DOI:
10.1039/C0SC00602E
(Edge Article)
Chem. Sci., 2011,
2, 1145-1150
Effect of anchoring groups in zinc phthalocyanine on the dye-sensitized solar cell performance and stability†
Received
1st December 2010
, Accepted 10th March 2011
First published on 30th March 2011
Abstract
We have designed and developed an unsymmetrical zinc phthalocyanine (TT9) sensitizer that consists of three tert-butyl and two carboxylic acid groups that act as “push” and “pull”, respectively. The two carboxylic acid groups graft the sensitizer onto the semiconductor surface resulting in enhanced stability under heat and light compared to the similar unsymmetrical zinc phthalocyanine (TT1) sensitizer that consists of three tert-butyl and only one carboxylic acid groups. The solar cells containing the TT9 and TT1 sensitizers with non-volatile electrolyte were subjected to light soaking conditions at 60 °C. Under these conditions, the short circuit current of the TT1 sensitized solar cell after 1000 h decreases to half of its initial value where as the TT9 sensitized solar cell remained the same demonstrating the influence of number of anchoring groups on the stability of zinc phthalocyanine sensitized solar cells.
Introduction
The conversion of sunlight to electricity using dye-sensitized solar cells (DSCs) has been researched as one of the most promising methods for future low cost power production from renewable energy sources.1–3 In these cells the most successful charge transfer sensitizers employed are ruthenium polypyridyl complexes, yielding 10–11% solar-to-electric power conversion efficiencies under AM 1.5.4–7 The relatively high efficiency of the DSC is attributed to a combination of very fast electron injection from dye onto the semiconductor oxide, sluggish back-reaction of the electron in the oxide to either the dye cation or the oxidized redox species and fairly fast regeneration of the dye by the redox electrolyte. In spite of this, the main drawback of ruthenium-based sensitizers is the lack of absorption in the red region of the visible spectrum, where half of the available solar energy is placed above 700 nm. Therefore, it is imperative to harvest the near IR energy by engineering sensitizers that absorb in this region with high molar extinction coefficient.
Phthalocyanines (Pc)8 are well known for their intense absorption in the red/near-IR (Q band) regions, as well as showing electrochemical, photochemical and thermal stability. Therefore they are an excellent option to explore for solar cell applications and have been the focus of intense research for the development of efficient near-IR absorbers.8–15 Moreover, through rational design of their structure several groups have previously shown the possibility to control their electrochemical and photophysical properties.16–20 Despite the number of groups working on the development of near IR dyes for molecular photovoltaic devices, only recently have efficiencies higher than 3.5% under standard conditions (100 mW cm−2, AM 1.5) been reported.11,12,15 The unimpressive performances are mainly due to strong aggregation on the TiO2 surface and the lack of directionality in the excited state of the zinc phthalocyanine (ZnPc).21 To overcome these drawbacks, S. Mori et al., have reported a bulky ZnPc having diphenylphenoxy groups to prevent aggregation and yielding 4.6% power conversion efficiency.15 We have reported TT1 bearing tert-butyl groups in order to not only minimize the formation of molecular aggregates, but also increase the solubility in organic solvents and induce the directionality.
However, long-term stability based on ZnPc sensitized solar cell has never been reported. Long-term stability of DSC is one of the important requirements to its commercialization, therefore in this paper we aim to study the effect of the anchoring group on stability of DSC and the device performance properties of particularly designed unsymmetrical zinc phthalocyanine, TT9 (see molecular structure in Fig. 1) as compared with TT1.
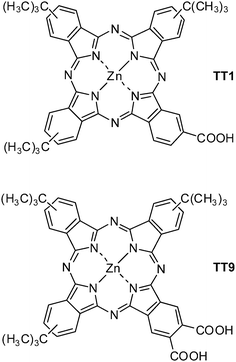 |
| | Fig. 1 Molecular structure of zinc carboxyphthalocyanines TT1 and TT9. | |
Results and discussion
Phthalocyanine
2 was prepared by metallation of free base phthalocyanine 122 using ZnCl2 and DBU as a base, in a 63% yield, after chromatographic purification (Scheme 1). On the other hand, dicarboxylic acid phthalocyanine TT9 was synthesized by hydrolysis of 2 as depicted in Scheme 1 in a 54% yield after chromatographic purification on a reverse stationary phase using a mixture of THF–H2O (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent.
1) as eluent.
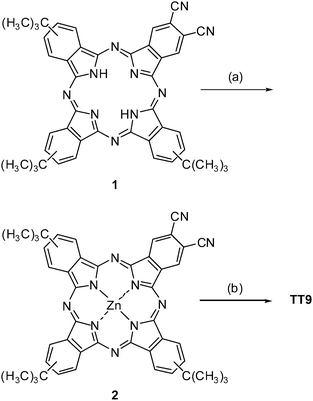 |
| | Scheme 1 Synthesis of phathalocyanine TT9. (a) ZnCl2, DBU, Pentanol, 60 °C; 63%. (b) i) KOH 0.5 M, MeOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) reflux. ii) AcOH–H2O (3 2) reflux. ii) AcOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 75 °C. iii) KOH 0.5 M, MeOH–H2O (5 2) 75 °C. iii) KOH 0.5 M, MeOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) reflux; 54%. 2) reflux; 54%. | |
The visible absorption spectrum of TT9 in THF solution shows a maximum at 684 nm (see Fig. 2), somewhat red-shifted (4 nm) when compared with the maximum showed by TT1 (680 nm), however in both cases, no significant aggregation was observed in THF solution of 1 × 10−4 concentration. The visible absorption spectrum of TT9/TiO2 shows a maximum at 689 nm, which is in good agreement with the solution absorption except for a slight red shift ∼5 nm due to the interaction of the anchoring groups to the surface.
Electrochemical properties of the TT1 and TT9 sensitizers were scrutinized by using cyclic voltammetry (CV) and differential pulse voltammetry (DPV) in dimethylformamide (DMF) solvent containing 0.1 M tetrabutylammonium hexafluorophosphate (TBAHFP). TT1 and TT9dyes show a reversible couple at 0.83 and 0.90 V vs.NHE due to the one electron oxidation couple. The difference of 70 mV between the TT1 and the TT9 sensitizers is due to the presence of one and two carboxylic acid groups, respectively, which act as electron acceptor groups stabilizing the HOMO level of TT9 slightly higher than the TT1. The LUMO levels of these two sensitizers is at −0.65 and −0.64 V vs.NHE, respectively, due to the reduction of TT1 and TT9.
Solar cell performance and stability
Fig. 3 shows the IPCE of TT1 and TT9 sensitized solar cells using non-volatile electrolyte. The IPCE data of both sensitizers showed a Q-band peak above 65%. Values of the integrated IPCE from 350 to 800 nm are 6.0 and 7.0 mA cm−2, respectively, which is consistent with current density (J)/voltage (V) performance in Table 1. Under AM 1.5 conditions, the TT1 sensitized solar cell yielded 5.9 mA cm−2 of the short circuit current density (Jsc), 662 mV of the open-circuit voltage (Voc), and 0.76 of fill factor (ff) leading to 2.95% of the power conversion efficiency (η). Where as the TT9 sensitized solar cell showed 7.0 mA cm−2 of Jsc, 634 mV of Voc, 0.74 of ff, and 3.20% of η. It is noted that the efficiencies of TT1 and TT9 sensitizers using a volatile electrolyte composed of 0.6 M N-methyl-N-butyl imidiazolium iodide, 0.04 M iodine, 0.05 M LiI, 0.05M GuNCS and 0.28 M 4-tert-butylpyridine in 15/85 (v/v) mixture of valeronitrile and acetonitrile, are 4.0 and 3.5%, respectively (see Table 1). The lower Voc with volatile electrolyte is plausibly attributed to the presence of LiI, which is a well known species to give rise to a downward shift movement of the band edge.23 However, the shift could give rise to an increase in injection efficiency from excited sensitizer to TiO2 leading to high Jsc.23
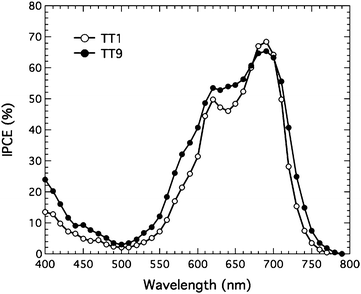 |
| | Fig. 3 Incident photon-to-current conversion efficiency (IPCE) of TT1 and TT9 sensitized solar cells based on non-volatile electrolyte. | |
Table 1 Change in J/V characteristics of TT1 and TT9 sensitized solar cell with volatile electrolyte and with non-volatile electrolyte during successive 1 sun visible-light soaking at 60 °C
| Sensitizer |
Time (h) |
J
sc
(mA cm−2) |
V
oc
(mV) |
ff
|
η (%) |
|
With volatile electrolyte.
With non-volatile electrolyte.
|
|
TT1
a
|
|
7.55 |
617 |
0.750 |
3.55 |
|
TT9
a
|
|
9.37 |
605 |
0.725 |
4.10 |
|
TT1
b
|
0 |
5.90 |
662 |
0.760 |
2.95 |
| 500 |
5.98 |
511 |
0.730 |
2.56 |
| 1000 |
4.48 |
455 |
0.710 |
1.48 |
|
TT9
b
|
0 |
7.00 |
631 |
0.750 |
3.20 |
| 500 |
8.67 |
542 |
0.700 |
3.33 |
| 1000 |
7.45 |
490 |
0.700 |
2.56 |
The cells with the non-volatile electrolyte were kept in successive light soaking with 60 °C and their efficiencies were regularly checked during 1000 h. Under light soaking with 60 °C, the TT1 sensitized cell lost 207 mV in Voc and 1.40 mA cm−2 in Jsc resulting in the power conversion efficiency of the TT1 sensitized solar cell to be half of its initial value (see Table 1 and Fig. 4 open markers). By contrast, under the same conditions, the TT9 sensitized solar cell showed a 141 mV drop in Voc without loss of Jsc (see Table 1 and Fig. 4 closed markers) resulting in 80% of the initial power conversion efficiency. The stable current in the TT9 sensitized solar cell compared to the TT1 elucidates a concomitant stability ascribed to two carboxylic acid of TT9.
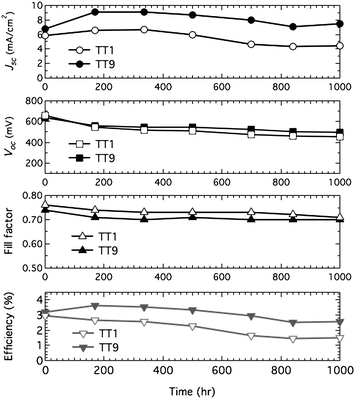 |
| | Fig. 4 Photovoltaic parameter (Jsc, Voc, ff and η) variations with aging time for the device based on TT1 (open markers) and TT9 (closed markers) sensitized film with non-volatile electrolyte during successive 1 sun visible-light soaking at 60 °C. | |
Photovoltage and photocurrent transient
In order to speculate the different degradations under the light soaking condition, photovoltage and photocurrent transients were performed at regular time intervals during 1000 h. Fig. 5 (upper part) shows the electron lifetime, which is the reciprocal recombination rate of the injected electron leading to a change of electron density in TiO2 and then Voc change of DSCs. The TT1 sensitized solar cell shows longer electron lifetime than the TT9cell over the whole range of charge density. The higher Voc of the TT1 sensitized DSCs is ascribed to its longer electron lifetime. After the 1000 h stability test, the electron lifetimes of both cells show a clear change. The TT1 sensitized solar cell shows >6 times decrease in the electron life time, while the TT9 sensitized solar cell shows <2 times decrease. Another important factor that has an influence on the voltage of DSCs is upward or downward shift movement of the TiO2 conduction band (CB) edge. From the transient Voc measurement, the Voc of the DSC at light soaking are compared at the same level of capacitance in the film as shown in Fig. 5 (lower part). Obviously, the Voc detected as aging is decreasing and finally, ∼150 mV lower than that of both cells before light soaking, while no difference between both cells are found. This low Voc implies a downward shift of the band edge towards more positive potentials.24–26 In a recent study, proton intercalation to TiO2 has been proposed to lead to the downward shift under light soaking.27 In this study, we observed no difference in the capacitance of TT1 and TT9 sensitized solar cells. Hence, the decrease in Voc of TT1 and TT9 sensitized solar cells is attributed to the band edge shift and the shortened electron lifetime. The bigger loss in Voc of the TT1 sensitized solar cell is attributed to a bigger drop in the electron lifetime of TT1 sensitized solar cells when compared to TT9 sensitized solar cells. The result elucidates the advantage of two anchoring groups in the light of long-term stability of DSCs. Recently, we found out the interfacial dynamics dependence with dye loading, where more exposed TiO2 surface due to poor coverage of dyes could result in fast recombination process.27 In this work, the small change in the interfacial electron lifetime of the TT9 sensitized solar cells could be attributed to either higher dye loading or stable anchoring on TiO2 due to TT9's two carboxylic acid groups (see supporting information†).
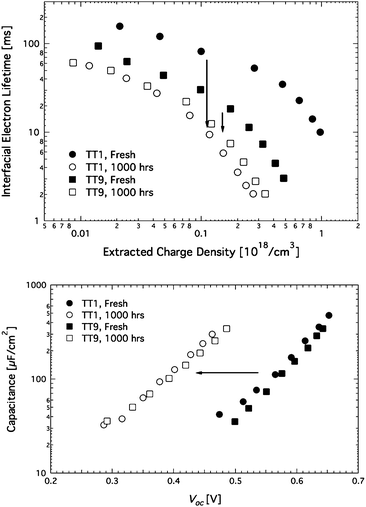 |
| | Fig. 5
Upper part – Changes in interfacial electron lifetime as a function of photo-induced charge density. Lower part – Capacitance as a function of Voc of TT1 and TT9 sensitized solar cells with non-volatile electrolyte under light soaking conditions. | |
Experimental
Synthesis and characterization
Phthalocyanine
TT1 was prepared as reported earlier.11
9(10),16(17),23(24)-Tri-tert-butyl-2,3-dicyano-5,28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 14,19-diimino-7,12
14,19-diimino-7,12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2)-N29,N30,N31,N32zinc(II) (mixture of regioisomers) (2).
A solution of phthalocyanine 122 (200 mg, 0.27 mmol), ZnCl2 (112 mg, 0.82 mmol, 3.0 equiv) and DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) (125 mg, 0.82 mmol, 3.0 equiv) in pentanol (10 mL) was heated at 60 °C under Ar atmosphere. After 15 min, the solution was poured into 200 mL of H2O and a blue-greenish solid precipitated. The solid was filtered and washed with water (2 × 50 mL) and mixtures of H2O–MeOH (3
21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2)-N29,N30,N31,N32zinc(II) (mixture of regioisomers) (2).
A solution of phthalocyanine 122 (200 mg, 0.27 mmol), ZnCl2 (112 mg, 0.82 mmol, 3.0 equiv) and DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) (125 mg, 0.82 mmol, 3.0 equiv) in pentanol (10 mL) was heated at 60 °C under Ar atmosphere. After 15 min, the solution was poured into 200 mL of H2O and a blue-greenish solid precipitated. The solid was filtered and washed with water (2 × 50 mL) and mixtures of H2O–MeOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 100 mL each). The product was finally purified by column chromatography using CH2Cl2/2-ethoxyethanol (50
1, 100 mL each). The product was finally purified by column chromatography using CH2Cl2/2-ethoxyethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent affording 2 as a green solid (135 mg, 63%). Mp: >250 °C. 1H-NMR (300 MHz, [D6]DMSO, 25 °C, TMS): δ 9.6–7.4 (m, 11H; PcH), 1.9–1.5 (broad s, 27H; C(CH3)3) ppm. FT-IR (KBr): ν 3490, 2963, 2902, 2230, 1616, 1528, 1370, 1086, 924, 837, 756, 695, 533 cm−1. UV/Vis (THF): λmax (log ε) 703 (5.19), 658 (4.90), 635 (4.79), 590 (4.29), 348 (4.81) nm. MS (MALDI-TOF, dithranol): m/z 894.3–800.3 [M]+(100%). HR MALDI-TOF MS, dithranol: m/z 794.25669 [M+], calcd for C46H38N10Zn: 794.25757.
1) as eluent affording 2 as a green solid (135 mg, 63%). Mp: >250 °C. 1H-NMR (300 MHz, [D6]DMSO, 25 °C, TMS): δ 9.6–7.4 (m, 11H; PcH), 1.9–1.5 (broad s, 27H; C(CH3)3) ppm. FT-IR (KBr): ν 3490, 2963, 2902, 2230, 1616, 1528, 1370, 1086, 924, 837, 756, 695, 533 cm−1. UV/Vis (THF): λmax (log ε) 703 (5.19), 658 (4.90), 635 (4.79), 590 (4.29), 348 (4.81) nm. MS (MALDI-TOF, dithranol): m/z 894.3–800.3 [M]+(100%). HR MALDI-TOF MS, dithranol: m/z 794.25669 [M+], calcd for C46H38N10Zn: 794.25757.
9(10),16(17),23(24)-Tri-tert-butyl-2,3-dicarboxy-5,28![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 14,19-diimino-7,12
14,19-diimino-7,12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2)-N29, N30, N31, N32zinc(II) (mixture of regioisomers) TT9.
Phthalocyanine
2 (100 mg, 0.126 mmol) was added slowly, in a few portions, to a stirred solution of KOH (0.5 M) in a mixture of MeOH–H2O (5
21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2)-N29, N30, N31, N32zinc(II) (mixture of regioisomers) TT9.
Phthalocyanine
2 (100 mg, 0.126 mmol) was added slowly, in a few portions, to a stirred solution of KOH (0.5 M) in a mixture of MeOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (10 mL). The solution was then refluxed for 12 h. The resulting mixture was extracted with THF, washed with brine, dried over MgSO4 and filtered. The solvent was evaporated and the crude was heated at 75 °C for 12 h in a 10 mL mixture of AcOH–H2O (3
2) (10 mL). The solution was then refluxed for 12 h. The resulting mixture was extracted with THF, washed with brine, dried over MgSO4 and filtered. The solvent was evaporated and the crude was heated at 75 °C for 12 h in a 10 mL mixture of AcOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The solvent was removed under vacuum and the solid obtained was refluxed again into a solution of KOH 0.5 M in a mixture of MeOH–H2O (5
2). The solvent was removed under vacuum and the solid obtained was refluxed again into a solution of KOH 0.5 M in a mixture of MeOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (10 mL) for 8 h. Finally, the solution was extracted with THF and washed with brine (1 × 40 mL), acidified until pH < 3 with H3PO4, dried over MgSO4 and filtered. After evaporation of the organic extracts, the crude obtained was purified on a chromatographic column (reverse phase, THF–water (2
2) (10 mL) for 8 h. Finally, the solution was extracted with THF and washed with brine (1 × 40 mL), acidified until pH < 3 with H3PO4, dried over MgSO4 and filtered. After evaporation of the organic extracts, the crude obtained was purified on a chromatographic column (reverse phase, THF–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) affording TT9 as a green solid (135 mg, 63%). Mp: >250 °C. 1H NMR (300 MHz, [D6]DMSO, 25 °C, TMS): δ 10.2–9.8 (m, 2H; PcH), 9.5–9.2 (m, 6H; PcH), 8.5–8.3 (m, 3H; PcH) 2.0–1.8 (broad s, 27H; C(CH3)3) ppm. FT-IR (KBr): ν 3481, 2955, 1715, 1620, 1485, 1364, 1283, 1094, 922, 748 cm−1. UV/Vis (THF): λmax (log ε) 684 (5.1), 676 (5.0), 614 (4.4), 350 (4.8) nm. MS (MALDI-TOF, dithranol): m/z: 832.3–839.3 [M]+ (100%). HR MALDI-TOF MS, dithranol: m/z 832.24585 [M+], calcd for C46H40N8O4Zn: 832.24597.
1)) affording TT9 as a green solid (135 mg, 63%). Mp: >250 °C. 1H NMR (300 MHz, [D6]DMSO, 25 °C, TMS): δ 10.2–9.8 (m, 2H; PcH), 9.5–9.2 (m, 6H; PcH), 8.5–8.3 (m, 3H; PcH) 2.0–1.8 (broad s, 27H; C(CH3)3) ppm. FT-IR (KBr): ν 3481, 2955, 1715, 1620, 1485, 1364, 1283, 1094, 922, 748 cm−1. UV/Vis (THF): λmax (log ε) 684 (5.1), 676 (5.0), 614 (4.4), 350 (4.8) nm. MS (MALDI-TOF, dithranol): m/z: 832.3–839.3 [M]+ (100%). HR MALDI-TOF MS, dithranol: m/z 832.24585 [M+], calcd for C46H40N8O4Zn: 832.24597.
Solar cell fabrication and characterization
The photoanodes composed of nanocrystalline TiO2 were prepared using a previously reported procedure.24 The TiO2 electrodes composed of a ∼7 μm transparent layer (20 nm) and a ∼5 μm scattering layer (400 nm, CCIC, HPW-400). The TiO2 electrodes were immersed into the TT1 and TT9 solutions composed of 0.1 mM ethanol with 60 mM chenodeoxycholic acid and kept at room temperature for ∼5 h. The non-volatile electrolyte was composed of 1.0 M 1-propyl-3-methylimidazolium iodide (PMII), 0.15 M iodine, 0.1 M guanidinium thiocyanate (GuNCS), and 0.5 M 1-butyl-1H-benzimidazole (NBB) in 3-methoxypropionitrile.28 The dye-adsorbed TiO2 electrode and thermally platinized counter electrode were assembled into a sealed sandwich type cell with a gap of hot-melt ionomer film, Surlyn (25 μm, Du-Pont) for light soaking and heat soaking condition, respectively. In order to reduce scattered light from the edge of the glass electrodes of the dyed TiO2 layer, a light shading mask was used on the DSC, so the active area of the DSC was fixed to 0.2 cm2. For photovoltaic measurements of the DSCs, the irradiation source was a 450 W xenon light source (Osram XBO 450, Germany) with a filter (Schott 113), whose power was regulated to the AM 1.5 G solar standard by using a reference Si photodiode equipped with a colour matched filter (KG-3, Schott) in order to reduce the mismatch in the region of 350–750 nm between the simulated light and AM 1.5 G to less than 4%. The measurement of incident photon-to-current conversion efficiency (IPCE) was plotted as a function of excitation wavelength by using the incident light from a 300 W xenon lamp (ILC Technology, USA), which was focused through a Gemini-180 double monochromator (Jobin Yvon Ltd.). The measured time delay of the J-V characteristics of the DSC was fixed at intervals of 80 ms. In order to investigate stability of DSCs, the values for short circuit current density (Jsc), open circuit voltage (Voc), fill factor (ff) and power conversion efficiency (η) were recorded over a period of more than 1000 h under 1 sun light soaking with 60 °C. During the period, all DSCs were kept under open circuit conditions.
Photovoltage and photocurrent transient
Photovoltage transients were observed by using a pump pulse generated by 4 red light emitting diodes controlled by a fast solid-state switch with a white light bias. The pulse of red light with widths of 50 ms was incident on the photoanode side of the cell, and its intensity was controlled to keep a suitably low level to generate the exponential voltage decay where the charge recombination rate constants are obtained directly from the exponential decay rate.29 A white bias light, also incident on the same side of the device, was supplied by white diodes. The photo-induced charge density as a function of the white light bias intensity was obtained by charge extraction measurements where the stored charges under open circuit conditions were extracted by placing the cell under short circuit conditions.30,31 Small perturbation transient photocurrent measurements were performed in a similar manner to the open circuit voltage decay measurement.
Conclusions
In conclusion, the data demonstrate design strategies of sensitizers useful for solar energy conversion, particularly by zinc phthalocyanine. To the best of our knowledge, this is the first report on the stability of zinc phthalocyanine under light soaking with 60 °C conditions. The stable current in the TT9 sensitized solar cell compared to the TT1 is attributed to the effect of two carboxylic acid groups, which anchor onto TiO2 and turns out to give a dramatic improvement in stability. The aging brought a decrease in Voc, which is mainly attributed to the band edge shift of TiO2. This preliminary result shows a key tool on the way to molecular engineering of an efficient and stable dye in dye-sensitized solar cells.
Acknowledgements
This work has been supported by the COST Action D35, and the EU Project ROBUST DSC, FP7-Energy-2007-1-RTD, N° 212792. Financial support by the MEC, Spain (CTQ2008-00418/BQU, CTQ2007-60746-BQU, CONSOLIDER-INGENIO 2010 CDS 2007-00010, and CONSOLIDER-INGENIO HOPE 0007-2007, PLE2009-0070, PSE-120000-2009-008 FOTOMOL), and CAM (MADRISOLAR-2, S2009/PPQ/1533) is gratefully acknowledged. We also thank the Korea Foundation for International Cooperation of Science and Technology through the Global Research Lab. (GRL) Program funded by the Ministry of Education, Science and Technology, Republic of Korea, and the World Class University (WCU) programme funded by the Ministry of Education Science and Technology (Grant No. R31-2008-000-10035-0). EP thanks ICREA and ICIQ for financial support. We thank Dr Davide Di Censo and Mr Pascal Compte for their kind assistance.
Notes and references
-
M. Grätzel, Handbook of nanostructured materials and nanotechnology, Academic Press, 2000, vol. 3 Search PubMed.
-
M. K. Nazeeruddin, Special issue: Michael Graetzel Festschrift, a tribute for his 60th Birthday: Dye Sensitized Solar Cells, Elsevier, Amsterdam, 2004, vol. 248 Search PubMed.
- L. M. Gonçalves, V. Z. Bermudez, H. A. Ribeiro and A. M. Mendes, Energy Environ. Sci., 2008, 1, 655 RSC.
- M. K. Nazeeruddin, R. Humphry-Baker, P. Liska and M. Grätzel, J. Phys. Chem. B, 2003, 107, 8981 CrossRef CAS.
- Y. Chiba, A. Islam, Y. Watanabe, R. Komiya, N. Koide and L. Y. Han, Jpn. J. Appl. Phys., 2006, 45, L638 CrossRef CAS.
- F. Gao, Y. Wang, D. Shi, J. Zhang, M. K. Wang, X. Y. Jing, R. Humphry-Baker, P. Wang, S. M. Zakeeruddin and M. Gratzel, J. Am. Chem. Soc., 2008, 130, 10720 CrossRef CAS.
- Y. M. Cao, Y. Bai, Q. J. Yu, Y. M. Cheng, S. Liu, D. Shi, F. F. Gao and P. Wang, J. Phys. Chem. C, 2009, 113, 6290 CrossRef CAS.
- G. De la Torre, C. G. Claessens and T. Torres, Chem. Commun., 2007, 2000 RSC.
- J. He, G. Benkoe, F. Korodi, T. Polivka, R. Lomoth, B. Kermark, L. Sun, A. Hagfeldt and V. Sundstroem, J. Am. Chem. Soc., 2002, 124, 4922 CrossRef CAS.
- K. Kato, M. Hirano, F. Takahashi, K. Shinbo, F. Kaneko and T. Wakamatsu, Trans. Mater. Res. Soc. Jpn., 2004, 29, 775 Search PubMed.
- J.-J. Cid, J.-H. Yum, S.-R. Jang, M. K. Nazeeruddin, E. Martinez-Ferrero, E. Palomares, J. Ko, M. Graetzel and T. Torres, Angew. Chem., Int. Ed., 2007, 46, 8358 CrossRef CAS.
- P. Y. Reddy, L. Giribabu, C. Lyness, H. J. Snaith, C. Vijaykumar, M. Chandrasekharam, M. Lakshmikantam, J.-H. Yum, K. Kalyanasundaram, M. Graetzel and M. K. Nazeeruddin, Angew. Chem., Int. Ed., 2007, 46, 373 CrossRef CAS.
- H. Imahori, T. Umeyama and S. Ito, Acc. Chem. Res., 2009, 42, 1809 CrossRef CAS.
-
(a)
M. V. Martínez-Díaz and T. Torres, On the significance of phthalocyanines in solar cells in Handbook of Porphyrin Science, ed. K. Kadish and K. M. Smith, Guilard, World Scientific Press, Singapore, 2010, vol. 10, pp. 141–181 Search PubMed;
(b) M. V. Martínez-Díaz, G. de la Torre and T. Torres, Chem. Commun., 2010, 46, 7090 RSC;
(c) M. G. Walter, A. B. Rudine and C. C. Wamser, J. Porphyrins Phthalocyanines, 2010, 14, 759 CrossRef CAS;
(d) M. V. Martínez-Díaz, M. Ince and T. Torres, Monatsh. Chem., 2011 DOI:10.1007/s00706-010-0431-0.
- S. Mori, M. Nagata, Y. Nakahata, K. Yasuta, R. Goto, M. Kimura and M. Taya, J. Am. Chem. Soc., 2010, 132, 4054 CrossRef CAS.
- E. Palomares, M. V. Martinez-Diaz, S. A. Haque, T. Torres and J. R. Durrant, Chem. Commun., 2004, 2112 RSC.
- A. Morandeira, I. López-Duarte, M. V. Martínez-Díaz, B. O'Regan, C. Shuttle, N. A. Haji-Zainulabidin, T. Torres, E. Palomares and J. R. Durrant, J. Am. Chem. Soc., 2007, 129, 9250 CrossRef CAS.
- B. C. O'Regan, I. López-Duarte, M. V. Martínez-Díaz, A. Forneli, J. Albero, A. Morandeira, E. Palomares, T. Torres and J. R. Durrant, J. Am. Chem. Soc., 2008, 130, 2906 CrossRef CAS.
-
(a) J. J. Cid, M. Garcia-Iglesias, J. H. Yum, A. Forneli, J. Albero, E. Martinez-Ferrero, P. Vazquez, M. Grätzel, M. K. Nazeeruddin, E. Palomares and T. Torres, Chem.–Eur. J., 2009, 15, 5130 CrossRef CAS;
(b) M. García-Iglesias, J.-J. Cid, J.-H. Yum, A. Forneli, P. Vázquez, M. Kd. Nazeeruddin, E. Palomares, M. Grätzel and T. Torres, Energy Environ. Sci., 2011, 4, 189 RSC.
-
(a) B. E. Hardin, E. T. Hoke, P. B. Armstrong, J. H. Yum, T. Torres, J. M. J. Fréchet, M. K. Nazeeruddin, M. Grätzel and M. D. McGehee, Nat. Photonics, 2009, 3, 406 CrossRef CAS;
(b) B. E. Hardin, J.-H. Yum, E. T. Hoke, T. Torres, Md. K. Nazeeruddin, M. Grätzel and M. D. McGehee, Nanolett., 2010, 10, 3077 CrossRef CAS;
(c) J.-H. Yum, B. E. Hardin, E. T. Hoke, E. Baranoff, S. M. Zakeeruddin, Md. K. Nazeeruddin, T. Torres, M. D. McGehee and M. Grätzel, ChemPhysChem, 2011, 12, 657 CrossRef CAS.
- M. K. Nazeeruddin, R. Humphry-Baker, M. Grätzel, D. Wöhrle, G. Schnurpfeil, G. Schneider, A. Hirth and N. Trombach, J. Porphyrins Phthalocyanines, 1999, 3, 230 CrossRef CAS.
- G. De la Torre, M. V. Martínez-Díaz and T. J. Torres, J. Porphyrins Phthalocyanines, 1999, 3, 560 CrossRef CAS.
- S. E. Koops, B. C. O'Regan, P. R. F. Barnes and J. R. Durrant, J. Am. Chem. Soc., 2009, 131, 4808 CrossRef CAS.
- J. H. Yum, S. R. Jang, R. Humphry-Baker, M. Grätzel, J. J. Cid, T. Torres and M. K. Nazeeruddin, Langmuir, 2008, 24, 5636 CrossRef CAS.
- Z. P. Zhang, S. M. Zakeeruddin, B. C. O'Regan, R. Humphry-Baker and M. Grätzel, J. Phys. Chem. B, 2005, 109, 21818 CrossRef CAS.
- N. Kopidakis, N. R. Neale and A. J. Frank, J. Phys. Chem. B, 2006, 110, 12485 CrossRef CAS.
- J. H. Yum, R. Humphry-Baker, S. M. Zakeeruddin, M. K. Nazeeruddin and M. Grätzel, Nano Today, 2010, 5, 91 CrossRef CAS.
- D. B. Kuang, C. Klein, S. Ito, J. E. Moser, R. Humphry-Baker, N. Evans, F. Duriaux, C. Grätzel, S. M. Zakeeruddin and M. Grätzel, Adv. Mater., 2007, 19, 1133 CrossRef CAS.
- B. C. O'Regan and F. Lenzmann, J. Phys. Chem. B, 2004, 108, 4342 CrossRef CAS.
- N. W. Duffy, L. M. Peter, R. M. G. Rajapakse and K. G. U. Wijayantha, Electrochem. Commun., 2000, 2, 658 CrossRef CAS.
- M. Bailes, P. J. Cameron, K. Lobato and L. M. Peter, J. Phys. Chem. B, 2005, 109, 15429 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental details of the new compounds. See DOI: 10.1039/c0sc00602e |
| ‡ Both authors contributed equally to the research. |
|
| This journal is © The Royal Society of Chemistry 2011 |
Click here to see how this site uses Cookies. View our privacy policy here. 
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent.
1) as eluent.

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) reflux. ii) AcOH–H2O (3
2) reflux. ii) AcOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 75 °C. iii) KOH 0.5 M, MeOH–H2O (5
2) 75 °C. iii) KOH 0.5 M, MeOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) reflux; 54%.
2) reflux; 54%.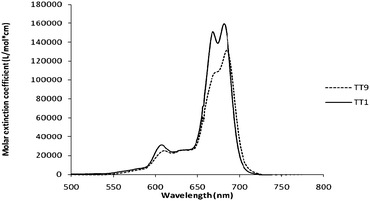



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 14,19-diimino-7,12
14,19-diimino-7,12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2)-N29,N30,N31,N32zinc(II) (mixture of regioisomers) (2).
A solution of phthalocyanine 122 (200 mg, 0.27 mmol), ZnCl2 (112 mg, 0.82 mmol, 3.0 equiv) and DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) (125 mg, 0.82 mmol, 3.0 equiv) in pentanol (10 mL) was heated at 60 °C under Ar atmosphere. After 15 min, the solution was poured into 200 mL of H2O and a blue-greenish solid precipitated. The solid was filtered and washed with water (2 × 50 mL) and mixtures of H2O–MeOH (3
21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2)-N29,N30,N31,N32zinc(II) (mixture of regioisomers) (2).
A solution of phthalocyanine 122 (200 mg, 0.27 mmol), ZnCl2 (112 mg, 0.82 mmol, 3.0 equiv) and DBU (1,8-diazabicyclo[5.4.0]undec-7-ene) (125 mg, 0.82 mmol, 3.0 equiv) in pentanol (10 mL) was heated at 60 °C under Ar atmosphere. After 15 min, the solution was poured into 200 mL of H2O and a blue-greenish solid precipitated. The solid was filtered and washed with water (2 × 50 mL) and mixtures of H2O–MeOH (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 2
1 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 100 mL each). The product was finally purified by column chromatography using CH2Cl2/2-ethoxyethanol (50
1, 100 mL each). The product was finally purified by column chromatography using CH2Cl2/2-ethoxyethanol (50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) as eluent affording 2 as a green solid (135 mg, 63%). Mp: >250 °C. 1H-NMR (300 MHz, [D6]DMSO, 25 °C, TMS): δ 9.6–7.4 (m, 11H; PcH), 1.9–1.5 (broad s, 27H; C(CH3)3) ppm. FT-IR (KBr): ν 3490, 2963, 2902, 2230, 1616, 1528, 1370, 1086, 924, 837, 756, 695, 533 cm−1. UV/Vis (THF): λmax (log ε) 703 (5.19), 658 (4.90), 635 (4.79), 590 (4.29), 348 (4.81) nm. MS (MALDI-TOF, dithranol): m/z 894.3–800.3 [M]+(100%). HR MALDI-TOF MS, dithranol: m/z 794.25669 [M+], calcd for C46H38N10Zn: 794.25757.
1) as eluent affording 2 as a green solid (135 mg, 63%). Mp: >250 °C. 1H-NMR (300 MHz, [D6]DMSO, 25 °C, TMS): δ 9.6–7.4 (m, 11H; PcH), 1.9–1.5 (broad s, 27H; C(CH3)3) ppm. FT-IR (KBr): ν 3490, 2963, 2902, 2230, 1616, 1528, 1370, 1086, 924, 837, 756, 695, 533 cm−1. UV/Vis (THF): λmax (log ε) 703 (5.19), 658 (4.90), 635 (4.79), 590 (4.29), 348 (4.81) nm. MS (MALDI-TOF, dithranol): m/z 894.3–800.3 [M]+(100%). HR MALDI-TOF MS, dithranol: m/z 794.25669 [M+], calcd for C46H38N10Zn: 794.25757.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 14,19-diimino-7,12
14,19-diimino-7,12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2)-N29, N30, N31, N32zinc(II) (mixture of regioisomers) TT9.
Phthalocyanine
2 (100 mg, 0.126 mmol) was added slowly, in a few portions, to a stirred solution of KOH (0.5 M) in a mixture of MeOH–H2O (5
21,26-dinitrilo-tetrabenzo[c,h,m,r][1,6,11,16]tetraazacycloeicosinato-(2)-N29, N30, N31, N32zinc(II) (mixture of regioisomers) TT9.
Phthalocyanine
2 (100 mg, 0.126 mmol) was added slowly, in a few portions, to a stirred solution of KOH (0.5 M) in a mixture of MeOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (10 mL). The solution was then refluxed for 12 h. The resulting mixture was extracted with THF, washed with brine, dried over MgSO4 and filtered. The solvent was evaporated and the crude was heated at 75 °C for 12 h in a 10 mL mixture of AcOH–H2O (3
2) (10 mL). The solution was then refluxed for 12 h. The resulting mixture was extracted with THF, washed with brine, dried over MgSO4 and filtered. The solvent was evaporated and the crude was heated at 75 °C for 12 h in a 10 mL mixture of AcOH–H2O (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). The solvent was removed under vacuum and the solid obtained was refluxed again into a solution of KOH 0.5 M in a mixture of MeOH–H2O (5
2). The solvent was removed under vacuum and the solid obtained was refluxed again into a solution of KOH 0.5 M in a mixture of MeOH–H2O (5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) (10 mL) for 8 h. Finally, the solution was extracted with THF and washed with brine (1 × 40 mL), acidified until pH < 3 with H3PO4, dried over MgSO4 and filtered. After evaporation of the organic extracts, the crude obtained was purified on a chromatographic column (reverse phase, THF–water (2
2) (10 mL) for 8 h. Finally, the solution was extracted with THF and washed with brine (1 × 40 mL), acidified until pH < 3 with H3PO4, dried over MgSO4 and filtered. After evaporation of the organic extracts, the crude obtained was purified on a chromatographic column (reverse phase, THF–water (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)) affording TT9 as a green solid (135 mg, 63%). Mp: >250 °C. 1H NMR (300 MHz, [D6]DMSO, 25 °C, TMS): δ 10.2–9.8 (m, 2H; PcH), 9.5–9.2 (m, 6H; PcH), 8.5–8.3 (m, 3H; PcH) 2.0–1.8 (broad s, 27H; C(CH3)3) ppm. FT-IR (KBr): ν 3481, 2955, 1715, 1620, 1485, 1364, 1283, 1094, 922, 748 cm−1. UV/Vis (THF): λmax (log ε) 684 (5.1), 676 (5.0), 614 (4.4), 350 (4.8) nm. MS (MALDI-TOF, dithranol): m/z: 832.3–839.3 [M]+ (100%). HR MALDI-TOF MS, dithranol: m/z 832.24585 [M+], calcd for C46H40N8O4Zn: 832.24597.
1)) affording TT9 as a green solid (135 mg, 63%). Mp: >250 °C. 1H NMR (300 MHz, [D6]DMSO, 25 °C, TMS): δ 10.2–9.8 (m, 2H; PcH), 9.5–9.2 (m, 6H; PcH), 8.5–8.3 (m, 3H; PcH) 2.0–1.8 (broad s, 27H; C(CH3)3) ppm. FT-IR (KBr): ν 3481, 2955, 1715, 1620, 1485, 1364, 1283, 1094, 922, 748 cm−1. UV/Vis (THF): λmax (log ε) 684 (5.1), 676 (5.0), 614 (4.4), 350 (4.8) nm. MS (MALDI-TOF, dithranol): m/z: 832.3–839.3 [M]+ (100%). HR MALDI-TOF MS, dithranol: m/z 832.24585 [M+], calcd for C46H40N8O4Zn: 832.24597.
