Sulfonated graphene as water-tolerant solid acid catalyst†
Junyi
Ji
,
Guanghui
Zhang
,
Hongyu
Chen
,
Shulan
Wang
,
Guoliang
Zhang
,
Fengbao
Zhang
and
Xiaobin
Fan
*
School of Chemical Engineering & Technology, Tianjin University, Tianjin, PR China. E-mail: xiaobinfan@tju.edu.cn; Fax: +86-22-27408778; Tel: +86-22-27408778
First published on 3rd December 2010
Abstract
Acid catalysts are essential for various chemical reactions in the industrial hydrocarbon chemistry. Sulfonated carbon has shown promising application as a new, cheap and environmentally friendly solid acid catalyst. In this study, we prepared the sulfonated graphene acid catalyst, and it was characterized by electron microscopy, Raman spectroscopy, solid state 13C MAS NMR, energy dispersive spectroscopy (EDS) and X-ray photoelectron spectroscopy (XPS). Catalytic hydrolysis of ethyl acetate shows that the sulfonated graphene has highly catalytic activity and can be repetitively used as a water-tolerant solid acid catalyst.
Introduction
Acid catalysts are essential for various chemical reactions in industrial hydrocarbon chemistry. Traditional liquid-acid catalysts (such as sulfuric acid) are highly efficient but nonrecyclable. It is also costly and difficult to separate the liquid-acid catalysts from the homogeneous reaction mixture, thus resulting in abundant nonrecyclable acid waste.Many solid acids have shown great potential to replace traditional liquid acids as recyclable and environmentally-safe acid catalysts.1–5 Among them, sulfonated carbon has emerged as a stable and highly active protonic acid catalyst. Hara et al. prepared the carbon-based solid acid by incomplete carbonization of sulfonated aromatic compounds for the first time.6 Later, they prepared sulfonated carbon by the sulfonation of incompletely carbonized sugars and proved that its activity was comparable to that of liquid sulfuric acid in the esterification of higher fatty acids.7 By covalent attachment of sulfonic acid-containing aryl radicals, Wang et al. obtained sulfonated ordered mesoporous carbon acid catalysts with an acid density of 1.93 mmol H+ g−1 and impressive catalytic activity.8Graphene has excellent mechanical properties, a large surface area and a distinctive two-dimensional structure,9,10 providing an ideal platform to anchor a large amount of acidic functional groups with accessible active sites.
Despite graphene and its derivatives (graphene oxide) having inherent catalytic activities or having been used as supports for nano-metal catalysts,11–14 the graphene-based solid acid catalyst has never been reported. In this study, we prepared the sulfonated graphene acid catalysts and investigated its catalytic performance. Considering the irreversible destruction to graphene surfaces and its low efficiency of sulfonation with sulfuric acid, we prepared sulfonated graphene by direct anchoring of sulfonic acid-containing aryl radicals to the surfaces of reduced graphene (Scheme 1).8,15
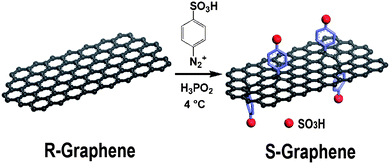 | ||
| Scheme 1 Illustration for the preparation of sulfonated graphene. | ||
Experimental
Preparation of graphene-based solid acid catalyst
Graphene oxide (GO) was prepared and purified by Hummers method.16 The resulting GO suspension (180 mL, 1.0 mg mL−1) was reduced with 80% hydrazine hydrate (1.8 mL) at 100 °C for 24 h followed by washing with deionized water several times. The yielded suspension was dialyzed for 48 h to get a purified reduced graphene suspension.4-Benzenediazoniumsulfonate was synthesized by diazotization of sulfanilic acid. Sulfanilic acid (5.2 g, 0.03 mol) was dispersed in 1 M HCl aqueous solution (300 mL) in a three-necked ground flask. The flask was then moved to an ice water bath, and the temperature was controlled at 3–5 °C with continuous stirring. Then, 10% excess of 1 M NaNO2 (33 mL, aqueous solution) was added dropwise, and a clear solution was obtained after all the NaNO2 was added. After stirring for another 1 h at the same temperature, the white precipitate formed was filtered off and washed with deionized water.
4-Benzenediazoniumsulfonate obtained was moved to a three-necked ground flask with deionized water (60 mL) and ethanol (60 mL). Then, reduced graphene (180 mg) was added at 3–5 °C. Subsequently, 50 wt% H3PO2 aqueous solution (60 mL) was added. After stirring for 30 min, the same amount of H3PO2 aqueous solution (60 mL) was added again and stirred for 1 h. The obtained sulfonated graphene was intensively washed with deionized water and then dried in the vacuum overnight.
Catalytic ability
The catalytic ability of the sulfonated graphene was examined through the hydrolysis of ethyl acetate. In brief, hydrolysis of ethyl acetate (70 °C) was carried out in a mixture of deionized water (6.25 mL) and ethyl acetate (0.375 mL) in the N2 atmosphere for 6 h. The catalysts (except the sulfuric acid) were evacuated at 100 °C for 1 h before the reactions, and the same amount of each catalyst (20 mg) was used. The resulting solution was analyzed by gas chromatography using a capillary column.Characterization
The sulfonated graphene was characterized by scanning electron microscopy (SEM) (Hitachi S4800), energy dispersive X-ray spectroscopy (EDS) (Hitachi S4800), high-resolution transmission electron microscopy (HRTEM) (Philips Tecnai G2 F20), Raman spectroscopy (NT-MDT NTEGRA Spectra), solid state 13C MAS NMR (Infinity plus 300WB) and X-ray photoelectron spectroscopy (XPS) (PerkinElmer, PHI 1600 spectrometer). The hydrolysis results were measured by GC (Agilent 6890N GC-FID system).Results and discussion
Scanning electron microscopy (SEM) images show the structures of reduced graphene before (Fig. 1a) and after (Fig. 1b) sulfonation. Compared with the reduced graphene before sulfonation, the sulfonated graphene shows similar crumpling features with clear layers. Transmission electron microscopy (TEM) verified that the microstructure of the graphene sheets was not destroyed by the sulfonate reaction. This crumpling feature may have potential advantages in acid-catalyzed reactions, as the reactants can easily access the active sites on both sides of the two dimensional graphene sheets. Though the two-dimensional structure may cause diffusion hindrance during the reaction, the reduced graphene sheets are small enough (about 5 μm × 1 nm in size) to be well dispersed under stirring conditions. The crumpling feature with scarce micropores may also facilitate the diffusion of the product molecules.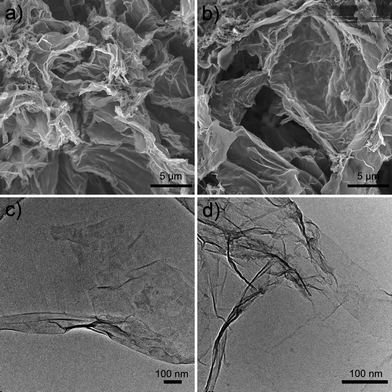 | ||
| Fig. 1 SEM images of reduced graphene (a) and graphene after sulfonation (b), TEM images of reduced graphene (c) and graphene after sulfonation (d). | ||
Raman spectroscopy results of the reduced graphene and sulfonated graphene are shown in Fig. 2. The Raman spectra of reduced graphene displays two characteristic bands around 1366 cm−1 and 1624 cm−1, corresponding to the defects induced D band and the first-order scattering of the E2g mode for sp2carbon lattice (G band), respectively.17 Usually, the I(D)/I(G) intensity ratio of graphene could be used to evaluate the structural changes during the chemical processing. In our study, the I(D)/I(G) intensity ratio of sulfonated graphene (Fig. 2b) is slightly higher than that of the reduced graphene (Fig. 2a), due to the introduction of abundant –SO3H groups to the sp2carbon network. Further, the G band of sulfonated graphene shifts slightly from 1624 cm−1 to 1630 cm−1 after sulfonation. Considering a red shift of the G band is expected with increasing strain,18 the introduced strain in the graphene sheet after sulfonation should not be the cause of the blue shift (6 cm−1) here. Therefore, the blue shift of the G band should be attributed to the doping, which introduces abundant aryl radicals to the graphene sheets.19 The change of alternating pattern of single—double carbon bonds within the sp2carbon sheets after sulfonation may also cause the blue shift of the G band.20 The structure of the sulfonated graphene is further confirmed by its solid state 13C NMR spectrum (Fig. S1, see ESI†), which shows a small peak at 136 ppm, corresponding to the carbons in the covalently attached phenyl-SO3H groups.21
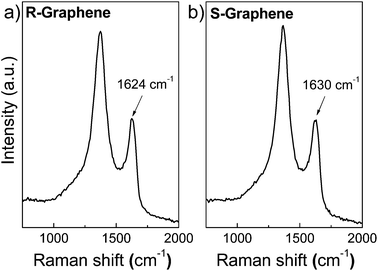 | ||
| Fig. 2 Raman spectra of reduced graphene (a) and sulfonated graphene (b). | ||
The density and distribution of the –SO3H groups on the sulfonated graphene are evaluated by quantitative energy dispersive X-ray spectroscopy (EDS) mapping. As can be seen in Fig. 3, rather than only located at the edges of graphene sheets, the element S is found to be homogeneously distributed on the whole surface. Quantitative analysis (Fig. 4) shows the element mass ratios of carbon, oxygen and sulfur in the sulfonated graphene are 83.35%, 10.25% and 6.40%, respectively. That is, the density of –SO3H group on the sulfonated graphene is calculated to be ∼2.0 mmol g−1. The actual acid exchange capacity of the sulfonated graphene was finally determined by neutralization titration to be 1.55 mmol H+ g−1. This value is significantly higher than those of commercial solid acid catalysts (e.g., Nafion NR50, 0.80 mmol H+ g−1). Considering the O/S atom ratio (3.2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) is higher than 3
1) is higher than 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, residual epoxide, hydroxyl and carboxyl groups are also expected on the sulfonated graphene sheets (Fig. S2, see ESI†).
1, residual epoxide, hydroxyl and carboxyl groups are also expected on the sulfonated graphene sheets (Fig. S2, see ESI†).
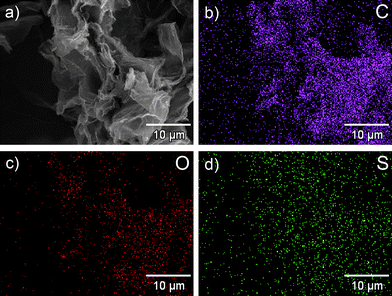 | ||
| Fig. 3 The SEM image of the sulfonated graphene (a), and corresponding quantitative EDS element mapping of C (b), O (c) and S (d). | ||
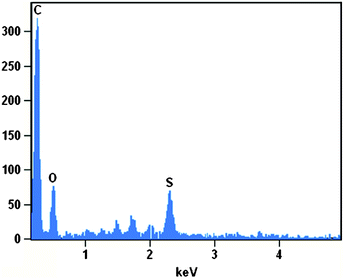 | ||
| Fig. 4 The EDS spectrum of the sulfonated graphene. | ||
Due to the severe poisoning of the acid sites by water, most solid acids will lose their catalytic activities in aqueous solutions, and only a few solid acids show decent activity and stability in a water participating reaction (such as hydrolysis, hydration, and esterification).1 Therefore, hydrolysis of ethyl acetate was chosen to evaluate the activity and stability of the sulfonated graphene, with concentrated sulfuric acid and a conventional solid acid catalyst (Nafion NR50) as controls. Fig. 5a shows the hydrolysis rates of ethyl acetate by equal amounts of sulfonated graphene, concentrated sulfuric acid and Nafion NR50. The sulfonated graphene shows comparable activity to the concentrated sulfuric acid and is obviously superior to the Nafion NR50, a commercial solid acid known for its excellent activity in reactions even in a high-temperature environment.2 The high catalytic activity of the sulfonated graphene here should be attributed to its high acid exchange capacity with abundant accessible active sites, as the reactants (ethyl acetate) can easily access the –SO3H groups on both sides of the two dimensional graphene sheets. The stability of the sulfonated graphene as a solid acid catalyst was examined by recycling experiments. Five run experiments show the catalytic activity of the sulfonated graphene was unchanged with an averaged hydrolysis rate of 64.0% (Fig. 5b). SEM image and EDS analysis (Fig S3 and Fig S4, see ESI†) of the sulfonated graphene after repeated experiments show that the element ratios of carbon, oxygen and sulfur have no noticeable change, indicating there should be no loss of the –SO3H groups. The excellent stability of the covalently bonded –SO3H groups may be attributed to the stable C–C bond with the conjugated graphene sheet and the aromatic ring. Experimentally, the C–C bond to graphene has been found to be stable even when heated to 200 °C.22
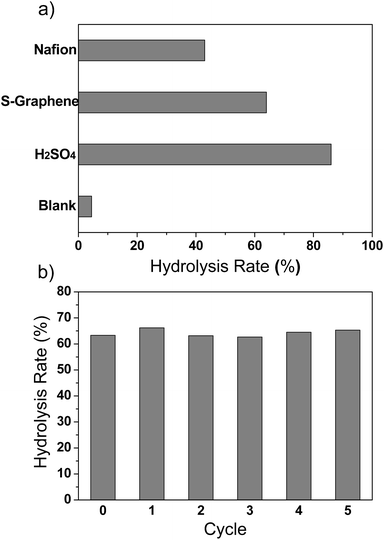 | ||
| Fig. 5 Hydrolysis rate of ethyl acetate at 343 K by using 20 mg of catalyst (a) and the reuse activity of the sulfonated graphene catalyst (b). | ||
Conclusions
In summary, we have prepared the sulfonated graphene and evaluated its application as a solid acid catalyst. This graphene-based solid acid catalyst shows high catalytic activity in the hydrolysis reaction. In addition, the sulfonated graphene shows desirable stability in water and can be repeatedly used without an obvious decrease to its catalytic activity. Considering its high acid exchange capacity and excellent tolerance in water, the sulfonated graphene may be also used in various acid-catalyzed reactions, such as esterification. Considering a liquid-acid catalyst is usually nonrecyclable, the graphene-based solid acid here shows promising applications as a cheap, low cost and environmentally friendly acid catalyst in industrial hydrocarbon chemistry.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No: 20776095), the Innovation Fund of Tianjin University and by the Program of Introducing Talents of Discipline to Universities (China, No: B06006).Notes and references
- T. Okuhara, Chem. Rev., 2002, 102, 3641 CrossRef CAS
.
- G. Busca, Chem. Rev., 2007, 107, 5366 CrossRef CAS
.
- J. H. Clark, Acc. Chem. Res., 2002, 35, 791 CrossRef CAS
.
- B. M. Reddy and M. K. Patil, Chem. Rev., 2009, 109, 2185 CrossRef CAS
.
- A. Stein, Z. Y. Wang and M. A. Fierke, Adv. Mater., 2009, 21, 265 CrossRef CAS
.
- M. Hara, T. Yoshida, A. Takagaki, T. Takata, J. N. Kondo, S. Hayashi and K. Domen, Angew. Chem., Int. Ed., 2004, 43, 2955 CrossRef CAS
.
- M. Toda, A. Takagaki, M. Okamura, J. N. Kondo, S. Hayashi, K. Domen and M. Hara, Nature, 2005, 438, 178 CrossRef CAS
.
- X. Q. Wang, R. Liu, M. M. Waje, Z. W. Chen, Y. S. Yan, K. N. Bozhilov and P. Y. Feng, Chem. Mater., 2007, 19, 2395 CrossRef CAS
.
- K. S. Novoselov, A. K. Geim, S. V. Morozov, D. Jiang, Y. Zhang, S. V. Dubonos, I. V. Grigorieva and A. A. Firsov, Science, 2004, 306, 666 CrossRef CAS
.
- M. J. Allen, V. C. Tung and R. B. Kaner, Chem. Rev., 2010, 110, 132 CrossRef CAS
.
- G. M. Scheuermann, L. Rumi, P. Steurer, W. Bannwarth and R. Mulhaupt, J. Am. Chem. Soc., 2009, 131, 8262 CrossRef CAS
.
- Y. H. Lu, M. Zhou, C. Zhang and Y. P. Feng, J. Phys. Chem. C, 2009, 113, 20156 CrossRef CAS
.
- Y. J. Song, K. G. Qu, C. Zhao, J. S. Ren and X. G. Qu, Adv. Mater., 2010, 22, 2206 CrossRef CAS
.
- D. R. Dreyer, H. P. Jia and C. W. Bielawski, Angew. Chem., Int. Ed., 2010, 49, 6813 CAS
.
- J. R. Lomeda, C. D. Doyle, D. V. Kosynkin, W. F. Hwang and J. M. Tour, J. Am. Chem. Soc., 2008, 130, 16201 CrossRef CAS
.
- A. Lerf, H. Y. He, M. Forster and J. Klinowski, J. Phys. Chem. B, 1998, 102, 4477 CrossRef CAS
.
- A. C. Ferrari, J. C. Meyer, V. Scardaci, C. Casiraghi, M. Lazzeri, F. Mauri, S. Piscanec, D. Jiang, K. S. Novoselov, S. Roth and A. K. Geim, Phys. Rev. Lett., 2006, 97, 187401 CrossRef CAS
.
- T. M. G. Mohiuddin, A. Lombardo, R. R. Nair, A. Bonetti, G. Savini, R. Jalil, N. Bonini, D. M. Basko, C. Galiotis, N. Marzari, K. S. Novoselov, A. K. Geim and A. C. Ferrari, Phys. Rev. B, 2009, 79, 205433 CrossRef
.
- M. Lazzeri and F. Mauri, Phys. Rev. Lett., 2006, 97, 266407 CrossRef
.
- K. N. Kudin, B. Ozbas, H. C. Schniepp, R. K. Prud'homme, I. A. Aksay and R. Car, Nano Lett., 2008, 8, 36 CrossRef CAS
.
- Y. C. Si and E. T. Samulski, Nano Lett., 2008, 8, 1679 CrossRef CAS
.
- E. Bekyarova, M. E. Itkis, P. Ramesh, C. Berger, M. Sprinkle, W. A. de Heer and R. C. Haddon, J. Am. Chem. Soc., 2009, 131, 1336 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Solid state 13C NMR spectrum, X-ray photoelectron spectrum, C 1s electron region of the X-ray photoelectron spectrum, scanning electron microscope and energy dispersive X-ray spectroscopy. See DOI: 10.1039/c0sc00484g |
| This journal is © The Royal Society of Chemistry 2011 |
