Passivating surface states on water splitting hematite photoanodes with alumina overlayers†
Florian
Le Formal
,
Nicolas
Tétreault
,
Maurin
Cornuz
,
Thomas
Moehl
,
Michael
Grätzel
and
Kevin
Sivula
*
Ecole Polytechnique Fédérale de Lausanne, Laboratoire de photonique et interfaces, CH-1015, Lausanne, Switzerland. E-mail: kevin.sivula@epfl.ch; Fax: +41 21 693 4111; Tel: +41 21 693 3669
First published on 24th January 2011
Abstract
Hematite is a promising material for inexpensive solar energy conversion viawater splitting but has been limited by the large overpotential (0.5–0.6 V) that must be applied to afford high wateroxidation photocurrent. This has conventionally been addressed by coating it with a catalyst to increase the kinetics of the oxygen evolution reaction. However, surface recombination at trapping states is also thought to be an important factor for the overpotential, and herein we investigate a strategy to passivate trapping states using conformal overlayers applied by atomic layer deposition. While TiO2 overlayers show no beneficial effect, we find that an ultra-thin coating of Al2O3 reduces the overpotential required with state-of-the-art nano-structured photo-anodes by as much as 100 mV and increases the photocurrent by a factor of 3.5 (from 0.24 mA cm−2 to 0.85 mA cm−2) at +1.0 V vs. the reversible hydrogen electrode (RHE) under standard illumination conditions. The subsequent addition of Co2+ ions as a catalyst further decreases the overpotential and leads to a record photocurrent density at 0.9 V vs. RHE (0.42 mA cm−2). A detailed investigation into the effect of the Al2O3 overlayer by electrochemical impedance and photoluminescence spectroscopy reveals a significant change in the surface capacitance and radiative recombination, respectively, which distinguishes the observed overpotential reduction from a catalytic effect and confirms the passivation of surface states. Importantly, this work clearly demonstrates that two distinct loss processes are occurring on the surface of high-performance hematite and suggests a viable route to individually address them.
Introduction
Photoelectrochemical (PEC) cells offer the ability to convert electromagnetic energy from our largest renewable source, the Sun, to stored chemical energy through the splitting of water into molecular oxygen and hydrogen.1Hematite (α-Fe2O3) has emerged as a promising photo-electrode material due to its significant light absorption (bandgap, Eg = 2.1 eV), chemical stability in aqueous environments, and ample abundance. Its performance as a water-oxidizing photoanode has been crucially limited by a very short electron–hole pair lifetime and poor minority charge carrier mobility which gives rise to a high recombination rate of photo-generated carriers in the bulk. This limitation can be overcome with various nanostructuring approaches that position the hematite in high proximity to the semiconductor-liquid junction (SCLJ) while maintaining a sufficient amount of material for complete light harvesting.2 Nanostructured and silicon-doped hematite thin films grown by a simple atmospheric pressure chemical vapor deposition (APCVD) technique, developed by our group, have recently demonstrated solar water-oxidation photocurrents superior to any other oxide-based photoanode material under standard testing conditions.3Despite these nanostructuring efforts, a second crucial drawback of hematite remains: a bias of over 1 V vs. the reversible hydrogen electrode (RHE) must be applied to afford significant water splitting photocurrent density. The bias is partly attributed to the position of the conduction band energy in hematite, which is 0.4–0.5 eV too positive to allow water reduction into hydrogen. While this can nominally be overcome using a tandem cell approach,4 the additional high overpotential (0.5–0.6 V) required to initiate water oxidation represents a significant energy loss. Thus there is a clear challenge to reduce the overpotential and afford the highest water splitting photocurrent density at the lowest possible applied potential. Typically, the overpotential is attributed to poor oxygen evolution reaction (OER) kinetics and has been addressed by attaching various catalyst materials.3,5 Yet even when using IrO2—the most effective water oxidation catalyst—only 0.2 V of the overpotential was eliminated.3 Consequently, while a photocurrent density of over 3.0 mA cm−2 was observed at +1.23 V vs. RHE in these electrodes, only 10% of that (0.31 mA cm−2) was available at 0.9 V.
The inability of a high-performance catalyst to completely eliminate the overpotential suggests that factors other than slow OER kinetics play an important role. Indeed, several groups have suggested surface traps as an additional loss mechanism in hematite photoelectodes prepared using various methods.6 Further investigation of these surface states using sacrificial electron donors in the electrolyte, such as pyrogallol,6e have suggested that hole traps at the surface can be eliminated. However, a feasible method to passivate surface states on hematite without sacrificial agents remains elusive. In addition, the relative roles of both possible loss mechanisms—poor OER kinetics and surface traps—at the surface of nanostructured water splitting hematite photoanodes are not clear. In this article we investigate the presence of surface trapping states on hematite prepared by APCVD and report a method to passivate surface states without using sacrificial components. To accomplish this we use atomic layer deposition (ALD) to coat the hematite with thin oxide overlayers. Recently ALD has been gaining interest as a powerful research tool for creating highly conformal layers on nanostructured electrodes7 and has even been used for surface state passivation of TiO2 in dye sensitized solar cells.8 Herein we show that an ultra-thin layer of Al2O3 deposited by ALD can decrease the overpotential for water photo-oxidation with nanostructured hematite. This observation and our supporting experiments confirm that there are two distinct loss processes at the surface of state-of-the-art hematite photoanodes.
Results and discussion
Our nanostructured and silicon-doped hematite thin-films grown by APCVD exhibit a cauliflower-like nanostructure formed by the agglomeration of small Fe2O3 particles (ca. 5 nm) nucleated in the gas phase.3 This nanostructure was conformally coated with Al2O3 or TiO2 by ALD.‡ The number of ALD cycles was varied for each material to obtain a corresponding thickness of about 0.1, 0.5, 1.0 or 2.0 nm (as measured by spectroscopic ellipsometry on Si). Control electrodes were subjected to identical conditions but without precursor pulses. While the TiO2 overlayer had no positive influence on the Fe2O3 (see Electronic Supplementary Information, ESI†), even the thinnest application of Al2O3 (1 cycle corresponding to 0.15 nm on the Si reference) had a significant impact on the photoelectrochemical properties of the iron oxide as shown by the photocurrent density vs. voltage characteristics (at AM 1.5 G 100 mW cm−2 simulated illumination) presented in Fig. 1. Before ALD treatment, the electrode exhibits the typical response of an APCVD grown hematite sample (black trace, circle markers). Water oxidation photocurrent onsets9 at +1.03 V vs. RHE in 1 M NaOH electrolyte (pH 13.6), the current density then increases rapidly, attaining approximately 2 mA cm−2 at 1.23 V vs. RHE bias and reaches a plateau of about 3.1 mA cm−2 from 1.4 V to 1.6 V vs. RHE before the dark current rises. After one ALD cycle, the onset of the photocurrent is dramatically shifted cathodically (red trace with square markers in Fig. 1) but a resistive behavior characterized by a slow and linear rise of the photocurrent with applied voltage is observed. This behavior is possibly due to a high degree of disorder in the amorphous Al2O3 overlayer that inhibits hole injection from Fe2O3 into the electrolyte. After annealing in air at 300 °C (green trace, triangle markers, Fig. 1), the electrode's photocurrent curve regains a shape similar to before the ALD. However, the photocurrent onset potential shifts dramatically by ca. 80 mV to 0.95 V vs. RHE while the current density reaches 2.3 mA cm−2 at 1.23 V and 3.3 mA cm−2 at 1.43 V vs. RHE. These values are superior to that of the control sample over the full bias range. Further annealing at 400 °C shows an additional cathodic shift in the onset potential to 0.93 V vs. RHE, but a lower plateau (3.0 mA cm−2 at 1.43 V) compared to control samples. | ||
| Fig. 1 Current densities, in mA cm−2 of the prepared photoanodes in the dark (broken lines) and under simulated solar illumination (AM 1.5G 100 mW cm2, solid curves) are shown as a function of the applied potential, V, with respect to the RHE. An APCVD sample covered with 1 ALD cycle of Al2O3 has been measured after deposition (red squares), after annealing for 20 min at 300 °C (green triangles) and after annealing for 20 min at 400 °C (blue diamonds) are compared to a control, which is the same sample before ALD (black circles). | ||
This decrease in photocurrent plateau is probably due to an increase of the feature size of the hematite nanoparticles when annealing at the higher temperature, which results in a loss of active surface area.10 Interestingly, this effect is found to subside when using thicker Al2O3 overlayers as shown in Fig. 2.
 | ||
| Fig. 2 Current densities, in mA cm−2 of the prepared photoanodes at 1.03V (yellow triangles), at 1.23 V (orange squares) and at 1.43 V vs. RHE (red circles), are presented for a control sample and samples covered by different thickness of alumina (deposition with 1, 3, 6 and 13 ALD cycles). All samples have been measured before and after ALD as well as after annealing at 300 °C and 400 °C. | ||
Fig. 2 shows the water oxidation photocurrent density attained at 1.03 V (yellow triangles), 1.23 V (orange squares) and 1.43 V (red circles) vs. RHE, with the different thicknesses of Al2O3 compared to the control sample. The photocurrent density of the control sample shows its expected evolution with little change until 400 °C when an increased crystallinity and loss of surface area cause a small increase in the photocurrent at 1.03 V and decrease at 1.43 V, respectively. In contrast, the Al2O3 overlayer has a strong effect on the photocurrent-voltage characteristics of the hematite photoanodes regardless of its thickness. Overall, the electrodes with an Al2O3 overlayer show: i) a lower photocurrent onset potential resulting in a higher photocurrent at 1.03 V, ii) a decrease in photocurrent at higher potentials after ALD deposition, iii) the recovery (at 1.23 and 1.43 V) and further increase of the photocurrent (1.03 V) after annealing in air at 300 °C, and iv) a slight decrease in photocurrent at 1.43 V vs. RHE after annealing at 400 °C. The latter effect is reduced with increasing Al2O3 thickness, indicating that the overlayer may prevent the hematite particle growth through the same mechanism observed when using SiO2.11
Importantly, the observed thickness-dependant behavior also suggests that the alumina is not dissolving under the basic conditions used for the PEC measurement (pH 13.6) despite the known thermodynamic instability of Al2O3 at this pH. Indeed, we found that several scans of the photoanodes exposed to the electrolyte and under illumination as well as the prolonged exposure to the electrolyte in the dark showed no change in the photoanode performance (see Figure S2, ESI†). However, long term exposure (ca. 1 h) in 1M NaOH under an applied potential of 1.03 V vs. RHE and 1 sun illumination caused a modified electrode to recover its initial photocurrent onset behavior due to the photoelectrochemical dissolution of the Al2O3 layer (Figure S3, ESI†). Despite this long-term instability, the Al2O3 was sufficiently stable for further characterization to scrutinize the nature of the photocurrent onset reduction.
Photocurrent transient measurements were performed in order to assess the dynamics of water oxidation and charge recombination at the hematite/electrolyte interface before and after the ALD of the Al2O3 overlayer. To summarize, samples are held at key bias potentials and the time-resolved photocurrent is recorded while illumination was turned on and off. The current density is shown with respect to time in Fig. 3 for a hematite photoanode covered with 3 cycles of aluminaALD (red, thick line) and a control photoanode (blue, thin line). Previous studies of photoanode transients have commented on the typically-observed behavior:6a,b,d,12 when light reaches the sample, photogenerated holes travel to the SCLJ and accumulate because of the slow OER kinetics,6d or because carriers oxidize trap states in the bulk6c and on the surface.6b This induces a sharp anodic current spike that decays as the accumulation process perturbs the charge distribution of the space charge region until equilibrium is eventually reached between water oxidation and charge recombination. Conversely, the cathodic transient peak observed when the light is turned off has been assigned to electrons diffusing from the external circuit and recombining with the accumulated holes at the SCLJ. The appearance of transients diminishes at higher potential as a larger proportion of holes have sufficient potential to oxidize water. For each potential, an estimate of the number of holes accumulated at the SCLJ can be obtained from the area, A, set between the actual photocurrent spectrum and the square wave obtained if the photocurrent had immediately risen to the steady state value.13 In Fig. 3, we qualitatively observe that A is smaller for the electrode with an overlayer than for the control at all bias potentials. By integration of the photocurrent at 1.1 V vs.RHE with respect to time, we can confirm from the estimated values of A = 71.2 μC cm−2 and A = 198 μC cm−2, for the coated hematite and the control, respectively, that the Al2O3 overlayer causes fewer holes to accumulate at the SCLJ.
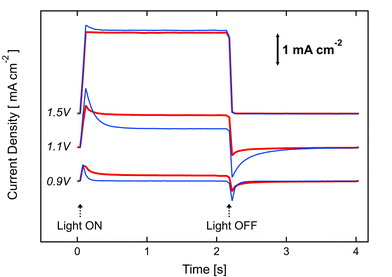 | ||
| Fig. 3 Transient photoresponse shown by light chopping current densities (light on/light off) as a function of time. The bias potential, with respect to the RHE, were applied during 4 s (2 s with light ON and 2 s with light OFF) at 0.9, 1.1 and 1.5 V as indicated. Comparison of the transient behavior of iron oxide photoanodes with 3 ALD cycles of Al2O3 overlayer (red, thick line) and without (blue, thin line). | ||
However, at this stage both the reduction of charge accumulation at the SCLJ implied by the transient results and the cathodic shift of the photocurrent onset potential afforded by the alumina overlayer could be explained as well by a catalytic argument; i.e. the Al2O3 could simply increase the rate of the OER at the SCLJ. However, three additional details indicate this is not an appropriate explanation. First, upon the surface adsorption of the common Co2+catalyst,5a we observe an additional cathodic shift of the overpotential to less than +0.9 V vs. RHE. This is shown in Fig. 4 and leads to photocurrent density of 0.42 mA cm−2 at 0.9 V vs. RHE. To our knowledge, this is the highest water oxidation photocurrent reported for a hematite photoanode at 0.9 V vs. RHE and under standard illumination conditions.
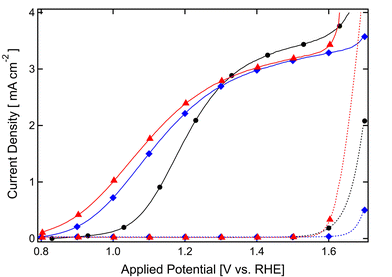 | ||
| Fig. 4 Current densities, in mA cm−2 of the prepared photoanodes in the dark (broken lines) and under simulated solar illumination (AM 1.5G 100 mW cm−2, solid curves) are shown as a function of the applied potential, V vs. RHE. A hematite photoanode has been characterized before ALD treatment (black circles), after 3 ALD cycles of alumina and annealed to 400 °C (blue diamonds) and after subsequent cobalt treatment (red triangles). | ||
The second observation discrediting a catalytic explanation is that the treatment of the hematite photoanode with an aqueous solution containing Al3+ species, analogous to the Co2+ treatment, does not improve the onset potential. Third and finally, the deposition of TiO2 overlayers (0.1–2 nm) by ALD on the nanostructured hematite photoanode does not shift cathodically the onset potential despite the superior OER kinetics known with this material.6b These latter two arguments are shown in the ESI as Figures S4 and S5, respectively.† Since the beneficial photochemical effects observed after ALD of the alumina overlayer cannot be explained by an improved catalytic process, it is reasonable to assume that the surface of the nanostructured Fe2O3 photoanodes present a high density of trap states, which are partially passivated by the deposited Al2O3 overlayer.
In order to further support this hypothesis, the effect of the Al2O3 overlayer was investigated by electrochemical impedance spectroscopy (EIS) and photoluminescence spectroscopy. EIS was performed in dark conditions on electrodes covered with 3 ALD cycles and a control electrode (without alumina) both annealed at 300 °C for 20 min. All samples showed, depending on the applied potential, one or two time constant features in the Nyquist plots (Figure S7†). Due to the large surface areas and high carrier concentrations in doped nanostructured electrodes like the APCVD samples used here, the capacitance of the space-charge region, CSC, can be the same order of magnitude as the Helmholtz capacitance, CH. Therefore it cannot be neglected when fitting the EIS data.14 For that reason, we used an equivalent circuit composed of 2 RC elements in series, which account for the semiconductor and surface processes (Fig. 5a).14,15 Since electronic processes in the bulk are normally faster than charge transfer processes or diffusion of ions in solution, the low frequency response was assigned to the semiconductor-electrolyte charge transfer resistance (RCT) together with CH while the high frequency response was accordingly designated to events occuring in the semiconductor with a resistance RSC and its accompanying CSC (see ESI†). This element is the combination of resistances and capacitances related to transport in the oxide (bulk) layer, charge diffusion in the space charge layer and surface trap charging by electrons and holes. Varying with the applied potential, each of these processes have nevertheless similar time constants. This make them difficult to distinguish and it leads to the observation of only one semicircle at high frequency on the Nyquist plot.16 In Fig. 5b, RCT (red circles) and RSC (blue squares) are plotted versus the applied potential for one electrode before (plain lines) and after ALD treatment (broken lines). The resistances are stable around 105 and 103 Ω, respectively, before the dark current starts to rise at about 1.6–1.65 V vs. RHE causing RCT to drop steeply. We note a decrease in RCT after the ALD treatment, which is consistent with the observed reduction of the overpotential. However, the considerable error associated with fitting such large resistances prohibits any definite conclusions on such a relatively small change in RCT. In contrast, more convincing information on the impact and acting mechanism of the Al2O3 overlayer is presented in Fig. 5c that compares CSC (blue triangles) and CH (red diamonds) before and after ALD on the same electrode (plain and broken lines, respectively).
 | ||
| Fig. 5 a) Electronic equivalent circuit representing the photoanode/electrolyte system used for EIS data modeling. RS represents a circuit series resistance (constant about 30–40 ohms). The other elements are defined in the main text. b) Representation of the different resistances determined by EIS and plotted versus the applied potential. The semiconductor resistance (blue circles) and the charge transfer resistance (red squares) are plotted before (full markers, plain line) and after (empty markers, broken line) 3 ALD cycles of Al2O3. c) Representation of the different capacitances determined by EIS and plotted versus the applied potential. The space charge capacitance (blue triangles) and the Helmholtz capacitance (red, diamonds) are plotted before (full markers, plain line) and after (empty markers, broken line) 3 ALD cycles of Al2O3. | ||
The change in CSC with respect to the applied potential before ALD shows the expected relationship: decreasing with increasing applied bias potential because of an increasing space charge (depletion) width. Just as the electrode enters a region of inversion that results in increased capacitance, charge transfer begins due to water oxidation and the capacitance drops suddenly. In accordance with other reports, CH shows a similar relationship with the applied potential to that of CSC,14 but the behavior of CH in highly doped nanostructured devices is not fully understood. Nevertheless, the effect of the Al2O3 overlayer becomes clear when comparing CSC and CH before and after ALD: CSC increases and CH decreases significantly. This effect was consistently observed on the samples tested. To rationalize this change we first recall that a capacitance is, by definition, the ability of a body to hold charges, and is most simply exemplified by a parallel plate capacitor: C = Q/V, with Q for the charge and V for the voltage. Thus the decrease of CH after the ALD treatment must be caused by a modification of the voltage or charge distribution at the SCLJ. This could be explained by improved charge screening of the anions in the Helmholtz layer due to the relatively high dielectric alumina overlayer or by a reduction of the charge density on the surface due to the passivation of surface states. Both effects would lead to an increase in voltage V, or decrease in charge density Q, and ultimately, would lower the capacitance according to the parallel-plate model. The increase of the space charge capacitance can also be understood by this simplification as well: in the absence of surface states, charges (an increase in Q) are depleted from the space-charge layer (rather than from surface states) to balance the applied voltage. Even though the parallel-plate model represents a significant simplification of the photoelectrochemical processes, it remains clear that the large change in the charge distribution, evidenced by change in capacitances, occurs over the entire voltage range cathodic of the water oxidation onset (i.e. 0.7–1.6 V vs. RHE). This would not be expected if the overlayer was merely acting to enhance the catalytic activity.
Recently, Hu et al.17 explained the reduced overpotential they observed when treating hematite photoanodes with CoF3 by a cathodic shift of the conduction and valence band energies evidenced by a modification of the flat band potential, Vfb. To investigate this possibility, we used the obtained value of CSC in a Mott-Schottky plot to extract Vfb along with the donor density, ND (see Figure S6, ESI†). We found Vfb = 0.53 V and 0.52 V vs. RHE, and ND = 7 × 1020 and 1 × 1021 cm−3 for the electrode before and after the Al2O3 ALD, respectively. These values are in good agreement with our previous results18 for similar hematite nanostructures and flat band potentials found in the literature.6b,6d,19 In addition, the Mott-Schottky analysis confirms that the alumina overlayer does indeed decrease the overpotential for the photocurrent onset while leaving the flat band potential unchanged as expected for the case of surface-state passivation.
Our inferred passivation of surface traps by the Al2O3 overlayer is expected to affect the recombination pathways of the photo-excited holes at the SCLJ. This effect is exemplified by an increased photoluminescence quantum yield typically observed in semiconductor quantum dots when coated with a conformal shell to eliminate surface trapping states.20 Various studies on nanocrystalline Fe2O3 particles have confirmed that photoluminescence in hematite due to direct hole-electron recombination is highly dependent on surface quality and, especially, on surface trap density.21 The absorption of a photon with energy greater than the band gap in hematite can be considered to create highly-localized excitonic states.21a In the bulk these excitons do not exhibit photoluminescence due to resonant energy transfer between cations and efficient lattice and magnetic relaxation pathways. Accordingly, photoluminescence is generally not observed in hematite except in nanocrystalline systems with specific surface capping agents.21b Since surface traps play a role in the nonradiative recombination of excitons present at the surface in hematite, photoluminescence should be enhanced if surface states are even partially eliminated by the alumina overlayer. Fig. 6 shows the results of a photoluminescence study of our hematite films with and without the ALD overlayer measured in air and without applied bias.
 | ||
| Fig. 6 Photoluminescence emission spectra (excitation wavelength = 520 nm) of a hematite cauliflower-type nanostructure photoanode before (red circles) and after 3 ALD cycles of Al2O3, on its surface (blue squares). | ||
While the control photoanode shows no presence of photoluminescence when excited at 520 nm, the electrode with 3 ALD cycles shows a weak and broad emission beginning at 580 nm (2.1 eV), peaking at 620 nm and tailing off at 720 nm to match the increasing background of the control sample. The onset of the emission at 2.1 eV is similar to other reports of photoluminescence in hematite,21b and corresponds well with the reported optical band gap. Moreover, this emission shows that a portion of photogenerated excitons are recombining radiatively. The induced photoluminescence after ALD is strong evidence that a fraction of the inter-bandgap surface states are passivated by the Al2O3 overlayer. Finally, no photoluminescence was observed when a control α-Fe2O3electrode was treated only with the Co2+catalyst indicating that surface states are not passivated by this treatment and further discounting a possible catalytic role of the alumina overlayer.
The results obtained from the various analytical techniques confirm the presence of surface trapping states on the nanostructured hematite photoanodes prepared by APCVD. Physically, the energetic traps could be a result of a disordered crystalline surface10 creating dangling bonds or oxygen vacancies. The filling of oxygen vacancies by the alumina overlayer is likely given that Al2O3 is generally thought to have negligible nonstoichiometry22 while oxygen vacancies are often observed in Fe2O3 as the presence of Fe2+.6b,23 Although the alumina overlayers showed a cathodic shift in the onset of the photocurrent, there remains a significant overpotential even after the addition of the Co2+catalyst (attempts to attach recently reported IrO2nanoparticles3 to the Al2O3 modified surface were unsuccessful) suggesting that all of the traps are not passivated by the ALD treatment, bulk trapping states are a limiting factor, or a limitation due to tunneling and OER surface intermediates is present.24 This, and the long-term instability of Al2O3 under the basic testing conditions will motivate the future investigation of new materials for the overlayer-based passivation, the mechanism of charge transfer and the OER with these materials, and the continued study of the factors limiting promising hematite photoanodes for solar water splitting.
Conclusions
In conclusion, the coating of nanostructured hematite photoanodes with a very thin layer (0.1 to 2 nm) of alumina by ALD resulted in a significant decrease in the overpotential required to photo-oxidize water. The photocurrent onset was shifted cathodically by as much as 100 mV and a record water oxidation photocurrent density of 0.42 mA cm−2 was obtained at 0.9 V vs. RHE under standard testing conditions after applying a Co2+catalyst. Further investigation by analyzing transient photocurrents, electrochemical impedance, and photoluminescence spectra provided convincing evidence that the cathodic shift afforded by the Al2O3 overlayer is due to the passivation of surface trapping states.Importantly, this study identifies two distinct causes—surface traps and slow oxidation kinetics—for the high overpotential observed in state-of-the-art nanostructured hematite photoanodes. Future studies will continue to focus on such surface treatments of hematite in order to further reduce the overpotential as well as the application of these overlayers to other metal oxide surfaces in order to facilitate interfacial electron transfer in different systems. In general, such post-treatments of metal oxide surfaces, like the one reported here, are of great interest to many research fields such as fuel cells, photovoltaics, sensors, and light-emitting electrochemical cells. The realization of high efficiency electron transfer at nanostructured interfaces in these systems will ultimately allow the use of inexpensive materials and processing techniques for high performance devices.
Acknowledgements
We gratefully acknowledge the Swiss Federal Office of Energy (Project number 102326, PECHouse), the Marie Curie Research Network “Hydrogen” (Contract number: MRTN-CT-2006-032474), the King Abdullah University of Science and Technology (KAUST, Award No KUS-C1-015-21), and Toyota Motor Corporation for financial support.Notes and references
- M. Grätzel, Nature, 2001, 414, 338–344 CrossRef CAS.
- (a) N. Beermann, L. Vayssieres, S. E. Lindquist and A. Hagfeldt, J. Electrochem. Soc., 2000, 147, 2456–2461 CAS; (b) H. E. Prakasam, O. K. Varghese, M. Paulose, G. K. Mor and C. A. Grimes, Nanotechnology, 2006, 17, 4285–4291 CrossRef CAS; (c) S. K. Mohapatra, S. E. John, S. Banerjee and M. Misra, Chem. Mater., 2009, 21, 3048–3055 CrossRef CAS; (d) K. Sivula, F. Le Formal and M. Grätzel, Chem. Mater., 2009, 21, 2862–2867 CrossRef CAS; (e) K. Sivula, R. Zboril, F. Le Formal, R. Robert, A. Weidenkaff, J. Tucek, J. Frydrych and M. Grätzel, J. Am. Chem. Soc., 2010, 132, 7436–7444 CrossRef CAS.
- S. Tilley, M. Cornuz, K. Sivula and M. Grätzel, Angew. Chem., Int. Ed., 2010, 49, 6405–6408 CrossRef CAS.
- J. Brillet, M. Cornuz, F. Le Formal, J.-H. Yum, M. Grätzel and K. Sivula, J. Mater. Res., 2010, 25, 17–24 CrossRef CAS.
- (a) A. Kay, I. Cesar and M. Grätzel, J. Am. Chem. Soc., 2006, 128, 15714–15721 CrossRef CAS; (b) D. K. Zhong, J. Sun, H. Inumaru and D. R. Gamelin, J. Am. Chem. Soc., 2009, 131, 6086–6087 CrossRef CAS.
- (a) P. Iwanski, J. Curran, W. Gissler and R. Memming, J. Electrochem. Soc., 1981, 128, 2128–2133 CrossRef CAS; (b) M. Dareedwards, J. Goodenough, A. Hamnett and P. Trevellick, J. Chem. Soc., Faraday Trans. 1, 1983, 79, 2027–2041 RSC; (c) G. Horowitz, J. Electroanal. Chem., 1983, 159, 421–436 CrossRef CAS; (d) C. Sanchez, K. Sieber and G. Somorjai, J. Electroanal. Chem., 1988, 252, 269–290 CrossRef CAS; (e) S. Ahmed, J. Leduc and S. Haller, J. Phys. Chem., 1988, 92, 6655–6660 CrossRef CAS; (f) J. Leduc and S. Ahmed, J. Phys. Chem., 1988, 92, 6661–6665 CrossRef CAS.
- H. Kim, H. B. R. Lee and W. J. Maeng, Thin Solid Films, 2009, 517, 2563–2580 CrossRef CAS.
- T. C. Li, M.r. S. Goćes, F. Fabregat-Santiago, J. Bisquert, P. R. Bueno, C. Prasittichai, J. T. Hupp and T. J. Marks, J. Phys. Chem. C, 2009, 113, 18385–18390 CrossRef CAS.
- The photocurrent onset potential is defined for this work as the potential where the line tangent to the inflection point of the photocurrent rise intersects the dark current. This method overestimates the onset potential as compared to visual inspection, but provides a quantitative measure.
- I. Cesar, K. Sivula, A. Kay, R. Zboril and M. Grätzel, J. Phys. Chem. C, 2009, 113, 772–782 CrossRef CAS.
- J. Brillet, M. Grätzel and K. Sivula, Nano Lett., 2010, 10, 4155–4160 CrossRef CAS.
- F. Le Formal, M. Grätzel and K. Sivula, Adv. Funct. Mater., 2010, 20, 1099–1107 CrossRef.
- By integrating the measured photocurrent density minus the steady-state value of the photocurrent (C cm−2s−1) with respect to time, we obtain a value proportional to the number of positive charges accumulated at the surface (i.e. C cm−2 s−1× s = C cm−2) in analogy to calculating the charge accumulated at a capacitor by integrating i(t) dt.
- J. Wielant, V. Goossens, R. Hausbrand and H. Terryn, Electrochim. Acta, 2007, 52, 7617–7625 CrossRef CAS.
- (a) N. Mcalpine and R. Fredlein, J. Electroanal. Chem., 1988, 252, 61–69 CrossRef CAS; (b) V. Aroutiounian, V. Arakelyan, G. Shahnazaryan, G. Stepanyan, J. Turner and S. Kocha, Electrochim. Acta, 2000, 45, 1999–2005 CrossRef CAS; (c) V. Aroutiounian, V. Arakelyan, G. Shahnazaryan, G. Stepanyan, E. Khachaturyan and J. Turner, C. R. Chim., 2006, 9, 325–331 CrossRef CAS.
- W. H. Leng, Z. Zhang, J. Q. Zhang and C. N. Cao, J. Phys. Chem. B, 2005, 109, 15008–15023 CrossRef CAS.
- Y.-S. Hu, A. Kleiman-Shwarsctein, G. D. Stucky and E. W. McFarland, Chem. Commun., 2009, 2652–2654 RSC.
- I. Cesar, K. Sivula, A. Kay, R. Zboril and M. Grätzel, J. Phys. Chem. C, 2009, 113, 772–782 CrossRef CAS.
- J. Kennedy and K. Frese, J. Electrochem. Soc., 1978, 125, 723–726 CrossRef CAS.
- P. Reiss, M. Protiere and L. Li, Small, 2009, 5, 154–168 CrossRef CAS.
- (a) D. M. Sherman and T. D. Waite, Am. Miner., 1985, 70, 1262–1269 CAS; (b) B. S. Zou and V. Volkov, J. Phys. Chem. Solids, 2000, 61, 757–764 CrossRef CAS.
- K. J. W. Atkinson, R. W. Grimes, M. R. Levy, Z. L. Coull and T. English, J. Eur. Ceram. Soc., 2003, 23, 3059–3070 CrossRef CAS.
- (a) R. F. G. Gardner, D. W. Tanner and F. Sweett, J. Phys. Chem. Solids, 1963, 24, 1183 CrossRef CAS; (b) K. M. Rosso, D. M. A. Smith and M. Dupuis, J. Chem. Phys., 2003, 118, 6455–6466 CrossRef CAS.
- The conformal nature of ALD and the large number of cycles used for the thickest case suggest that a tunneling mechanism is necessary for eletron transfer. A recent report with TiO2 electrodes and a tunnel-barrier overlayer of MgO ( L. Guo, D. Hung, W. Wang, W. Shen, L. Zhu, C.-L. Chien and P. C. Searson, Appl. Phys. Lett., 2010, 97, 063111–063113 Search PubMed ) showed that high water oxidation photocurrents can be obtained through a tunnel barrier without a transition metal oxide surface. The OER reaction presumably occurs through the homolytic decomposition of OH– groups as was shown by F. Freund, N. Scheikh-ol-Eslami and H. Gentsch, Angew. Chem., Int. Ed. Engl., 1975, 8, 568 CrossRef.
- R. Puurunen, J. Appl. Phys., 2005, 97, 121301 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details and additional tests indicated in the main text. See DOI: 10.1039/c0sc00578a |
| ‡ Atomic layer deposition and photoelectrochemical evaluation: The as-prepared APCVD films (heated at 300 °C in air to remove any surface contamination) were first evaluated for their PEC water oxidation performance under standard conditions (sweeping potentiometry in 1 M NaOH electrolyte, AM 1.5 G simulated solar illumination 100 mW cm−2) before the conformal deposition of Al2O3 or TiO2 by atomic layer deposition (ALD). For Al2O3, successive pulses of trimethylaluminium (TMA) and water in a nitrogen carrier gas were performed in a closed chamber heated at 200 °C.25 The number of cycles (pulse of TMA/vacuum/pulse of water/vacuum) applied to the sample was adjusted to 1, 3, 6, or 13 to give a corresponding thickness of 0.15, 0.45, 0.9 or 1.95 nm (as measured by ellipsometery on a Si reference under identical conditions). For samples covered with TiO2, 6, 33, 66 and 132 cycles (pulse of titanium isopropoxide/vacuum/pulse of water/vacuum) were necessary to give similar thicknesses due to the lower reactivity of the precursor at 200 °C. The hematite electrodes were then tested under standard conditions, annealed in air for 20 min at 300 °C, tested, annealed at 400 °C, and tested once more. A control electrode was subjected to the same set of conditions but without any deposition. The transient photocurrent was realized by setting the desired potential and modulating the light with a computer controlled chopper. Additional experimental details are provided in the Supporting Information.†Electrochemical Impedance Spectroscopy (EIS): The ac-impedance was measured in 1M NaOH as electrolyte using Ag/AgCl in saturated KCl as a reference electrode, using a EG&G 273 potentiometer combined with a frequency response analyzer (FRA) from Solartron. A sinusoidal voltage perturbation of 10 mV amplitude was superimposed on a bias voltage, with a frequency that ranged from 100 kHz to 0.1 Hz. The bias potential was scanned cathodically from 0.8 to −0.3 V vs. Ag/AgCl with a 50 mV interval and is reported again versus the reversible hydrogen electrode (RHE). The raw data were fitted using the Zview software from Scribner Associate Inc.Photoluminescence spectra (PL): Photoluminescence experiment was carried on a Horiba Fluorolog 3–22 spectrofluorometer using a 520 nm excitation wavelength (5 nm bandpass for both entrance and exit) and front-face detection with a standard photomultiplier detector. The emission was scanned from 510 nm to 850 nm with 1 nm increments and a 1 s integration time was used. |
| This journal is © The Royal Society of Chemistry 2011 |
