Amino olefin nickel(I) and nickel(0) complexes as dehydrogenation catalysts for amine boranes†‡
Matthias
Vogt
a,
Bas
de Bruin
b,
Heinz
Berke
c,
Mónica
Trincado
*a and
Hansjörg
Grützmacher
*a
aDepartment of Chemistry and Applied Biosciences, ETH, Hönggerberg, 8093, Zürich, Switzerland. E-mail: gruetzmacher@inorg.chem.ethz.ch; Web: http://www.gruetzmacher.ethz.ch
bUniversiteit van Amsterdam, Van’t Hoff Institute for Molecular Sciences, Science Park 904, 1098 XH, Amsterdam, The Netherlands
cUniversität Zürich, Anorganisch-chemisches Institut, CH-8057, Zürich, Switzerland
First published on 17th January 2011
Abstract
A rare paramagnetic organometallic nickel(I) olefin complex can be isolated using the ligand bis(5H-dibenzo[a,d]cyclohepten-5-yl)amine. This complex and related nickel(0) hydride complexes show very high catalytic activity in the dehydrogenation of dimethylamino borane with release of one equivalent of dihydrogen.
Introduction
Recent years have seen a significant effort to use amine boranes, R3−xHxN–BHyRy−3, as chemical hydrogen storage materials with relatively high storage capacities (ideally up to 19.6 wt% in the conversion of ammonia borane to boronitride structures).1 This approach may be questioned as long as boranes and amines are not produced from their natural resources using eco-friendly energy sources and the direct “refuelling” of ‘BN’ compounds remains problematic without pre-digestion of the ‘BN’ material.2 The generation of reactive intermediates through dehydrogenations offers interesting perspectives for “waste-free” atom economic synthetic methods. Efforts along these lines also include the development of catalysts free of rare and expensive precious metals which enable fast release of H2.3Several mechanisms for the dehydrogenation of amine borane compounds have been proposed4 and a variety of metal complexes with, Ti,5 Rh,6–8 Ir,9 Ru,10 Re11 and more recently Mg12 and Pd,13 promote this reaction. Two results are especially noteworthy with respect to this work: Baker et al. reported that N-heterocyclic carbene (NHC) complexes of nickel(0) show a higher catalytic activity compared to Rh(NHC) or Ru(NHC) analogs.14 Possible reaction mechanisms for the ammonia borane dehydrogenation catalyzed by nickel carbene have been calculated.15a–c It has been suggested that a dissociated free carbene may participate in the process.15d Further, ruthenium-amido catalysts have been introduced with the highest reported activity to date in the dehydrogenation of amine boranes.10
According to Fagnou et al.,10a the amido group plays the role of a cooperative ligand16 and the dehydrogenation proceeds according to a Noyori–Morris-related mechanism.17 Schneider et al. observed and isolated an inactive Ru–N–B–H metallacycle in a side reaction to rationalize the quick release of only 70% of H2.10b,c
We discovered that amino olefin ligands, specifically bis(5H-dibenzo[a,d]cyclohepten-5-yl)amine = bistropylidenylamine = trop2NH,18 give rise to structurally well-defined rhodium amido complexes like B, which serve as superb transfer hydrogenation catalysts.19 Furthermore B, which is simply derived through deprotonation of the very stable amine complex [Rh(trop2NH)(PPh3)]OTf A is a highly active catalyst in the dehydrogenative coupling (DHC) of primary alcohols to carboxylic derivatives under various reaction conditions.19 Here we report on novel bistropylidenylamine nickel(I) and nickel(0) complexes which can be applied in the highly efficient catalytic dehydrogenation of Me2HN–BH34.
Results and discussion
The dark green nickel(I) complex 2 was prepared by addition of trop2NH17 to nickel(II) trifluoroacetate 1 in the presence of Zn powder. The dark red nickel(0) complex 3 was prepared by further reducing the nickel(I) complex in the presence of PPh3 (Scheme 1). This reaction can be performed in one step, where [NiII(OOCCF3)2] 1 is directly converted to 3 when the reduction of 1 with Zn is performed in the presence of trop2NH and PPh3. While the reduction of 2 in the presence of PPh3 proceeds smoothly with Zn powder, a cyclic voltammogram (CV) of complex 2 in THF shows the onset potential for a reduction at rather negative values (−1.99 V vs. Fc+/Fc; the peak potential of the irreversible reduction wave is seen at −2.42 V). On the contrary, the CV of complex [Ni(trop2NH)(PPh3)] 3 shows a reversible redox wave at −1.28 V for the nickel(I)/nickel(0) couple.The molecular structures of 2 and 3 were determined by a single crystal X-ray diffraction study. The results are shown in Fig. 1A and 1B, respectively. Complex 2 is a very rare example of a nickel(I) olefin complex.20 The nickel(I) center is pentacoordinated in a coordination sphere formed through the trop2NH ligand and the non-symmetrically κ2–binding trifluoroacetate [Ni–O1: 2.176(1) Å; Ni–O2: 2.265(1) Å]. In [Ni(trop2NH)(PPh3)] 3 the nickel(0) center resides in a distorted pseudo-tetrahedral coordination sphere. With respect to the Ni(trop2NH) moiety, the structures of 2 and 3 are rather similar. Not unexpected, the Ni–N bond is slightly longer in the nickel(0) complex 3 (2.1939(2) Å vs. 2.061(2) Å in 2). The Ni–ct1 and Ni–ct2 (ct1 and ct2 represent the centroids of the C4–C5 and C19–C20 double bonds, respectively) distances are marginally shorter and the coordinated C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ctrop bonds (C4
Ctrop bonds (C4![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C5, C19
C5, C19![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C20) are slightly longer in the nickel(0) complex 3 indicating more metal-to-ligand back donation of the d10-nickel(0) center into the π-accepting olefinic units. This is also reflected in the NMR data; the 13C NMR resonances of the C
C20) are slightly longer in the nickel(0) complex 3 indicating more metal-to-ligand back donation of the d10-nickel(0) center into the π-accepting olefinic units. This is also reflected in the NMR data; the 13C NMR resonances of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ctrop nuclei are observed at rather low frequencies (13C NMR δ = 67.9, 69.5 ppm). In comparison, the corresponding resonances in the cationic d8-RhI complex A are observed at 74.0 ppm and 74.2 ppm in the 13C NMR spectra. Note that in A the NH group of the trop2NH ligand is rather acidic (1H NMR δ = 5.66 ppm; estimated pKa DMSO ≈ 17). The nickel(0) complex 3 has a reduced acidity (1H NMR δ = 1.09 ppm) compared to A. Indeed, adding MeOD to solutions of 3 did not show any evidence for a proton-deuteron exchange and no deprotonation reaction is observed between 3 and KOtBu.
Ctrop nuclei are observed at rather low frequencies (13C NMR δ = 67.9, 69.5 ppm). In comparison, the corresponding resonances in the cationic d8-RhI complex A are observed at 74.0 ppm and 74.2 ppm in the 13C NMR spectra. Note that in A the NH group of the trop2NH ligand is rather acidic (1H NMR δ = 5.66 ppm; estimated pKa DMSO ≈ 17). The nickel(0) complex 3 has a reduced acidity (1H NMR δ = 1.09 ppm) compared to A. Indeed, adding MeOD to solutions of 3 did not show any evidence for a proton-deuteron exchange and no deprotonation reaction is observed between 3 and KOtBu.
![A) ORTEP plot of 2 at 50% ellipsoid probability (hydrogen atoms (apart from N1–H) and solvent (THF) molecule are omitted for clarity). Selected bond lengths [Å] and angles [°]: Ni1–N1 2.061(2), Ni1–O1 2.176(1), Ni1–O2 2.265(1), Ni1–ct1 1.993, Ni–ct2 2.001, C4–C5 1.388(3), C19–C20 1.392(3), O1–C31 1.249(2), O2–C31 1.249(2); N1–Ni1–O1 104.91(6), N1–Ni1–O2 164.94(5), O1–Ni1–O2 60.05(5), ct1–Ni–ct2 133.67, ct1–Ni–O1 112.33, ct2–Ni1–O1 109.41. B) ORTEP plot of 3 at 50% ellipsoid probability hydrogen atoms (apart from N1–H) are omitted for clarity. Selected bond lengths [Å] and angles [°]: Ni1–N1 2.194(2), Ni1–P1 2.1636(5), Ni1–ct1 1.925, Ni–ct2 1.925, C4–C5 1.409(3), C19–C20 1.411(3); N1–Ni1–P1 119.18(4), ct1–Ni–ct2 132.00, ct1–Ni–P1 108.14, ct2–Ni1–P1 111.54.](/image/article/2011/SC/c0sc00483a/c0sc00483a-f1.gif) | ||
| Fig. 1 A) ORTEP plot of 2 at 50% ellipsoid probability (hydrogen atoms (apart from N1–H) and solvent (THF) molecule are omitted for clarity). Selected bond lengths [Å] and angles [°]: Ni1–N1 2.061(2), Ni1–O1 2.176(1), Ni1–O2 2.265(1), Ni1–ct1 1.993, Ni–ct2 2.001, C4–C5 1.388(3), C19–C20 1.392(3), O1–C31 1.249(2), O2–C31 1.249(2); N1–Ni1–O1 104.91(6), N1–Ni1–O2 164.94(5), O1–Ni1–O2 60.05(5), ct1–Ni–ct2 133.67, ct1–Ni–O1 112.33, ct2–Ni1–O1 109.41. B) ORTEP plot of 3 at 50% ellipsoid probability hydrogen atoms (apart from N1–H) are omitted for clarity. Selected bond lengths [Å] and angles [°]: Ni1–N1 2.194(2), Ni1–P1 2.1636(5), Ni1–ct1 1.925, Ni–ct2 1.925, C4–C5 1.409(3), C19–C20 1.411(3); N1–Ni1–P1 119.18(4), ct1–Ni–ct2 132.00, ct1–Ni–P1 108.14, ct2–Ni1–P1 111.54. | ||
Magnetic susceptibility measurements (SQUID, T = 2–300 K) of [NiI(trop2NH)(OOCCF3)] 2 gave an effective magnetic moment of μeff = 1.81 μB in accordance with the existence of one unpaired electron.
Inspection of the EPR spectrum of 2 in a frozen 2-Me-THF solution clearly reveals besides the major species 2a (∼90% of the total spectral intensity), the presence of a second minor species 2b (∼5%), and a detailed line shape analysis suggests the presence of even a third minor species, 2c (∼5%) (Fig. 2).21
![Top: Experimental and simulated X-band EPR spectrum of [NiI(trop2NH)(OOCCF3)] in frozen 2-methyl THF. Experimental conditions: 120 K, microwave frequency 9.500720 GHz, microwave power 2.012 mW, modulation amplitude 1.00 Gauss. Bottom: Individual components 2a (90%), 2b (4%) and 2c (6%) contributing to the EPR spectrum. (The simulation was obtained using the parameters in Table 3 of the ESI).](/image/article/2011/SC/c0sc00483a/c0sc00483a-f2.gif) | ||
| Fig. 2 Top: Experimental and simulated X-band EPR spectrum of [NiI(trop2NH)(OOCCF3)] in frozen 2-methyl THF. Experimental conditions: 120 K, microwave frequency 9.500720 GHz, microwave power 2.012 mW, modulation amplitude 1.00 Gauss. Bottom: Individual components 2a (90%), 2b (4%) and 2c (6%) contributing to the EPR spectrum. (The simulation was obtained using the parameters in Table 3 of the ESI†). | ||
The major species 2a must correspond to [NiI(trop2NH)(κ2-OOCCF3)], as the DFT calculated parameters of the optimized geometry of 2 correspond well to the experimental parameters of 2a (for details concerning the EPR spectra and DFT calculations see the ESI†).
Although trop2NH may be regarded as a redox non-innocent ligand when coordinated to late transition metals,16,18 it behaves “innocently” in 2. The EPR and DFT data clearly reveal metal-centered spin density (ρDFTNi > 80%).
The nickel complexes 2 and 3 and the rhodium amide B were investigated for the catalytic dehydrogenation of Me2HN–BH34 in THF at room temperature under an inert atmosphere. The amidoborane salt K[Me2NBH3] 5 was used as a co-catalyst, added either in substance22 or prepared in situ from Me2HN–BH34 and tBuOK, (Scheme 2). The nickel(I) complex [Ni(trop2NH)(OOCCF3)] 2 is an especially active catalyst and even with 0.3 mol% and 1 mol% of co-catalyst, one equivalent of H2 is released in less than one minute from 1 M solutions of substrate 4 in THF (Fig. 3).23 To our knowledge this is the highest rate for the dehydrogenation of 4 mediated by any non-noble metal catalyst under mild conditions reported to date. The nickel(0) phosphane complex [Ni(trop2NH)(PPh3)] 3 is significantly slower and no conversion is observed when the catalysis is performed in the presence of an excess of PPh3.24
![Catalytic dehydrogenation of Me2HN–BH34 mediated by complexes 2 and 3 and catalytic amounts of K[Me2NBH3] 5 or tBuOK.](/image/article/2011/SC/c0sc00483a/c0sc00483a-s2.gif) | ||
| Scheme 2 Catalytic dehydrogenation of Me2HN–BH34 mediated by complexes 2 and 3 and catalytic amounts of K[Me2NBH3] 5 or tBuOK. | ||
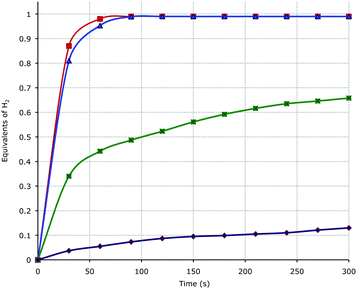 | ||
| Fig. 3 Volumetric measurement of H2 release from Me2HN–BH34 mediated by complexes 2 (■ 1 mol%; ▲ 0.3 mol%), 3 (■ 1 mol%), and B (♦ 0.2 mol%). | ||
As final products, the cyclic 1,3-diaza-diboretane 6 (11B NMR δ = 5.10 ppm, t, 1JBH = 112 Hz) is quantitatively obtained and traces of the monomer Me2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2 (11B NMR δ = 37.2 ppm, 1JBH = 121 Hz) are detected. In the slower reaction with 3 as the catalyst, the linear N–B–N–B product 7 is the kinetically controlled product which is converted in a slower follow-up reaction to 6.25
BH2 (11B NMR δ = 37.2 ppm, 1JBH = 121 Hz) are detected. In the slower reaction with 3 as the catalyst, the linear N–B–N–B product 7 is the kinetically controlled product which is converted in a slower follow-up reaction to 6.25
Given the significance of paramagnetic nickel complexes in hydrogenase enzymes,26 it is tempting to assume that the nickel(I) species and radical pathways are involved in the H2 release. However, presently we cannot exclude that nickel(0) complexes are involved in the catalytic cycle based on the following observations from reactions performed under non-catalytic conditions: (i) [NiI(trop2NH)(OOCCF3)] 2 is cleanly reduced to [Ni0(trop2NH)(PPh3)] 3 with one equivalent of K[Me2HN–BH3] 5 in the presence of one equivalent PPh3. In this reaction, the monomer Me2N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BH2 is clearly detected which dimerizes over time to the 1,3-diaza-diboretane 6. (ii) The reaction between the nickel(I) complex 2 and a slight excess of amido borane 5 results in a brown solution, showing similar appearance to the catalytic reaction, in which a bridging nickel hydride complex 8 (1H NMR δ = −8.12 ppm) is observed beside amine borane 4 and the heterocycle 6. The hydride species could not be isolated but was characterized by NMR and we propose the C2 symmetric structure shown in Scheme 3 (see the ESI† for details). (iii) If K[Me2NBH3] is formed in situ from 4 with an excess of KOtBu, the reaction leads to an additional nickel hydride 9 (1H NMR δ = −4.59 ppm) which after a short time becomes the main product. So far we have not been able to isolate this species but on the basis of the 1H and 13C NMR data we have assigned the structure 9.27 (iv) Finally, the mixture containing both hydrides 8 and 9 also catalyzes comparably fast hydrogen release from amine borane 4.
BH2 is clearly detected which dimerizes over time to the 1,3-diaza-diboretane 6. (ii) The reaction between the nickel(I) complex 2 and a slight excess of amido borane 5 results in a brown solution, showing similar appearance to the catalytic reaction, in which a bridging nickel hydride complex 8 (1H NMR δ = −8.12 ppm) is observed beside amine borane 4 and the heterocycle 6. The hydride species could not be isolated but was characterized by NMR and we propose the C2 symmetric structure shown in Scheme 3 (see the ESI† for details). (iii) If K[Me2NBH3] is formed in situ from 4 with an excess of KOtBu, the reaction leads to an additional nickel hydride 9 (1H NMR δ = −4.59 ppm) which after a short time becomes the main product. So far we have not been able to isolate this species but on the basis of the 1H and 13C NMR data we have assigned the structure 9.27 (iv) Finally, the mixture containing both hydrides 8 and 9 also catalyzes comparably fast hydrogen release from amine borane 4.
![Different reaction pathways of [NiI(trop2NH)(OOCCF3)] 2 to Ni0 complexes 3, 8, and 9 in the presence of amine borane 4 and amido borane 5.](/image/article/2011/SC/c0sc00483a/c0sc00483a-s3.gif) | ||
| Scheme 3 Different reaction pathways of [NiI(trop2NH)(OOCCF3)] 2 to Ni0 complexes 3, 8, and 9 in the presence of amine borane 4 and amido borane 5. | ||
Conclusions
In summary, we were able to isolate and fully characterize a very rare example of a nickel(I) olefin complex. This species serves as a precursor to remarkably active non-noble metal catalysts for the dehydrogenation of amine boranes.28 Again, olefinic binding sites proved suitable as steering ligands in a catalytic reaction while in reactions with transition metal complexes from the fourth period they commonly serve only as place holders for “vacant” coordination sites.Acknowledgements
This work was supported by the joint SNF-DFG project “Unconventional Approaches to the Activation of Dihydrogen” (2OPA21-126711/1) and the ETH Zürich.Notes and references
- For recent reviews concerning ammonia borane and related B–N compounds as emerging candidates for reversible hydrogen storage materials, see: (a) F. H. Stephens, V. Pons and R. T. Baker, Dalton Trans., 2007, 2613 RSC; (b) T. B. Marder, Angew. Chem., 2007, 119, 8262 CrossRef; T. B. Marder, Angew. Chem., Int. Ed., 2007, 46, 8116 CrossRef CAS; (c) H. W. Langmi and G. S. McGrady, Coord. Chem. Rev., 2007, 251, 925 CrossRef CAS; (d) C. W. Hamilton, R. T. Baker, A. Staubitz and I. Manners, Chem. Soc. Rev., 2009, 38, 279 RSC.
- (a) B. L. Davis, D. A. Dixon, E. B. Garner, J. C. Gordon, M. H. Matus, B. Scott and F. H. Stephens, Angew. Chem., Int. Ed., 2009, 48, 6812 CrossRef CAS; B. L. Davis, D. A. Dixon, E. B. Garner, J. C. Gordon, M. H. Matus, B. Scott and F. H. Stephens, Angew. Chem., 2009, 121, 6944 CrossRef; (b) S. Hausdorf, F. Baitalow, G. Wolf and F. Mertens, Int. J. Hydrogen Energy, 2008, 33, 608 CrossRef CAS; (c) N. C. Smythe and J. C. Gordon, Eur. J. Inorg. Chem., 2010, 509 CrossRef CAS; (d) A. D. Sutton, B. L. Davis, K. X. Bhattacharyya, B. D. Ellis, J. C. Gordon and P. P. Power, Chem. Commun., 2010, 46, 148 RSC; (e) A. Sutton, J. C. Gordon, K. C. Ott, A. K. Burrell, US 2010/0272622 A1, Los Alamos National Security, LLC, USA, 2010.
- For a comprehensive revision of dihydrogen reactivity, see: (a) H. Berke, Chem. Phys. Chem., 2010, 11, 1837 CAS. For a recent review of the current status of the hydrogen storage research and targets in the hydrogen storage materials, see: (b) S. Satyapal, J. Petrovic, C. Read, G. Thomas and G. Ordaz, Catal. Today, 2007, 120, 246 CrossRef CAS.
- For an overview about transition-metal-catalyzed DHC reactions of amine boranes, see: T. J. Clark, K. Lee and I. Manners, Chem.–Eur. J., 2006, 12, 8634 Search PubMed.
- (a) T. J. Clark, C. A. Russell and I. Manners, J. Am. Chem. Soc., 2006, 128, 9582 CrossRef CAS; (b) D. Pun, E. Lobkovsky and P. J. Chirik, Chem. Commun., 2007, 3297 RSC; (c) Y. Luo and K. Ohno, Organometallics, 2007, 26, 3597 CrossRef CAS; (d) M. I. Sloan, A. Staubitz, T. C. Clark, C. A. Russell, G. C. Lloyd-Jones and I. Manners, J. Am. Chem. Soc., 2010, 132, 3831 CrossRef CAS.
- (a) H. Dorn, R. A. Singh, J. A. Massey, J. M. Nelson, C. A. Jaska, A. J. Lough and I. Manners, J. Am. Chem. Soc., 2000, 122, 6669 CrossRef CAS; (b) C. A. Jaska, K. Temple, A. J. Lough and I. Manners, Chem. Commun., 2001, 962 RSC; (c) C. A. Jaska, K. Temple, A. J. Lough and I. Manners, J. Am. Chem. Soc., 2003, 125, 9424 CrossRef CAS; (d) C. A. Jaska and I. Manners, J. Am. Chem. Soc., 2004, 126, 9776 CrossRef CAS; (e) Y. Chen, J. L. Fulton, J. C. Linehan and T. Autrey, J. Am. Chem. Soc., 2005, 127, 3254 CrossRef CAS; (f) J. L. Fulton, J. C. Linehan, T. Autrey, M. Balasubramanian, Y. Chen and N. K. Szymczak, J. Am. Chem. Soc., 2007, 129, 11936 CrossRef CAS.
- M. E. Sloan, T. J. Clark and I. Manners, Inorg. Chem., 2009, 48, 2429 CrossRef CAS.
- (a) T. M. Douglas, A. B. Chaplin and A. S. Weller, J. Am. Chem. Soc., 2008, 130, 14432 CrossRef CAS; (b) T. M. Douglas, A. B. Chaplin, A. S. Weller, X. Z. Yang and M. B. Hall, J. Am. Chem. Soc., 2009, 131, 15440 CrossRef CAS; (c) R. Dallanegra, A. B. Chaplin and A. S. Weller, Angew. Chem., 2009, 121, 7007 CrossRef; R. Dallanegra, A. B. Chaplin and A. S. Weller, Angew. Chem., Int. Ed., 2009, 48, 6875 CrossRef CAS. For the isolation of hydrido complexes of base-stabilized boryl compounds that show a dimeric motif with three different amine borane activation modes, see: (d) A. B. Chaplin and A. S. Weller, Angew. Chem., 2010, 122, 591 CrossRef; A. B. Chaplin and A. S. Weller, Angew. Chem., Int. Ed., 2010, 49, 581 CAS; (e) A. B. Chaplin and A. S. Weller, Inorg. Chem., 2010, 49, 1111 CrossRef CAS.
- (a) M. C. Denney, V. Pons, T. J. Hebden, D. M. Heinekey and K. I. Goldberg, J. Am. Chem. Soc., 2006, 128, 12048 CrossRef CAS; (b) B. L. Dietrich, K. I. Goldberg, D. M. Heinekey, T. Autrey and J. C. Linehan, Inorg. Chem., 2008, 47, 8583 CrossRef CAS; (c) T. J. Hebden, M. C. Denney, V. Pons, P. M. B. Piccoli, T. F. Koetzle, A. J. Schultz, W. Kaminsky, K. I. Goldberg and D. M. Heinekey, J. Am. Chem. Soc., 2008, 130, 10812 CrossRef CAS; (d) A. Paul and C. B. Musgrave, Angew. Chem., 2007, 119, 8301 CrossRef; A. Paul and C. B. Musgrave, Angew. Chem., Int. Ed., 2007, 46, 8153 CrossRef CAS.
- (a) N. Blaquiere, S. Diallo-Garcia, S. I. Gorelsky, D. A. Black and K. Fagnou, J. Am. Chem. Soc., 2008, 130, 14034 CrossRef CAS; (b) M. Käβ, A. Friedrich, M. Drees and S. Schneider, Angew. Chem., 2009, 121, 922 CrossRef; M. Käβ, A. Friedrich, M. Drees and S. Schneider, Angew. Chem., Int. Ed., 2009, 48, 905 CrossRef; (c) A. Friedrich, M. Drees and S. Schneider, Chem.–Eur. J., 2009, 15, 10339 CrossRef CAS; (d) For an example of bis(σ–B–H) monomeric aminoborane complexes derived by simple metal-assisted dehydrogenation of amino boranes in a ruthenium catalyzed dehydrogenation process, see: G. Alcaraz, L. Vendier, E. Clot and S. Sabo-Etienne, Angew. Chem., 2010, 122, 930 Search PubMed; G. Alcaraz, L. Vendier, E. Clot and S. Sabo-Etienne, Angew. Chem., Int. Ed., 2010, 49, 918 CrossRef.
- (a) Y. F. Jiang and H. Berke, Chem. Commun., 2007, 3571 RSC; (b) Y. F. Jiang, O. Blacque, T. Fox, C. M. Frech and H. Berke, Organometallics, 2009, 28, 5493 CrossRef CAS.
- D. J. Liptrot, M. S. Hill, M. F. Mahon and D. J. MacDougall, Chem.–Eur. J., 2010, 16, 8508 CrossRef CAS.
- S. Kwan Kim, W. S. Han, T. J. Kim, T. Y. Kim, S. W. Nam, M. Mitoraj, Ł. Piekos, A. Michalak, S. J. Hwang and S. O. Kang, J. Am. Chem. Soc., 2010, 132, 9954 CrossRef.
- R. J. Keaton, J. M. Blacquiere and R. T. Baker, J. Am. Chem. Soc., 2007, 129, 1844 CrossRef CAS.
- (a) X. Yang and M. B. Hall, J. Am. Chem. Soc., 2008, 130, 1798 CrossRef CAS; (b) V. Pons, R. T. Baker, N. K. Szymczak, D. J. Heldebrant, J. C. Linehan, M. H. Matus, D. J. Grant and D. A. Dixon, Chem. Commun., 2008, 6597 RSC; (c) P. M. Zimmerman, A. Paul and C. B. Musgrave, Inorg. Chem., 2009, 48, 5418 CrossRef CAS; (d) P. M. Zimmerman, Z. Zhang and C. B. Musgrave, Angew. Chem., 2009, 121, 2235 CrossRef; P. M. Zimmerman, Z. Zhang and C. B. Musgrave, Angew. Chem., Int. Ed., 2009, 48, 2201 CrossRef CAS.
- H. Grützmacher, Angew. Chem., 2008, 120, 1838 CrossRef; H. Grützmacher, Angew. Chem., Int. Ed., 2008, 47, 1814 CrossRef.
- (a) S. E. Clapham, A. Hadzovic and R. H. Morris, Coord. Chem. Rev., 2004, 248, 2201 CrossRef CAS; (b) T. Ikariya, K. Murata and R. Noyori, Org. Biomol. Chem., 2006, 4, 393 RSC; (c) J. S. M. Samec, J.-M. Backvall, P. G. Andersson and P. Brandt, Chem. Soc. Rev., 2006, 35, 237 RSC.
- For the synthesis of the ligand trop2NH, see: P. Maire, T. Büttner, F. Breher, P. Le Floch and H. Grützmacher, Angew. Chem., 2005, 117, 6477 Search PubMed; P. Maire, T. Büttner, F. Breher, P. Le Floch and H. Grützmacher, Angew. Chem., Int. Ed., 2005, 44, 6318 CrossRef.
- (a) T. Zweifel, J. Naubron, T. Büttner, T. Ott and H. Grützmacher, Angew. Chem., 2008, 120, 3289 CrossRef; T. Zweifel, J. Naubron, T. Büttner, T. Ott and H. Grützmacher, Angew. Chem., Int. Ed., 2008, 47, 3245 CrossRef CAS; (b) T. Zweifel, J. Naubron and H. Grützmacher, Angew. Chem., 2009, 121, 567 CrossRef; T. Zweifel, J. Naubron and H. Grützmacher, Angew. Chem., Int. Ed., 2009, 48, 559 CrossRef CAS; (c) M. Trincado, H. Grützmacher, F. Vizza and C. Bianchini, Chem.–Eur. J., 2010, 16, 2751 CrossRef CAS.
- Several nickel(I)-arene complexes have been structurally characterized: (a) Y. F. Chen, C. Sui-Seng and D. Zargarian, Angew. Chem., 2005, 117, 7899 CrossRef; Y. F. Chen, C. Sui-Seng and D. Zargarian, Angew. Chem., Int. Ed., 2005, 44, 7721 CrossRef CAS; (b) M. Ito, T. Matsumoto and K. Tatsumi, Inorg. Chem., 2009, 48, 2215 CrossRef CAS; (c) A. Velian, S. Lin, A. J. M. Miller, M. W. Day and T. Agapie, J. Am. Chem. Soc., 2010, 132, 6296 CrossRef CAS. Several nickel(I)-olefin complexes have been investigated by EPR spectroscopy: (d) L. Bonneviot, D. Olivier and M. Che, J. Mol. Catal., 1983, 21, 415 CrossRef CAS; (e) V. V. Saraev, P. B. Kraikivskii, V. V. Annenkov, S. N. Zelinskiy, D. A. Matveev, A. I. Vilms, E. N. Danilovtseva and K. Lammertsma, Arkivoc, 2005, 44 CAS; (f) V. V. Saraev, P. B. Kraikivskii, S. N. Zelinskii, A. I. Vil'ms, D. A. Matveev, A. Y. Yunda, A. A. Fedonina and K. Lammertsma, Russ. J. Coord. Chem., 2006, 32, 397 CrossRef CAS. The structurally well-defined example of a coordinated η2-H2CCPh2 to NiI is given in: (g) G. Bai, P. Wei and D. W. Stephan, Organometallics, 2005, 24, 5901 CrossRef CAS.
- The minor species 2b and 2c are perhaps penta-coordinated solvent adducts and/or species with a κ1-OOCCF3 coordination mode instead of the κ2-OOCCF3 coordination mode. The isotropic g-value (2.190) measured at 298 K (featureless, relatively broad isotropic signal) corresponds roughly with the weighted averaged anisotropic g-values of the species 2a, 2b and 2c, suggesting that the rapidly frozen solution spectrum reflects approximately the kinetically trapped equilibrium distribution of these same species in solution.
- K[Me2NBH3] 5 was prepared by treatment of Me2HN–BH34 with 1.0 equiv of KH in THF at room temperature, according to the method adapted from: R. O. Hutchins, K. Learn, F. El-Telbany and Y. P. Stercho, J. Org. Chem., 1984, 49, 2438 Search PubMed . Control experiments with only K[Me2NBH3] or KOtBu showed no H2 evolution. The base/catalyst ratio is crucial for the performance of the catalytic reaction. Reducing the amount of base with respect to the catalyst loading proved to have a detrimental effect in conversion of Me2HN–BH34.
- The catalytic reaction can be re-loaded with five charges of amine borane without loss of activity. No color change of the solution or precipitation of nickel metal was observed. Only after addition of the sixth charge of substrate, the release of H2 slowed down but went to completion (release of 1 equivalent of H2). The catalytic reaction performed in the presence of elemental mercury did not show any reduction of the activity of the catalytic system. This finding may suggest no participation of colloidal heterogeneous nickel metal catalysts.
- This observation indicates that PPh3 dissociation from 3 is necessary and an unsaturated Ni complex like [Ni0(trop2NH)] is the catalytically active species which may coordinate Me2HNBH3 (4) as substrate.
- It has been suggested that the thermal dehydrogenation of Me2HN–BH34 to (Me2N–BH2)26 proceeds via the linear N–B–N–B intermediate 7: (a) G. E. Ryschkewitsch and J. W. Wiggins, Inorg. Chem., 1970, 9, 314 CrossRef CAS; (b) O. T. Beachley, Inorg. Chem., 1967, 6, 870 CrossRef CAS.
- For reviews and recent work see: (a) W. Lubitz, E. Reijerse and M. van Gastel, Chem. Rev., 2007, 107, 4331 CrossRef CAS; (b) A. Volbeda and J. C. Fontecilla-Camps, Dalton Trans., 2003, 4030 RSC; (c) F. A. Armstrong and S. P. J. Albracht, Philos. Trans. R. Soc. London, Ser. A, 2005, 363, 937 CrossRef CAS; (d) O. Nilsson Lill and P. E. M. Siegbahn, Biochemistry, 2009, 48, 1056 CrossRef CAS; (e) J. M. Keith and M. B. Hall, Inorg. Chem., 2010, 49, 6378 CrossRef CAS.
- Dinuclear hydride nickel complexes have been reported briefly in the literature: (a) K. R. Porschke, W. Kleirnann, G. Wilke, K. H. Claus and C. Kruger, Angew. Chem., 1983, 105, 451; K. R. Porschke, W. Kleirnann, G. Wilke, K. H. Claus and C. Kruger, Angew. Chem., Int. Ed. Engl., 1983, 22, 991 CrossRef; (b) G. Longoni, M. Manasserob and M. Sansoni, J. Organomet. Chem., 1979, 174, C41 CrossRef CAS; (c) J. J. Garcia and W. D. Jones, Organometallics, 2000, 19, 5544 CrossRef CAS; (d) S. Pfirrmann, C. Limberg, C. Herwig, R. Stößer and B. Ziemer, Angew. Chem., Int. Ed., 2009, 48, 3357 CrossRef CAS; S. Pfirrmann, C. Limberg, C. Herwig, R. Stößer and B. Ziemer, Angew. Chem., 2009, 121, 3407 CrossRef. For interesting examples of H2 formation form dinuclear hydride nickel(II) complexes, see: (e) S. Pfirrmann, C. Limberg and B. Ziemer, Dalton Trans., 2008, 6689 RSC; (f) S. Pfirrmann, S. Yao, B. Ziemer, R. Stößer, M. Driess and C. Limberg, Organometallics, 2009, 28, 6855 CrossRef CAS.
- Preliminary results show that the parent amino borane, (NH3BH3), is dehydrogenated with equal efficiency under loss of one equivalent of H2. Further results using related substrates are also currently under investigation.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC reference numbers 767256–767257. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c0sc00483a |
| ‡ Dedicated to Prof. Dr José Barluenga on the occasion of his 70th birthday |
| This journal is © The Royal Society of Chemistry 2011 |

