
- (a) N. Akhtar, Y.-Z. Zheng, Y. Lan, V. Mereacre, C. E. Anson and A. K. Powell, Inorg. Chem., 2009, 48, 3502 CrossRef CAS; (b) A. M. Ako, V. Mereacre, R. Clérac, I. J. Hewitt, Y. Lan, G. Buth, C. E. Anson and A. K. Powell, Inorg. Chem., 2009, 48, 6713 CrossRef CAS; (c) M. Andruh, J.-P. Costes, C. Diaz and S. Gao, Inorg. Chem., 2009, 48, 3342 CrossRef; (d) J. Rinck, G. Novitchi, W. V. d. Heuvel, L. Ungur, Y. Lan, W. Wernsdorfer, C. E. Anson, L. F. Chibotaru and A. K. Powell, Angew. Chem., Int. Ed., 2010, 49, 7583 CrossRef CAS; (e) C. Papatriantafyllopoulou, W. Wernsdorfer, K. A. Abboud and G. Christou, Inorg. Chem., 2011, 50, 421 CrossRef CAS.
- (a) G. Karotsis, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, Angew. Chem., Int. Ed., 2009, 48, 9928 CrossRef CAS; (b) G. Karotsis, S. Kennedy, S. J. Teat, C. M. Beavers, D. A. Fowler, J. J. Morales, M. Evangelisti, S. J. Dalgarno and E. K. Brechin, J. Am. Chem. Soc., 2010, 132, 12983 CrossRef CAS.
- (a) C. Lampropoulos, T. C. Stamatatos, K. A. Abboud and G. Christou, Inorg. Chem., 2009, 48, 429 CrossRef CAS; (b) T. Shiga, T. Onuki, T. Matsumoto, H. Nojiri, G. N. Newton, N. Hoshinoa and H. Oshio, Chem. Commun., 2009, 3568 RSC; (c) T. C. Stamatatos, S. J. Teat, W. Wernsdorfer and G. Christou, Angew. Chem., Int. Ed., 2009, 48, 521 CrossRef CAS.
- (a) I. D. Brown and D. Altermatt, Acta Crystallogr., Sect. B: Struct. Sci., 1985, 41, 244 CrossRef; (b) W. Liu and H. H. Thorp, Inorg. Chem., 1993, 32, 4102 CrossRef CAS.
- O. Kahn, Molecular Magnetism, VCH Publishers, New York, 1993 Search PubMed.
- N. Ishikawa, M. Sugita, T. Ishikawa, S.-y. Koshihara and Y. Kaizu, J. Am. Chem. Soc., 2003, 125, 8694 CrossRef CAS.
- J.-P. Sutter and M. L. Kahn, Magnetism: molecules to materials; Miller, J. S.; Drillon, M. ed.; Wiley-VCH: Weinheim, 2005; Vol. 5, pp 161–188 Search PubMed.
- O. Waldmann, Inorg. Chem., 2007, 46, 10035 CrossRef CAS.
- L. Chibotaru and L. Ungur. Computer programs SINGLE_ANISO and POLY_ANISO, University of Leuven, 2006 Search PubMed.
- K. S. Cole and R. H. Cole, J. Chem. Phys., 1941, 9, 341 CrossRef CAS.
- J. A. Mydosh, Spin Glasses: An Experimental Introduction, Taylor and Francis, London, 1993 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: The ESI contains experimental details of the synthesis of 1-Dy, 2-Dy and 2-Y together with their X-ray structure determinations, magnetic susceptibility and ab initio calculations of the local magnetic properties on Dy sites in 1-Dy and 2-Dy. CCDC 779464 (1-Dy), 779465 (2-Dy), and 795483 (2-Y). For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1sc00166c |
| This journal is © The Royal Society of Chemistry 2011 |
